Abstract
Mitochondria are essential cytoplasmic organelles, critical for cell survival and death. Recent mitochondrial research revealed that mitochondrial dynamics – the balance of fission and fusion in normal mitochondrial dynamics – is an important cellular mechanism in eukaryotic cell and is involved in the maintenance of mitochondrial morphology, structure, number, distribution, and function. Research into mitochondria and cell function has revealed that mitochondrial dynamics is impaired in a large number of aging and neurodegenerative diseases, and in several inherited mitochondrial diseases, and that this impairment involves excessive mitochondrial fission, resulting in mitochondrial structural changes and dysfunction, and cell damage. Attempts have been made to develop molecules to reduce mitochondrial fission while maintaining normal mitochondrial fusion and function in those diseases that involve excessive mitochondrial fission. This review article discusses mechanisms of mitochondrial fission in normal and diseased states of mammalian cells and discusses research aimed at developing therapies, such as Mdivi, Dynasore and P110, to prevent or to inhibit excessive mitochondrial fission.
Introduction
Several lines of evidence suggest that abnormal mitochondrial dynamics is associated with aging; with neurodegenerative diseases, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), multiple sclerosis, and amyotrophic lateral sclerosis (ALS); and with several inherited mitochondrial diseases. Mitochondrial dynamics is the delicate balance between mitochondrial fission and mitochondrial fusion in mammalian cells, including neurons. Mitochondrial fission involves the division or fragmentation of one healthy mitochondrion into 2, and mitochondrial fusion involves the integration of 2 mitochondria (healthy or defective) into one elongated one. Fission and fusion are essential processes that maintain mitochondrial structure, size, shape, morphology, number, and distribution in mammalian cells, including neurons. Mitochondria are synthesized in the soma and travel along exons, dendrites, and synapses; and supply energy for many synaptic functions of neurons.
In healthy neurons, fission and fusion balance equally and maintain mitochondrial dynamics and distribution [1–2]. However, in neurons that express mutant proteins – such as amyloid beta (in AD) [3–9], Tau (in AD) [10–14], mutant huntingtin or mutant Htt (in HD) [15–20], mutant SOD1 (in ALS) [21–22], and mutant LRRK2 [23] or mutant parkin or mutant DJ1 (in PD) [24] and overexpression of α-synuclein [25] -- the balance of fission and fusionis altered, leading to structural and functional abnormalities in the mitochondria and, ultimately, to neuronal damage. Abnormal mitochondrial dynamics is caused by an imbalance in highly conserved GTPase genes, which are essential for mitochondrial fission and fusion. GTPase genes – dynamin-related protein 1 (Drp1), fission 1 (Fis1), mitofusins 1 and 2 (Mfn1, Mfn2), and optic atrophy 1 (Opa1) – regulate, maintain, and remodel mammalian mitochondria [2].
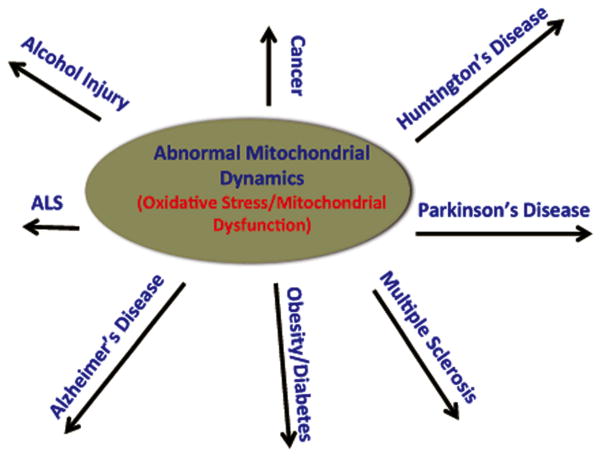
Recent research has revealed that abnormal mitochondrial dynamics is an early event in several diseases that involve oxidative stress and mitochondrial dysfunction, including AD [3–9,61], HD [15–20], PD [23–25], ALS [21–22] multiple sclerosis [26–31], cancer [33–34], diabetes [35–39], obesity [40–41], and alcohol injury [42–46] (Fig 1). Extensive recent research has also revealed that increased mitochondrial fission is a key factor in mitochondrial dysfunction, probably caused by mutant protein(s) interacting with Drp1, resulting in the initiation of abnormal mitochondrial fission. Recently, some progress has been made in developing molecules that are capable of inhibiting excessive mitochondrial fragmentation in order to treat patients with neurodegenerative diseases.
Figure 1.
Illustration showing human diseases that are involved with oxidative stress/mitochondrial dysfunction and abnormal mitochondrial dynamics as an early event in the disease process.
In research aimed at developing treatments for patients with neurological diseases, progress – although limited – has recently been made in developing molecules that are capable of inhibiting increased mitochondrial fission. The purpose of this article is to summarize recent progress in the development of therapiesto reduce increased mitochondrial fission and to maintain balanced mitochondrial dynamics, healthy mitochondrial function, and normal neuronal activity in neurodegenerative diseases, particularly in AD and HD. This article also highlights mechanisms of excessive mitochondrial fission in AD and HD, and discusses molecules that have been found to inhibit mitochondrial fission and enhance neuronal function.
Mitochondrial dynamics
Mitochondria are cytoplasmic organelles – bag-like structures found in eukaryotic cells. Mitochondria are present where multiple cellular processes occur, including respiration and energy production. Mitochondria are comprised of an outer and inner biolipid membranes, and a matrix. The outer membrane is highly porous and allows the flow of small molecules and metabolites into its inner-membrane space. The inner membrane is nonporous and does not allow ionic flow into the mitochondrial matrix. The electron transport chain is present within the inner membrane that participates in the oxidative phosphorylation (OXPHOS) and in the production of essential ATP for cellular functioning [47–49]. The reactive oxygen species (ROS) are generated due to electron leaks in the electron transport chain as a byproduct of OXPHOS and ATP synthesis. The mitochondrial matrixharbors beta-oxidation and the TCA cycle [47–49].
Mitochondria are constantly undergoing fission and fusion, and altering their size and shape while traveling through neurons, from the bodies to nerve terminals and synapses of neurons, the sites of high-energy demand. Fission and fusion are equally balanced in healthy cells. Mitochondrial fragmentation is generally referred in a disease state such as AD, HD and PD, in which mitochondria are excessively fragmented in affected neurons, may be due to increased oxidation and ROS production [48].
In the normal mitochondrial fusion process, metabolites, DNA, and proteins exchange and re-energize and survive longer and support essential energy in the form of ATP to cells. This fusion process is not found in cells that have increased mitochondrial fission, where researchers have found the increased expression levels of the fission genes Drp1 and Fis1 and/or the reduced expression of the fusion genes Mfn1, Mfn2, and Opa1 [1]. Genetic mutations in Mfn2 and Opa1 have been found to cause Char coat-Marie-Tooth disease type2A [1]. Further, Mfn2 and Opa1 have been found to cause dominant optic atrophy, and increased mitochondrial fusion found, leading to abnormal mitochondria function.
Normal mitochondrial fission is regulated and maintained by highly conserved two GTPase genes: Fis1 and Drp1. Drp1 is a cytosolic protein and is translocated to the outer membrane of the mitochondria and interacts with multiple proteins, including Fis1, mitochondrial fission factor (Mff) and MiD49 and Mid51 [1,50–52], and Mff, Fis1, MiD49 and Mid51act as receptors for Drp1 and completes mitochondrial division.
Mitochondrial fusion is regulated and maintained by the GTPase genes Mfn1, Mfn2, and Opa1. Opa1 is a protein located in the mitochondrial inner membrane, and Mfn1 and Mfn2 are proteins located in the mitochondrial outer membrane. The C-terminal part of Mfn1 mediates oligomerization between Mfn molecules of adjacent mitochondria and facilitates mitochondrial fusion [1,50–54]. Mitochondrial fusion protects cells from the toxic effects of mitochondrial DNA by allowing the functional complementation of mitochondrial DNA, proteins, and metabolites between two adjacent mitochondria.
