Abstract
Receptor editing, a major mechanism of B cell tolerance, can also lead to allelic inclusion at the immunoglobulin light chain loci and the development of B cells that co-express two different immunoglobulin light chains and, therefore, two antibody specificities. Most allelically included B cells express two κ chains, although rare dual-λ cells are also observed. Moreover, these cells typically co-express an autoreactive and a nonautoreactive antibody. Thus, allelically included B cells could operate like “Trojan horses”: expression and function of the nonautoreactive antigen receptors might promote their maturation, activation, and terminal differentiation into effector cells that also express and secrete autoantibodies. Indeed, dual-κ B cells are greatly expanded into effector B cell subsets in some autoimmune mice, thus indicating they might play an important role in disease.
Introduction
B cells develop in the bone marrow tissue in a step-wise process during which immunoglobulin heavy (Ig H) and light (Ig L) chain genes randomly assemble via the ordered joining of V, D and J gene segments at the Igh locus first followed by V and J joining at the L chain loci, Igk and Igλ. Each B cell productively rearranges, generally, only one Ig H and one L chain genes and, thus, expresses an antibody with a unique specificity, a phenomenon known as allelic exclusion (Box 1). The purpose of V(D)J recombination is to create a B cell population with a diverse and large antibody repertoire, however, this process also produces–at least in mice and humans–Ig genes that most frequently encode autoreactive antibodies [1,2]. Therefore, B cell selection (and tolerance) mechanisms have evolved to decrease the likelihood of developing self-reactive immune responses. Indeed, during their maturation from immature to effectors, B cells pass through tolerance checkpoints that eliminate most autoreactive cells from the functional repertoire [3,4]. The earliest checkpoint (referred to as central tolerance) takes place in the bone marrow when immature B cells first express their newly acquired Ig H + L chains on the cell surface in the form of an IgM antigen receptor (BCR). If this BCR binds a self-antigen with medium-to-high avidity, the cells undergo negative selection, otherwise, they enter the blood and differentiate into transitional and then mature B cells. Studies employing Ig transgenic and gene targeted (“knock-in”) mice have demonstrated that central B cell selection operates primarily via receptor editing and not clonal deletion [5–7]. Receptor editing is a process by which immature B cells perform secondary V-J gene recombination at the Ig L chain loci. These secondary rearrangement events not only remove the original gene encoding the autoreactive L chain, but also provide a new rearranged VL-JL gene encoding a nonautoreactive L chain [8–10]. H chain gene replacements are also an option during receptor editing, but they succeed less frequently [11**]. Receptor editing is an efficient process in both Ig “knock-in” mice [5,12] and wild-type mice [13], occurring in approximately 25% of immature B cells in a diverse repertoire [14,15].
Box 1. Glossary terms.
- V(D)J recombination
a stochastic process by which developing B cells use Rag1 and Rag2-mediated DNA rearrangement to juxtapose Ig gene segments and create an Ig variable region coding sequence or exon. The Ig H chain variable region is made via joining VH, DH, and JH gene segments, while the Ig L chain variable region is made by joining only VL and JL.
- Productive rearrangement
a V(D)J rearrangement that is in frame and, therefore, capable of encoding a protein. Stochastically, only about one third of the rearrangements are in frame, while the rest are out of frame and contain stop codons.
- RS (recombining sequence) recombination
a Rag-mediated recombination event that takes place between a recombination signal sequence (RSS) located downstream of the 3′ Igk enhancer and an RSS located either within the Jk-Ck intron or an upstream germline Vk gene. In both instances, RS recombination prevents further rearrangement and expression of the Igk allele because it deletes the Igk enhancers and the Ck gene region.
- Allelic exclusion
a process by which each B cell productively rearranges only one Ig H and one L chain allele and, thus, expresses one H and one L chain that pair in an antibody with one specificity.
- Allelic inclusion
when a B cell harbors two productively rearranged alleles at the Ig H or L chain locus and, thus, expresses two different H or L chains, respectively.
- Isotypic inclusion
when a B cell harbors productively rearranged Igk and Igλ alleles and, thus, expresses both κ and λ L chains. It differs from allelic inclusion by the fact that the rearrangements are not on alleles of the same gene.