Mitochondrial function is largely governed by mitochondrial fission and fusion in mammalian cells. Balanced fission and fusion is an important factor for the integrity, structure, and function of healthy mitochondria. Abnormal and/or impaired fission or fusion directly impacts mitochondrial function, for example resulting in excessive production of free radicals, altered mitochondrial enzymatic activities, impaired calcium homeostasis, low ATP production, and overall reduced energy metabolism in mammalian cells.
Abnormal mitochondrial dynamics in neurodegenerative diseases
Several recent mitochondrial studies of neurodegenerative diseases, such as AD, HD, ALS, and PD, revealed that excessive mitochondrial fission occurs mainly in neurons that produce high levels of ROS and that exhibit defective mitochondrial function. Further, several biochemical studies revealed that mutant proteins, including Aβ (in AD) [3–9], phosphorylated Tau (in AD) [10–14,54], mutant Htt (in HD) [15–20], and mutant LRRK2 [23] or mutantDJ1 (in PD) [24] and overexpression of α-synuclein [25] are interacted with mitochondria in affected neurons and that this interaction is primarily responsible for increased levels of free radicals, ultimately causing an imbalance between mitochondrial fission and fusion, and mitochondrial dysfunction and neuronal damage.
Abnormal mitochondrial dynamics and mitochondrial dysfunction in Alzheimer’s disease
Using biochemical, molecular, and ultra-structural studies, several groups are studied mitochondrial structure, morphology, and mitochondrial dynamics in postmortem brains from AD patients and from AD transgenic mice; primary neurons from AD mice; and neuronal cells transfected with mutant AD genes, such as APP, tau and cybrids from AD patients [3–9].
Silva et al. [9] modeled AD and mild cognitive impairment bioenergetic dysfunction by transferring mitochondria from mild cognitive impairment AD patients, and platelets from the brains of control subjects to mtDNA-depleted SH-SY5Y cells. Researchers found bioenergetic fluxes and bioenergetics-related infrastructures in the cells. Relative to the control cybrids, the AD and MCI cybrids showed changes in oxygen consumption, respiratory coupling and glucose utilization. The AD and MCI cybrids had higher ADP/ATP and lower NAD (+)/NADH ratios, and exhibited differences in proteins (including HIF1α, PGC1α, SIRT1, AMPK, p38 MAPK and mTORP) that monitor, respond to, or regulate cell bioenergetic fluxes. Several endpoints suggested mitochondrial mass increased in the AD cybrid group and, probably to a lesser extent, in the MCI cybrid group, and that the mitochondrial fission-fusion balance shifted towards increased fission in the AD and MCI cybrids.
Using postmortem brains from AD patients at different stages of AD progression and control subjects, and molecular and cell biology methods, Manczak et al. [6] studied mitochondrial fission and fusion in AD-affected neurons. We found increased expression of Drp1 and Fis1, and decreased expression of Mfn1, Mfn2, Opa1, and Tomm40. The matrix gene CypD was up-regulated in AD patients. Findings from these quantitative RT-PCR and immunoblotting analyses suggest that abnormal mitochondrial dynamics increase as AD progresses.
Using molecular, biochemical, and transmission electron microscopy methods, Manczak et al. [5,7] studied mRNA and protein levels of mitochondrial fission (Drp1 and Fis1) and fusion (Mfn1, Mfn2, and Opa1) genes in primary neurons from APP transgenic mice (Tg2576 line) and mouse neuroblastoma cells treated with the Aβ peptide. Similar to the results in studies using postmortem AD brains, we found increased levels of Drp1 and Fis1; reduced Mf1, Mfn2, and Opa1 mRNA. These results suggest the presence of impaired mitochondrial dynamics in AD neurons, mainly increased fission [6,7]. Further, using transmission electron microscopy, we found significantly increased numbers of small and rounded broken mitochondria in the Aβ-treated neurons. In addition, we found an increased accumulation of oligomeric Aβ and increased apoptotic neuronal death in the primary neurons from the AβPP mice relative to the non-transgenic wild-type neurons. These findings revealed an accumulation of intraneuronal oligomeric Aβ, leading to mitochondrial and synaptic deficiencies, and ultimately causing neurodegeneration in AβPP neurons [7].
Using electron and confocal microscopy, gene expression analysis, and biochemical methods, Manczak et al. [5] studied mitochondrial structure and function, and neurite outgrowth in mouse neuroblastoma (N2a) cells treated with Aβ or the Aβ peptide 25–35. In the N2a cells treated with only Aβ, we found increased expressions of Drp1 and Fis1, indicating increased mitochondrial fission, and they found decreased expressions of Mfn1, Mfn2, and Opa1, indicating decreased mitochondrial fusion. Thus, these results point to the presence of abnormal mitochondrial dynamics in AD neurons. Transmission electron microscopy of AD neurons treated with Aβ revealed a significant increase in mitochondrial fission, further supporting abnormal mitochondrial dynamics in AD. Manczak et al. [5] -- also found significantly decreased neurite outgrowth and decreased mitochondrial function in cells treated with Aβ peptide [5].
Using human neuroblastoma (M17) cells that are known to stably express mutant AβPP and produce Aβ levels, Wang et al. [3–4] investigated the effects of AβPP and Aβ on mitochondrial structural changes. In their 2008 publication, they reported that 40% of the M17 cells over expressing wild-type APP and 80% of the M17 cells overexpressing mutant AβPP displayed alterations in mitochondrial morphology, particularly fragmented mitochondria. They also found reduced levels of Drp1, Mfn1, Mfn2, and Opa1 proteins in the M17 cells. In their 2009 publication [4], they found that the involvement of Aβ in abnormal mitochondrial dynamics, by assessing changes in the expression of mitochondrial fission and fusion proteins in AD brains. They found that mitochondria were redistributed away fromaxons in the pyramidal neurons of AD brains; that levels of Drp1, Opa1, Mfn1, and Mfn2 were significantly reduced, whereas levels of Fis1 were significantly increased in the M17 cells and from AD brains; and that reduced mitochondrial density in the cell periphery of M17 cells and in the neuronal process of primary neurons correlated with reduced spine density in the neurites. Interestingly, oligomeric Aβ-derived diffusible ligands caused mitochondrial fragmentation and reduced mitochondrial density in the neuronal processes. Further, Aβ-derived diffusible ligands induced the loss of dendritic spine and postsynaptic density protein 95 puncta, the loss of which also correlated with an abnormal distribution of mitochondria in AD neurons [4].
Trushina and colleagues [8] studied early structural alterations in mitochondria and oxidative stress in APP, PS1, and APP/PS1 transgenic mouse models of AD. They found that each AD mutation model exhibited a unique mitochondrial motility, distribution, dynamics, morphology, and metabolomics profile. The PS1 and APP/PS1 mice also exhibited reduced mitochondrial trafficking in primary neurons, suggesting an increased susceptibility of neurons to excitotoxic cell death. Using electron micrographology, they 3-D reconstructed neurons and found swollen mitochondria that were connected to each other like beads on a string, suggesting that increased mitochondrial fission in AD neurons. Further, they found a loss of integrity in synaptic mitochondria and energy production in all 3 lines of mice [8].
In another set of studies investigating the relationship between Tau and mitochondria in AD neurons, researchers found increased mitochondrial fission caused by overexpressed N-terminal tau, caspase cleaved tau, and phosphorylated tau [10–13]. However, a recent Drosophila study investigating the role of Tau in mitochondrial dynamics revealed that full-length tau overexpression caused increased mitochondrial elongation through actin-stabilization-mediated mislocated Drp1 [14].
Overall, these findings suggest that Aβ, in association with mitochondria, causes excessive mitochondrial fission and reduced mitochondrial fusion, leading to mitochondrial dysfunction and neuronal damage in AD-affected neurons. These findings also suggest that inhibitors of excessive mitochondrial fission could be potential therapeutic targets in order to balance mitochondrial dynamics in AD neurons.
Mechanisms of excessive mitochondrial fission
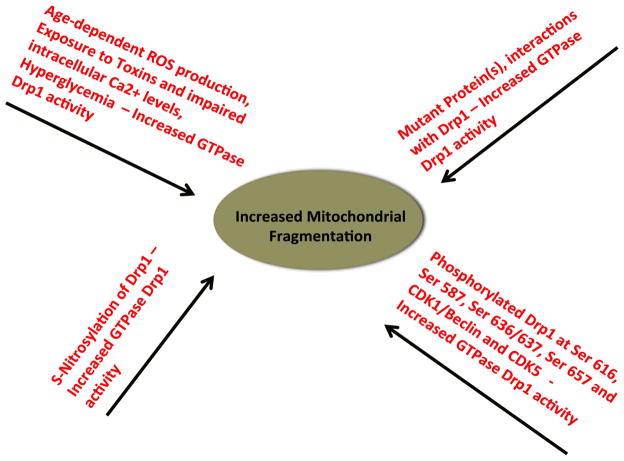
Although there is increasing evidence that mitochondrial fission is extensive in neurons affected by AD, HD, and, to some extent, PD, the precise mechanism underlying mitochondrial fission is not well understood. However, the following mechanisms have been proposed, based on recent studies using cellular and molecular biology, animal and cell models of AD, HD, and PD, and postmortem brains from patients with AD and HD (Fig. 2):
Figure 2.
Illustration showing mechanisms of excessive mitochondrial fragmentation in human diseases.
production of ROS is excessive, which activates fission proteins and increases GTPase Drp1 enzymatic activity, ultimately affecting the structural integrity of mitochondria and increasing mitochondrial fission,
a mutant protein(s), such as mutant Htt, Aβ, or DJ1/LRRK2, interacts with Drp1 and increases GTPase–Drp1 enzymatic activity, which increases mitochondrial fission and creates an imbalance in mitochondrial dynamics.
S-nitrosylation of Drp1 enhances GTPase Drp1 activity, causing excessive mitochondrial fission,
phosphorylated Drp1 at Ser 616, Ser 585, and Ser 637 sites elevates GTPase activity, causing increased mitochondrial fission.
Here we discuss these proposed mechanisms of mitochondrial fission.
-
An age-dependent increase in ROS production in mammalian cells, including neurons, has been extensively reported in the literature. This increase may be due to increased electron leaks in OXPHOS and defective OXPHOS and low synthesis of ATP. This increased leakage, in turn, might lead to increased energy demands in cells, resulting in increased mitochondrial fission and imbalanced mitochondrial dynamics. The overproduction of ROS has been found to occur in cells undergoing increased mitochondrial fission [55–58]. Further, altered mitochondrial structure has been reported in cells exposed to toxins, such as rotenone and nitric oxide [55–58] and high glucose levels [59].
An age-dependent increase in calcium within the mitochondria has been reported to induce ROS production [60], and this increase may ultimately cause excessive mitochondrial fission in AD neurons. In this study, cytosolic Drp1 was found to be translocated to the outer membrane of mitochondria, which formed punctate spots on mitochondria and initiated mitochondrial fission.
-
In postmortem brain specimens from AD [6], and HD [19–20] mutant protein(s)interacting with Drp1 have been reported to increase GTPase Drp1 enzymatic activity. As discussed above, using real-time RT-PCR and immunoblotting analysis, we studied mitochondrial dynamics in postmortem brains from patients [6] and from APP transgenic mice [7], and mitochondrial dynamics in mouse neuroblastoma cells treated with the Aβ25–35 peptide [5]. We found increased mRNA and increased levels of the fission proteins Drp1 and Fis1; reduced RNA and reduced levels of the fusion proteins Mfn1, Mfn2 and Opa1; and increased levels of CypD and VDAC1.
We also investigated the interaction between Drp1 and Aβ, and the interaction between phosphorylated tau and GTPase Drp1 enzymatic activity, using postmortem brain specimens from patients with AD and from AD mice (APP, APP/PS1, and 3XAD.Tg mice) [54]. Interestingly, we found that Drp1 interacted with Aβ and phosphorylated tau in the human and mouse brain specimens. Further, we found that this interaction progressively increased as AD progressed. In addition, we observed that the GTPase Drp1 enzymatic activity also progressively increased as AD progressed [54]. We concluded that Drp1, interacting with Aβ and phosphorylated Tau enhances GTPase Drp1 enzymatic activity and causes excessive mitochondrial fission, resulting in imbalanced mitochondrial dynamics, defective execution of mitochondrial functions, and neuronal damage in AD-affected neurons from humans and from mice (Fig. 4).
Similar to AD, our lab also found mutant Htt interaction with Drp1 in postmortem brains from HD patients and cortical tissues from BACHD mice, leading to increased levels of GTPase Drp1 enzymatic activity and causes excessive mitochondrial fission, imbalanced mitochondrial dynamics in HD-affected neurons.
Bossy-Wetzel group [20] also reported mutant Htt interaction with Drp1 using YAC-HD transgenic mice and HD postmortem brains and peripheral cells from HD patients, further supporting that abnormal interactions between Drp1 and mutant Htt causes excessive mitochondrial fission and defective mitochondrial function in HD neurons.
-
Recently, Lipton’s group [61–62] reported that S-nitrosylation of Drp1 caused mitochondrial fission in brain neurons from AD and HD patients. Cho et al. studied S-nitrosylation of Drp1 in AD neurons. They found increased S-nitrosylation of Drp1 and GTPase Drp1 activity in AD neurons. Cho et al also prevented the nitrosylation of Drp1 by introducing a cysteine mutation in the cDNA of Drp1, which reduced S-nitrosylation of Drp1 and abrogated neurotoxic events in the AD neurons [61].
Huan et al. [62] studied to determine whether the S-nitrosylation ofDrp1 contributed to the pathogenesis of HD. They found that mutant Htt protein in primary neurons triggered a significant increase in nitric oxide. Consistent with this result, increased levels of S-nitrosylation in Drp1 were found in the striatum of an HD transgenic mice as well as in the neurons from HD patients. Using specific fluorescence markers, they demonstrated that S-nitrosylation of Drp1 induced excessive mitochondrial fission, which was followed by loss of dendritic spines, signifying synaptic damage. They transfected cDNA with non-nitrosylatable mutant Drp1 (C644A), or they blocked the production of NO using an nitric oxide synthase inhibitor. They found that both of these therapies significantly reduced neurotoxic events. These findings suggest that S-nitrosylation of Drp1 is a key mediator of mutant Htt toxicity.
Based on findings from AD and HD studies, Lipton et al. [61–62] proposed that nitric oxide, produced in response to an increase in Aβ and/or mutant Htt, may be a key mediator of disease progression, mitochondrial fission, synaptic loss, and neuronal damage, in part via the S-nitrosylation of Drp1. However, Bossy-Wetzel’s group [63] followed the Cho et al. [61] procedures and could not reproduce the Lipton’s S-nitrosylation of Drp1 data. Additional research is needed to clarify the involvement of Drp1 S-nitrosylation in mitochondrial fission and neuronal death in AD and HD.
-
Several research groups recently reported the phosphorylation of Drp1 at Ser 616, Ser 637, Ser 585, and Ser 656; and increased GTPase activity and mitochondrial fission [64–70].
Cribbs and Strack [64] identified the phosphorylation of Drp1 at Ser 656, the site at which Drp1 is phosphorylated by the cyclic AMP-dependent protein kinase. The Ser 656 site is dephosphorylated by calcineurin, and the phosphorylated state of Ser 656 is controlled by sympathetic tone calcium levels and cell viability. De-phosphorylation of Drp1 by genetically mutating Ser 656into aspartic acid led to the elongation of mitochondria and conferred resistance against various pro-apoptotic insults. Further, the dephosphorylation of Ser 656 into the Ala mutant Drp1 promotes mitochondrial fission and increases cell vulnerability to cells. Cribbs and Strack concluded that the phosphorylation of Drp1 at Ser 656 may provide a mechanism for the integration of cAMP and calcium levels in the control of mitochondrial structure and viability, such as in terms of the shape of mitochondria and apoptosis.
Taguchi et al. [66] studied the phosphorylation of Drp1 at Ser 585, using an in vitro system (HeLA cells) and RNA-silencing of Drp1. They studied the mitochondrial network in HeLa cells. They found tubular mitochondria fragmented in early mitosis and Drp1 specifically phosphorylated in mitosis byCDK1/cyclin B on Ser 585. They also found an exogenous expression of unphosphorylated mutant Drp1 S585A, leading toreduced mitochondrial fission in HeLa cells. These results suggest that the phosphorylation of Drp1 at Ser-585 promotes mitochondrial fission in mitotic cells.
Using HeLa cells and biochemical methods, Chang and Blackstone [65] studied Drp1 phosphorylation and GTPase activity. They found that cAMP-dependent, protein kinase-dependent phosphorylation of Drp1 within the GED domain at Ser 637 and that this phosphorylated Drp1inhibited GTPase activity. This effect likely resulted from a decrease in the interaction of GTP-binding/middle domains with the GED domain because the phosphomimetic S637D mutation is known to impair intramolecular interactions but not Drp1-Drp1 intermolecular interactions. Further, using the phosphomimetic S637D substitution they found that mitochondrial fission was prominently inhibited in cells. Thus, the phosphorylation of Drp1 at Ser 637 resulted in alterations in Drp1 function and mitochondrial morphology that are likely involved in increased mitochondrial fission.
Phosphorylated Drp1at Ser 616 (antibody developed by Cell Signaling) has been extensively studied by several groups to clarify the role of Ser 616 in GTPase Drp1 activity and mitochondrial fission [4,67–69]. Wang et al. [4] reported increased phosphorylation of Drp1 at Ser616 in AD brains and in cells after Aβ-derived diffusible ligands treatment [4]. Overall, Drp1 phosphorylation has been found to be activated at Ser 616, leading to increased mitochondrial fission.
Using primary neuronal cultures, RNA silencing methods, and CDK5 inhibitors, Meuer et al. [70] studied the role of CDK5 in mitochondrial morphology, tubular networks, and mitochondrial fission. They found that mitochondrial fission is an early and kinetically invariant event during neuronal cell death, which causally contributes to cytochrome c release and neuronal apoptosis. Using a small molecule CDK5 inhibitor, as well as a dominant-negative CDK5 mutant and RNAi knockdown procedures, they identified CDK5 as an upstream signaling kinase that regulates mitochondrial fission during apoptosis of neurons. They also showed that mitochondrial fission is a modulator contributing to CDK5-mediated neurotoxicity [70].
It is possible that Drp1 phosphorylation at Ser 616 is triggered by CDK5 activation, leading to excessive mitochondrial fission and apoptotic cell death in neurons affected by AD and HD. Further research is needed to understand the molecular and cellular links between Drp1 phosphorylation at Ser 616 and CDK5 activation in diseased states.
Overall, these studies suggest that phosphorylated Drp1and elevated levels of GTPase enzymatic activity increase mitochondrial fission. Further research is still needed to understand the involvement of phosphorylated Drp1 at Ser 616 and CDK5 activation, if any, and mitochondrial fission in diseases states, such as AD and HD.
Inhibitors of mitochondrial fission as a therapeutic strategy
Since mitochondrial fission has been found to be increased in affected neurons of neurodegenerative diseases, inhibitors of mitochondrial fission may hold promise as therapeutic targets to treat patients with such neurodegenerative diseases as AD and HD. In the last 5 years, there has been some progress in identifying and developing inhibitors of mitochondrial fission. Three inhibitors have been identified: the molecules Mdivi [71], P110 [72], and Dynasore [73].
Recently, several studies used these molecules and investigated the efficacies and adverse effect, if any in rodent models of human diseases. We discussed the progress made so far below:
Mdivi
Using a yeast two-hybrid system, Cassidy et al. [71] screened several chemical libraries to identify molecules that may reduce mitochondrial fission and enhance the mitochondrial network. They identified Mdivi and found that it inhibited the assembly of Drp1 and GTPase Drp1 enzymatic activity in vitro while not inhibiting mitochondrial fusion. Mdivi binds outside the GTPase domain that is involved in oligomeric assembly, thereby inhibiting GTPase activity.
Xie et al. [74] studied the protective role of mdivi-1 in hippocampal neuron death after seizures induced by pilocarpine. They found that pretreatment of mice with mdivi-1 significantly attenuated the neuronal death in seizures that were initiated in the hippocampus. In addition, the seizures resulted in an up-regulation of Drp1 expression. The mdivi-1 treatment had no effect on Drp1 expression. They also found that mdivi-1 treatment reversed the release of the CytC translocation of apoptosis-inducing factorinduced by seizures while inhibiting the activated caspase-3 They concluded that mdivi-1 exerts neuroprotective effects against seizure-induced cell death of hippocampal neurons.
In an in vivo study, Qiu et al. [75] investigated whether mdivi-1 could suppress mitochondrial fission in an epileptic model of rats. They found that, after seizures, mitochondrial fission increased and mdivi-1 significantly attenuated oxidative stress and reduced neuronal loss after seizures, and the increased surviving neurons in the hippocampus. Mitochondrial fission was up-regulated in rats that underwent seizures, and that the inhibition of mitochondrial fission via Mdivi protects against injury. Findings from this study provide a mechanism that Mdivi protects against ROS and mitochondrial toxicity
Zhang et al. [76] investigated the protective effects of mdivi-1 on a cerebral ischemia/reperfusion injury in a cerebral artery occlusion mouse model. They found thatMdivi-1 significantly reduced cerebral damage that was induced by ischemia/reperfusion. The treatment of ischemia/reperfusion mice with Mdivi-1 blocked apoptotic death of cells involved in cerebral ischemia and reperfusion injury, and significantly decreased the level of Drp1 and CytoC in ischemia/reperfusion.
Wappler et al. [77] tested the effects of two Drp1 blockers – Mdivi-1 and 15-deoxy-Δ12,14-Prostaglandin J2 (PGJ2) – on mitochondrial dynamics and cell survival. One hour of oxygen glucose deprivation (OGD) hadminimal impact on neuronal viability, but mitochondria appeared condensed. Three hours of OGD treatment caused a 60% reduction in neuronal viability. After these 3 hours of treatment, a small percentage of the primarily tubular mitochondriamorphed into rounded mitochondria, but the same percentage of the round mitochondria remained round. This resulted in increased levels of VDAC, Complex V, and mtDNA, a reduction in Mfn2 expression that was not as great in comparison to control cells, an increase in Mfn1 expression compared to the increase in Mfn2, and no changes in either Opa1 or Fis1. Although PGJ2 increased the polymerization of Drp1, PGJ2 did not reduce cell death or alter mitochondrial morphology following OGD, and in this context, Mdivi-1 did not protect neurons against OGD.
Tang et al. [78] aimed to identify the effects of Mdivi on mitochondrial function and renal tubular cell apoptosis in rhabdomyolysis (RM)-induced AKI when Mdivi was used as a treatment to suppress the accumulation of Drp-1 in mitochondria. In RM model was induced by intramuscular injection of glycerol into Sprague Dawley rats. Twenty-four and 48 hours after intraperitoneal injections of Mdivi-the researchers studied kidney function, changes in kidney pathology the extent that Drp-1 accumulated in tubular mitochondria, mitochondrial function, and tubular epithelial cell apoptosis. RM was found to induce Drp1 accumulation, decrease ATP production, and increase ROS in mitochondria. In a time-dependent manner, as cytochrome c expression increased, so did cell apoptosis, but kidney function decreased. At both time points, although Mdivi-1 did not significantly influence the overall expression of Drp1, Mdivi-1 suppressed Drp-1 accumulation, inhibited the entry of proapoptotic Bax intomitochondria, and inhibited the release of cytochrome c thus ameliorating cell apoptosis. Tang et al. concluded that in RM-induced AKI, the suppression of Drp1 accumulation favored the maintenance of mitochondrial function and reduced the apoptosis of tubular cells.
Chlystum et al. [79] studied the role of AnxA6 in regulating mitochondrial morphogenesis. AnxA6 is a phospholipid binding protein that has been implicated in mediating the aggregation of endosomes and in having a role in vesicle fusion during exocytosis. They characterized fibroblasts from AnxA6 (−/−) mice. They found that, in cells lacking anxA6, the mitochondria were fragmented, respiration is impaired, and the membrane potential of mitochondria was reduced compared to wild-type fibroblasts. Mitochondrial Ca(2+) uptake in the fibroblasts was reduced and cytosolic Ca(2+) transients were elevated. These results led to the investigation of possible interactions between anxA6 and proteins with known roles in mitochondrial fusion and fission, in AnxA6(−/−) fibroblasts. Chlystum et al. found that anxA6 was associated with Drp1 and that mdivi-1 prevented the fission of mitochondria. In normal cells, the elevation of intracellular Ca(2+) disrupted the interaction between anxA6 and Drp1, resulting in the displacement of anxA6 to the plasma membrane and an increase in mitochondrial fission. These results suggest that anxA6 inhibits Drp1 activity and that Ca(2+)-binding to anxA6 relieves this inhibition, with the result that Drp1mediates mitochondrial fission.
Park et al. [80] studied whether acute elevation of intraocular pressure (IOP) alters Drp1 and whether mdivi-1 can a selectively inhibit Drp1, in order to block apoptotic cell death and thus to increase the survival of retinal ganglion cells in the retina of ischemic mice. The 57BL/6 mice received injections of mdivi-1 or vehicle, and then transient retinal ischemia was induced by the acute elevation of IOP. The survival of retinal ganglion cells was measured after FluoroGold labeling. Using Western blot and immunohistochemistry, Park et al. [80] assessed Drp1 and the expression and distribution of the glial fibrillary acidic protein at 12 hours after ischemia-reperfusion, and assessed apoptotic cell death by TUNEL staining. They found that Drp1 and the expression of the glial fibrillary acidic protein in the ischemic mouse retina were significantly higher within 12 hours of reperfusion. Mdivi-1 blocked apoptotic cell death in the ischemic retina and increased the survival of retinal ganglion cellsat 2 weeks after ischemia. In the ganglion cell layer, Drp1 immunoreactivity was strong in retinal ganglion cells. Drp1 protein expression increased at 12 hours, in the ganglion cell layer of those ischemic mice who received mdivi-1 treatment. The treatment of mice with Mdivi 1 did not affect this increase, but it did significantly decrease the expression of Drp1.
P110
Qi et al. [72] designed a Drp1 inhibitor, referred to as P110, to reduce aberrant mitochondrial fission. Initial studies have found that P110 blocks Drp1, inhibit Drp1 enzyme activity, decreases Fis1, and reduces excessive mitochondrial fission in vitro and in cultured neurons. Furthermore, P110 was found to be neuroprotective by its ability to inhibit mitochondrial fission and ROS production, and the subsequent improvement in the mitochondrial membrane potential and mitochondrial integrity. P110 increased neuronal cell viability by reducing apoptosis and autophagic cell death, and it reduced neurite loss of primary dopaminergic neurons in a PD cell culture model. Additional research is needed, but based on this early investigation P110 may be promising as strategy to treat neurodegenerative diseases that involve excessive mitochondrial fission and mitochondrial dysfunction.
Dynasore
There are limited number of published studies on the protective effects of Dynasore to inhibit mitochondrial fission and apoptotic cell death in animal models. Dynasore is a dynamin GTPase inhibitor of endocytic pathways that is known to prevent the division or formation of dynamin-dependent endocytic vesicles.
Marcia et al. [73] screened 16,000 small molecules to identify fission inhibitors and identified Dynasore that inhibits mitochondrial fission. They found that Dynasore interferes in vitro with the GTPase activity of Dynamin1, Dynamin 2, and Drp1. Two types of intermediates have been found to accumulate during Dynasore inhibition: (1) g-shaped, half formed pitsand (2) O-shaped, fully formed pits. Thus, Dynamin acts at two steps during clathrin coat formation GTP hydrolysis is probably needed at both steps.
Seil et al. [81] studied the interaction of lipopolysaccharide-primed murine peritoneal macrophages with Dynasore and ivermectin (a broad spectrum antiparacitic treatment). Murine peritoneal macrophages express P2X(4) receptors, which are mostly intracellular. In cells from P2X(7)-knockout mice, 10 μm ATP provoked a transient increase of the intracellular concentration of calcium. Ivermectin had no effect by itself, but potentiated the increase of the intracellular concentration of calcium by ATP. The combination of ATP plus ivermectin also decreased the intracellular concentration of potassium and promoted the secretion of IL-1β. Concentrations of Dynasore above 50 μm affected the integrity of mitochondria and of the plasma membrane. By itself, Dynasore was found to promote the release of potassium and the secretion of IL-1β after activation of caspase-1. In turn, this potentiation was found to trigger the release of IL-1β by macrophages As opposed to ivermectin, Dynasore had no effect on P2X (4) receptors.
Gao et al.[82] studied protective effects of Dynasore against ischemia/reperfusion injury in mice. Langendorff-perfused mouse hearts were subjected to ischemia/reperfusion (30 minutes of global ischemia followed by 1 hour of reperfusion). Pretreatment with 1 μM Dynasore prevented ischemia/reperfusion-induced elevation of diastolic pressure in the left ventricular end, indicating a significant and specific lusitropic effect for Dynasore. Dynasore also decreased cardiac troponin I efflux during reperfusion and reduced infarct size. In cultured adult mouse cardiomyocytes subjected to oxidative stress, Dynasore increased cardiomyocyte survival and viability, and reduced the depletion of cellular ATP. Moreover, the pretreatment of cultured cells with Dynasore protected mitochondrial fission induced by oxidative stress. Dynasore protected against cardiac lusitropy and limited cell damage through a mechanism that maintained mitochondrial morphology and intracellular ATP in the cells.
Overall, these studies suggest that Mdivi, Dynasore, and P110 are promising candidates to reduce excessive mitochondrial fission, to increase mitochondrial fusion, and to maintain mitochondrial function in neurons affected by AD, HD, and PD.
Excessive mitochondrial fusion and its effects on mitochondria
To determine the normal function of Drp1, researchers developed Drp1 knockout mice. These Drp1 homozygote knockout mice (Drp1−/−) have developmental abnormalities, particularly in the forebrain, and they die, on average, at embryonic days 11.5–12.5 [83–84]. Similarly, neural cell-specific Drp1−/− mice died shortly after birth, from brain hypoplasia. Primary culture of the neuronsal-specific-Drp1−/− mouse forebrain showed a decrease in neurites and the presence of the formation of defective synapses, the latter which was suggested to be due to aggregated mitochondria that failed to distribute properly within cell processes [84].
A recent characterization of heterozygote Drp1 knockout mice (Drp1+/−) revealed that Drp1+/− mice are normal in terms of lifespan, fertility, and viability [85], and phenotypically these mice are not different from wild-type mice. Further, a functional characterization of mitochondria in the Drp1+/− mice, compared to the wild-type Drp1+/+ mice, revealed that the mitochondrial and synaptic functions and GTPase Drp1 enzymatic activity of the Drp1+/− mice appeared to be normal but that their levels of hydrogen peroxide and lipid peroxidation were significantly reduced.
These studies suggest that Drp1 can reduce mitochondrial fission and elevate mitochondrial fusion. However, a partial reduction in Drp1 does not affect mitochondrial and synaptic viability and may have therapeutic value in diseases with oxidative stress and mitochondrial dysfunction.
Conclusions and future studies
Increasing evidence suggests that impaired mitochondrial dynamics is an early event in the progression of neurodegenerative diseases that involve excessive mitochondrial fission, mitochondrial dysfunction and neuronal damage. There has been progress in screening, identifying, and developing molecules as therapies to reduce mitochondrial fission while maintaining mitochondrial fusion and cell survival. Three molecules – Mdivi, Dynasore and P110 – have been found to be good candidates as therapeutic inhibitors to reduce mitochondrial fission. These candidate molecules need to be tested in genetic and experimental mouse models of AD, HD, and PD to determine their efficacy in reducing excessive mitochondrial fission and protecting cells from mutant proteins-induced neuronal damage, and as promising candidates to treat patients with AD, HD and PD.
Acknowledgments
This research was supported by NIH grants AG028072, AG042178, and RR000163, and a grant from the Medical Research Foundation of Oregon.
References
- 1.Chan DC. Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol. 2006;22:79–99. doi: 10.1146/annurev.cellbio.22.010305.104638. [DOI] [PubMed] [Google Scholar]
- 2.Chen H, Chan DC. Mitochondrial dynamics--fusion, fission, movement, and mitophagy--in neurodegenerative diseases. Hum Mol Genet. 2009;18:R169–176. doi: 10.1093/hmg/ddp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang X, Su B, Siedlak SL, Moreira PI, Fujioka H, Wang Y, Casadesus G, Zhu X. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc Natl Acad Sci U S A. 2008;105:19318–19323. doi: 10.1073/pnas.0804871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang X, Su B, Lee HG, Li X, Perry G, Smith MA, Zhu X. Impaired balance of mitochondrial fission and fusion in Alzheimer’s disease. J Neurosci. 2009;29:9090–9103. doi: 10.1523/JNEUROSCI.1357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manczak M, Mao MP, Calkins MJ, Cornea A, Reddy AP, Murphy MP, Szeto HH, Park B, Reddy PH. Mitochondria-targeted antioxidants protect against amyloid-beta toxicity in Alzheimer’s disease neurons. J Alzheimers Dis. 2010;20:S609–S631. doi: 10.3233/JAD-2010-100564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manczak M, Calkins MJ, Reddy PH. Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer’s disease: implications for neuronal damage. Hum Mol Genet. 2011;20:2495–2509. doi: 10.1093/hmg/ddr139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calkins MJ, Manczak M, Mao P, Shirendeb U, Reddy PH. Impaired mitochondrial biogenesis, defective axonal transport of mitochondria, abnormal mitochondrial dynamics and synaptic degeneration in a mouse model of Alzheimer’s disease. Hum Mol Genet. 2011;20:4515–4529. doi: 10.1093/hmg/ddr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trushina E, Nemutlu E, Zhang S, Christensen T, Camp J, Mesa J, Siddiqui A, Tamura Y, Sesaki H, Wengenack TM, Dzeja PP, Poduslo JF. Defects in mitochondrial dynamics and metabolomic signatures of evolving energetic stress in mouse models of familial Alzheimer’s disease. PLoS One. 2012;7:e32737. doi: 10.1371/journal.pone.0032737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silva DF, Selfridge JE, Lu J, EL, Roy N, Hutfles L, Burns JM, Michaelis EK, Yan S, Cardoso SM, Swerdlow RH. Bioenergetic flux, mitochondrial mass and mitochondrial morphology dynamics in AD and MCI cybrid cell lines. Hum Mol Genet. 2013;22:3931–3946. doi: 10.1093/hmg/ddt247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quintanilla RA, Matthews-Roberson TA, Dolan PJ, Johnson GV. Caspase-cleaved tau expression induces mitochondrial dysfunction in immortalized cortical neurons: implications for the pathogenesis of Alzheimer disease. J BiolChem. 2009;284:18754–18766. doi: 10.1074/jbc.M808908200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quintanilla RA, Dolan PJ, Jin YN, Johnson GV. Truncated tau and Aβ cooperatively impair mitochondria in primary neurons. Neurobiol Aging. 2012;33:619, e25–35. doi: 10.1016/j.neurobiolaging.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schulz KL, Eckert A, Rhein V, Mai S, Haase W, Reichert AS, Jendrach M, Müller WE, Leuner K. A new link to mitochondrial impairment in tauopathies. MolNeurobiol. 2012;46:205–216. doi: 10.1007/s12035-012-8308-3. [DOI] [PubMed] [Google Scholar]
- 13.Cagalinec M, Safiulina D, Liiv M, Liiv J, Choubey V, Wareski P, Veksler V, Kaasik A. Principles of the mitochondrial fusion and fission cycle in neurons. J Cell Sci. 2013;126:2187–2197. doi: 10.1242/jcs.118844. [DOI] [PubMed] [Google Scholar]
- 14.DuBoff B, Götz J, Feany MB. Tau promotes neurodegeneration via DRP1 mislocalization in vivo. Neuron. 2012;75:618–632. doi: 10.1016/j.neuron.2012.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang H, Lim PJ, Karbowski M, Monteiro MJ. Effects of overexpression of huntingtin proteins on mitochondrial integrity. Hum Mol Genet. 2009;18:737–752. doi: 10.1093/hmg/ddn404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim J, Moody JP, Edgerly CK, Bordiuk OL, Cormier K, Smith K, Beal MF, Ferrante RF. Mitochondrial loss, dysfunction and altered dynamics in Huntington’s disease. Hum Mol Genet. 2010;19:3919–3935. doi: 10.1093/hmg/ddq306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Costa V, Giacomello M, Hudec R, Lopreiato R, Ermak G, Lim D, Malorni W, Davies KJ, Carafoli E, Scorrano L. Mitochondrial fission and cristae disruption increase the response of cell models of Huntington’s disease to apoptotic stimuli. EMBOMol Med. 2010;2:490–503. doi: 10.1002/emmm.201000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shirendeb U, Reddy AP, Manczak M, Calkins MJ, Mao P, Tagle DA, Reddy PH. Abnormal mitochondrial dynamics, mitochondrial loss and mutant huntingtin oligomers in Huntington’s disease: implications for selective neuronal damage. Hum Mol Genet. 2011;20:1438–1455. doi: 10.1093/hmg/ddr024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shirendeb UP, Calkins MJ, Manczak M, Anekonda V, Dufour B, McBride JL, Mao P, Reddy PH. Mutant huntingtin’s interaction with mitochondrial protein Drp1 impairs mitochondrial biogenesis and causes defective axonal transport and synaptic degeneration in Huntington’s disease. Hum Mol Genet. 2012;21:406–420. doi: 10.1093/hmg/ddr475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song W, Chen J, Petrilli A, Liot G, Klinglmayr E, Zhou Y, Poquiz P, Tjong J, Pouladi MA, Hayden MR, Masliah E, Ellisman M, Rouiller I, Schwarzenbacher R, Bossy B, Perkins G, Bossy-Wetzel E. Mutant huntingtin binds the mitochondrial fission GTPase dynamin-related protein-1 and increases its enzymatic activity. Nat Med. 2011;17:377–382. doi: 10.1038/nm.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song W, Song Y, Kincaid B, Bossy B, Bossy-Wetzel E. Mutant SOD1G93A triggers mitochondrial fragmentation in spinal cord motor neurons: neuroprotection by SIRT3 and PGC-1α. Neurobiol Dis. 2013;51:72–81. doi: 10.1016/j.nbd.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magrané J, Sahawneh MA, Przedborski S, Estévez ÁG, Manfredi G. Mitochondrial dynamics and bioenergetic dysfunction is associated with synaptic alterations in mutant SOD1 motor neurons. J Neurosci. 2012;32:229–242. doi: 10.1523/JNEUROSCI.1233-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Yan MH, Fujioka H, Liu J, Wilson-Delfosse A, Chen SG, Perry G, Casadesus G, Zhu X. LRRK2 regulates mitochondrial dynamics and function through direct interaction with DLP1. Hum Mol Genet. 2012;21:1931–1944. doi: 10.1093/hmg/dds003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, Petrie TG, Liu Y, Liu J, Fujioka H, Zhu X. Parkinson’s disease-associated DJ-1 mutations impair mitochondrial dynamics and cause mitochondrial dysfunction. J Neurochem. 2012;121:830–839. doi: 10.1111/j.1471-4159.2012.07734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamp F, Exner N, Lutz AK, Wender N, Hegermann J, Brunner B, Nuscher B, Bartels T, Giese A, Beyer K, Eimer S, Winklhofer KF, Haass C. Inhibition of mitochondrial fusion by α-synuclein is rescued by PINK1, Parkin and DJ-1. EMBO J. 2010;29:3571–3589. doi: 10.1038/emboj.2010.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu F, Selak M, O’Connor J, Croul S, Lorenzana C, Butunoi C, Kalman B. Oxidative damage to mitochondrial DNA and activity of mitochondrial enzymes in chronic active lesions of multiple sclerosis. J NeurolSci. 2000;177:95–103. doi: 10.1016/s0022-510x(00)00343-9. [DOI] [PubMed] [Google Scholar]
- 27.Kalman B, Leist TP. A mitochondrial component of neurodegeneration in multiple sclerosis. Neuromolecular Med. 2003;3:147–58. doi: 10.1385/NMM:3:3:147. [DOI] [PubMed] [Google Scholar]
- 28.Singh V, Prajeeth CK, Gudi V, Bénardais K, Voss EV, Stangel M. 2-Chlorodeoxyadenosine (cladribine) induces apoptosis in human monocyte-derive dendritic cells. ClinExpImmunol. 2013;173:288–297. doi: 10.1111/cei.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mao P, Reddy PH. Is multiple sclerosis a mitochondrial disease? BiochimBiophysActa. 2010;1802:66–79. doi: 10.1016/j.bbadis.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Horssen J, Witte ME, Schreibelt G, de Vries HE. Radical changes in multiple sclerosis pathogenesis. BiochimBiophysActa. 2011;1812:141–50. doi: 10.1016/j.bbadis.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 31.Haider L, Fischer MT, Frischer JM, Bauer J, Höftberger R, Botond G, Esterbauer H, Binder CJ, Witztum JL, Lassmann H. Oxidative damage in multiple sclerosis lesions. Brain. 2011;134:1914–1924. doi: 10.1093/brain/awr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang B, Davidson MM, Zhou H, Wang C, Walker WF, Hei TK. Cytoplasmic irradiation results in mitochondrial dysfunction and DRP1-dependent mitochondrial fission. Cancer Res. 2013 doi: 10.1158/0008-5472.CAN-13-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farrand L, Kim JY, Im-Aram A, Suh JY, Lee HJ, Tsang BK. An improved quantitative approach for the assessment of mitochondrial fragmentation in chemoresistant ovarian cancer cells. PLoS One. 2013;8:e74008. doi: 10.1371/journal.pone.0074008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Oliveira MF, Amoêdo ND, Rumjanek FD. Energy and redox homeostasis in tumor cells. Int J Cell Biol. 2012;2012:593838. doi: 10.1155/2012/593838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siesjö BK, Katsura KI, Kristián T, Li PA, Siesjö P. Molecular mechanisms of acidosis-mediated damage. ActaNeurochir Suppl. 1996;66:8–14. doi: 10.1007/978-3-7091-9465-2_2. [DOI] [PubMed] [Google Scholar]
- 36.Ozawa T. Mitochondrial DNA mutations and age. Ann N Y AcadSci. 1998;854:128–154. doi: 10.1111/j.1749-6632.1998.tb09898.x. [DOI] [PubMed] [Google Scholar]
- 37.Joza N, Pospisilik JA, Hangen E, Hanada T, Modjtahedi N, Penninger JM, Kroemer G. AIF: not just an apoptosis-inducing factor. Ann N Y AcadSci. 2009;1171:2–11. doi: 10.1111/j.1749-6632.2009.04681.x. [DOI] [PubMed] [Google Scholar]
- 38.Romanello V, Sandri M. Mitochondrial biogenesis and fragmentation as regulators of muscle protein degradation. CurrHypertens Rep. 2010;12:433–439. doi: 10.1007/s11906-010-0157-8. [DOI] [PubMed] [Google Scholar]
- 39.Pangare M, Makino A. Mitochondrial function in vascular endothelial cell in diabetes. JSmooth Muscle Res. 2012;48:1–26. doi: 10.1540/jsmr.48.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knott AB, Bossy-Wetzel E. Impact of nitric oxide on metabolism in health and age-related disease. Diabetes ObesMetab Suppl. 2010;2:126–33. doi: 10.1111/j.1463-1326.2010.01267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Supale S, Thorel F, Merkwirth C, Gjinovci A, Herrera PL, Scorrano L, Meda P, Langer T, Maechler P. Loss of Prohibitin Induces Mitochondrial Damages Altering β-Cell Function and Survival and Is Responsible for Gradual Diabetes Development. Diabetes. 2013;62:3488–3499. doi: 10.2337/db13-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Das S, Hajnóczky N, Antony AN, Csordás G, Gaspers LD, Clemens DL, Hoek JB, Hajnóczky G. Mitochondrial morphology and dynamics in hepatocytes from normal and ethanol-fed rats. Pflugers Arch. 2012;464:101–109. doi: 10.1007/s00424-012-1100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–1585. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bailey SM, Cunningham CC. Contribution of mitochondria to oxidative stress associated with alcoholic liver disease. Free RadicBiol Med. 2002;32:11–16. doi: 10.1016/s0891-5849(01)00769-9. [DOI] [PubMed] [Google Scholar]
- 45.Hoek JB, Cahill A, Pastorino JG. Alcohol and mitochondria: a dysfunctional relationship. Gastroenterology. 2002;122:2049–2063. doi: 10.1053/gast.2002.33613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoek JB, Pastorino JG. Ethanol, oxidative stress, and cytokine-induced liver cell injury. Alcohol. 2002;27:63–68. doi: 10.1016/s0741-8329(02)00215-x. [DOI] [PubMed] [Google Scholar]
- 47.Reddy PH. Mitochondrial dysfunction in aging and Alzheimer’s disease: strategies to protect neurons. Antioxid Redox Signal. 2007;9:1647–1658. doi: 10.1089/ars.2007.1754. [DOI] [PubMed] [Google Scholar]
- 48.Reddy PH. Mitochondrial medicine for aging and neurodegenerative diseases. Neuromolecular Med. 2008;10:291–315. doi: 10.1007/s12017-008-8044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reddy PH, Tripathi R, Troung Q, Tirumala K, Reddy TP, Anekonda V, Shirendeb UP, Calkins MJ, Reddy AP, Mao P, Manczak M. Abnormal mitochondrial dynamics and synaptic degeneration as early events in Alzheimer’s disease: implications to mitochondria-targeted antioxidant therapeutics. BiochimBiophysActa. 2012;1822:639–649. doi: 10.1016/j.bbadis.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Knott AB, Perkins G, Schwarzenbacher R, Bossy-Wetzel E. Mitochondrial fragmentation in neurodegeneration. Nat Rev Neurosci. 2008;9:505–518. doi: 10.1038/nrn2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Otera H, Wang C, Cleland MM, Setoguchi K, Yokota S, Youle RJ, Mihara K. Mff is an essential factor for mitochondrial recruitment of Drp1 during mitochondrial fission in mammalian cells. J Cell Biol. 2010;191:1141–1158. doi: 10.1083/jcb.201007152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palmer CS, Osellame LD, Laine D, Koutsopoulos OS, Frazier AE, Ryan MT. MiD49 and MiD51, new components of the mitochondrial fission machinery. EMBO Rep. 2011;12:565–573. doi: 10.1038/embor.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reddy PH, Reddy TP, Manczak M, Calkins MJ, Shirendeb U, Mao P. Dynamin-related protein 1 and mitochondrial fragmentation in neurodegenerative diseases. Brain Res Rev. 2011;67:103–118. doi: 10.1016/j.brainresrev.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Manczak M, Reddy PH. Abnormal interaction between the mitochondrial fission protein Drp1 and hyperphosphorylated tau in Alzheimer’s disease neurons: implications for mitochondrial dysfunction and neuronal damage. Hum Mol Genet. 2012;21:2538–2547. doi: 10.1093/hmg/dds072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barsoum MJ, Yuan H, Gerencser AA, Liot G, Kushnareva Y, Gräber S, Kovacs I, Lee WD, Waggoner J, Cui J, White AD, Bossy B, Martinou JC, Youle RJ, Lipton SA, Ellisman MH, Perkins GA, Bossy-Wetzel E. Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. EMBO J. 2006;25:3900–3911. doi: 10.1038/sj.emboj.7601253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Benard G, Bellance N, James D, Parrone P, Fernandez H, Letellier T, Rossignol R. Mitochondrial bioenergetics and structural network organization. J Cell Sci. 2007;120:838–848. doi: 10.1242/jcs.03381. [DOI] [PubMed] [Google Scholar]
- 57.Liot G, Bossy B, Lubitz S, Kushnareva Y, Sejbuk N, Bossy-Wetzel E. Complex II inhibition by 3-NP causes mitochondrial fragmentation and neuronal cell death via an NMDA- and ROS-dependent pathway. Cell Death Differ. 2009;16:899–909. doi: 10.1038/cdd.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang X, Su B, Liu W, He X, Gao Y, Castellani RJ, Perry G, Smith MA, Zhu X. DLP1-dependent mitochondrial fragmentation mediates 1-methyl-4-phenylpyridinium toxicity in neurons: implications for Parkinson’s disease. Aging Cell. 2011;10:807–823. doi: 10.1111/j.1474-9726.2011.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu T, Robotham JL, Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. ProcNatlAcadSci U S A. 2006;103:2653–2658. doi: 10.1073/pnas.0511154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brown MR, Sullivan PG, Geddes JW. Synaptic mitochondria are more susceptible to Ca2+overload than nonsynaptic mitochondria. J BiolChem. 2006;281:11658–11668. doi: 10.1074/jbc.M510303200. [DOI] [PubMed] [Google Scholar]
- 61.Cho DH, Nakamura T, Fang J, Cieplak P, Godzik A, Gu Z, Lipton SA. S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science. 2009;324:102–105. doi: 10.1126/science.1171091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Haun F, Nakamura T, Shiu AD, Cho DH, Tsunemi T, Holland EA, La Spada AR, Lipton SA. S-Nitrosylation of Dynamin-Related Protein 1 Mediates Mutant Huntingtin-Induced Mitochondrial Fragmentation and Neuronal Injury in Huntington’s Disease. Antioxid Redox Signal. 2013;19:1173–1184. doi: 10.1089/ars.2012.4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bossy B, Petrilli A, Klinglmayr E, Chen J, Lütz-Meindl U, Knott AB, Masliah E, Schwarzenbacher R, Bossy-Wetzel E. S-Nitrosylation of DRP1 does not affect enzymatic activity and is not specific to Alzheimer’s disease. J AlzheimersDis. 2010;20(Suppl 2):S513–526. doi: 10.3233/JAD-2010-100552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cribbs JT, Strack S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep. 2007;8:939–944. doi: 10.1038/sj.embor.7401062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chang CR, Blackstone C. Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J BiolChem. 2007;282:21583–21587. doi: 10.1074/jbc.C700083200. [DOI] [PubMed] [Google Scholar]
- 66.Taguchi N, Ishihara N, Jofuku A, Oka T, Mihara K. Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J BiolChem. 2007;282:11521–11529. doi: 10.1074/jbc.M607279200. [DOI] [PubMed] [Google Scholar]
- 67.Rehman J, Zhang HJ, Toth PT, Zhang Y, Marsboom G, Hong Z, Salgia R, Husain AN, Wietholt C, Archer SL. Inhibition of mitochondrial fission prevents cell cycle progression in lung cancer. FASEB J. 2012;26:2175–2186. doi: 10.1096/fj.11-196543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Merrill RA, Dagda RK, Dickey AS, Cribbs JT, Green SH, Usachev YM, Strack S. Mechanism of neuroprotective mitochondrial remodeling by PKA/AKAP1. PLoSBiol. 2011;9:e1000612. doi: 10.1371/journal.pbio.1000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rambold AS, Kostelecky B, Elia N, Lippincott-Schwartz J. Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation. ProcNatlAcadSci U S A. 2011;108:10190–10195. doi: 10.1073/pnas.1107402108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meuer K, Suppanz IE, Lingor P, Planchamp V, Göricke B, Fichtner L, Braus GH, Dietz GP, Jakobs S, Bähr M, Weishaupt JH. Cyclin-dependent kinase 5 is an upstream regulator of mitochondrial fission during neuronal apoptosis. Cell Death Differ. 2007;14:651–661. doi: 10.1038/sj.cdd.4402087. [DOI] [PubMed] [Google Scholar]
- 71.Cassidy-Stone A, Chipuk JE, Ingerman E, Song C, Yoo C, Kuwana T, Kurth MJ, Shaw JT, Hinshaw JE, Green DR, Nunnari J. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev Cell. 2008;14:193–204. doi: 10.1016/j.devcel.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Qi X, Qvit N, Su YC, Mochly-Rosen D. A novel Drp1 inhibitor diminishes aberrant mitochondrial fission and neurotoxicity. J Cell Sci. 2013;126:789–802. doi: 10.1242/jcs.114439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T. Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell. 2006;10:839–850. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 74.Xie N, Wang C, Lian Y, Zhang H, Wu C, Zhang Q. A selective inhibitor of Drp1, mdivi-1, protects against cell death of hippocampal neurons in pilocarpine-induced seizures in rats. NeurosciLett. 2013;545:64–68. doi: 10.1016/j.neulet.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 75.Qiu X, Cao L, Yang X, Zhao X, Liu X, Han Y, Xue Y, Jiang H, Chi Z. Role of mitochondrial fission in neuronal injury in pilocarpine-induced epileptic rats. Neuroscience. 2013;245:157–165. doi: 10.1016/j.neuroscience.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 76.Zhang N, Wang S, Li Y, Che L, Zhao Q. A selective inhibitor of Drp1, mdivi-1, acts against cerebral ischemia/reperfusion injury via an anti-apoptotic pathway in rats. NeurosciLett. 2013;535:104–109. doi: 10.1016/j.neulet.2012.12.049. [DOI] [PubMed] [Google Scholar]
- 77.Wappler EA, Institoris A, Dutta S, Katakam PV, Busija DW. Mitochondrial dynamics associated with oxygen-glucose deprivation in rat primary neuronal cultures. PLoS One. 2013;8:e63206. doi: 10.1371/journal.pone.0063206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tang WX, Wu WH, Qiu HY, Bo H, Huang SM. Amelioration of rhabdomyolysis-induced renal mitochondrial injury and apoptosis through suppression of Drp-1 translocation. J Nephrol. 2013 Apr 3; doi: 10.5301/jn.5000268. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 79.Chlystun M, Campanella M, Law AL, Duchen MR, Fatimathas L, Levine TP, Gerke V, Moss SE. Regulation of mitochondrial morphogenesis by annexin A6. PLoS One. 2013;8:e53774. doi: 10.1371/journal.pone.0053774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Park SW, Kim KY, Lindsey JD, Dai Y, Heo H, Nguyen DH, Ellisman MH, Weinreb RN, Ju WK. A selective inhibitor of drp1, mdivi-1, increases retinal ganglion cell survival in acute ischemic mouse retina. Invest Ophthalmol Vis Sci. 2011;52:2837–2843. doi: 10.1167/iovs.09-5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Seil M, El Ouaaliti M, Dehaye JP. Secretion of IL-1β triggered by dynasorein murine peritoneal macrophages. Innate Immun. 2012;18:241–249. doi: 10.1177/1753425911399478. [DOI] [PubMed] [Google Scholar]
- 82.Gao D, Zhang L, Dhillon R, Hong TT, Shaw RM, Zhu J. Dynasore protects mitochondria and improves cardiac lusitropy in Langendorff perfused mouse heart. PLoS One. 2013;8:e60967. doi: 10.1371/journal.pone.0060967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wakabayashi J, Zhang Z, Wakabayashi N, Tamura Y, Fukaya M, Kensler TW, Iijima M, Sesaki H. The dynamin-related GTPase Drp1 is required for embryonic and brain development in mice. J Cell Biol. 2009;186:805–816. doi: 10.1083/jcb.200903065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ishihara N, Nomura M, Jofuku A, Kato H, Suzuki SO, Masuda K, Otera H, Nakanishi Y, Nonaka I, Goto Y, Taguchi N, Morinaga H, Maeda M, Takayanagi R, Yokota S, Mihara K. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat Cell Biol. 2009;11:958–966. doi: 10.1038/ncb1907. [DOI] [PubMed] [Google Scholar]
- 85.Manczak M, Sesaki H, Kageyama Y, Reddy PH. Dynamin-related protein 1 heterozygote knockout mice do not have synaptic and mitochondrial deficiencies. BiochimBiophysActa. 2012;1822:862–874. doi: 10.1016/j.bbadis.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]