Since its discovery, receptor editing was appreciated as being potentially dangerous, partly because autoreactive B cells survive for a few days while undergoing central tolerance [16] with the risk they might be selected for entry into the periphery. Also, receptor editing does not guarantee that the gene encoding the autoreactive Ig chain is disrupted, as the V(D)J machinery does not discriminate between Ig L chain alleles and targets the non-rearranged and rearranged alleles with similar frequency [17*]. Since there are no general mechanisms that prevent expression of a rearranged Ig allele, receptor editing has the potential to produce allelically or isotypically included B cells that express the original autoreactive antibody along with an edited nonautoreactive antibody (Box 1 and Figure 1). Indeed, Weigert and colleagues were the first to report that in anti-DNA Ig gene targeted mice receptor editing results in B cells expressing two L chains (or less often two H chains) [18]. In 3H9/56R anti-DNA mice these B cells represent 25% of the mature B cell compartment and co-express an autoreactive (λ) and a nonautoreactive (κ) L chain [19]. In these dual κ/λ B cells, the autoreactive antibody has a low avidity for the self-antigen, as also implied by its maintenance on the cell surface. However, this characteristic is not necessarily a feature of all allelically/isotypically included B cells. For example in the 3-83Igi mouse model, in which the autoreactive BCR has a high avidity for the H-2Kb self-antigen and is thus almost completely downmodulated from the cell surface, 15–20% of the B cells enter the mature peripheral pool retaining cytoplasmic expression of 3-83 while expressing a different Ig on the cell surface [20]. Thus, immature B cells with low or high avidity for self-antigens can give rise to mature B cells that co-express autoreactive and nonautoreactive antibodies (Figure 1). These allelically included B cells have the ability to bind a foreign antigen and differentiate into cells that also secrete autoantibodies and, thus, represent an enigma in respect to our understanding of B cell tolerance. The scope of this review is to discuss mouse studies that, in recent years, have investigated the development of allelically/isotypically included B cells in a diverse repertoire, the role of receptor editing in this process, and the function these cells have in the context of autoimmune responses.
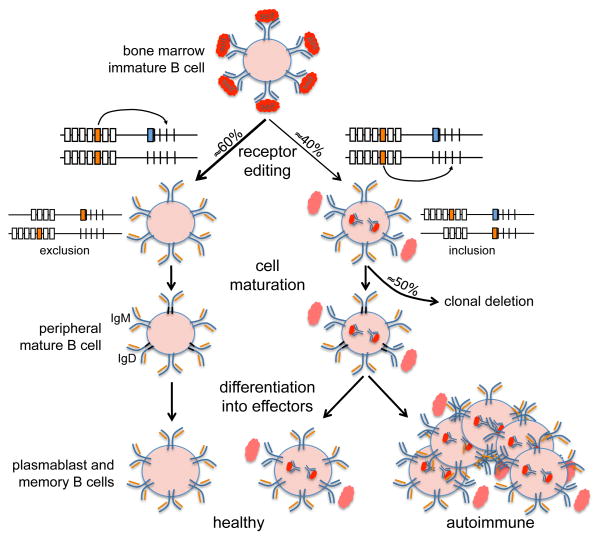
Figure 1.
Development of allelically included B cells in healthy and autoimmune conditions. Upon binding self-antigen, an autoreactive immature B cell activates receptor editing, which (left side of the diagram) leads to cells that have deleted the V-J gene encoding the original autoreactive L chain (depicted in blue) and replaced it with a new V-J gene encoding a nonautoreactive L chain (depicted in orange). Receptor editing can also operate by inclusion (right side of the diagram), resulting in dual-L chain B cells that retain the original rearrangement but also harbor a new V-J gene, and thus co-express an autoreactive and a nonautoreactive L chain. The amount of autoreactive antibody retained in the cytoplasm of allelically included B cells varies depending on the avidity for the self-antigen (or less likely the affinity for the H chain in relation to the second L chain). Despite their autoreactivity, at least half of dual L-chain B cells differentiate into mature B cells and participate in immune responses. Positive selection of allelically included B cells into effector B cell subsets (e.g., plasmablasts, memory B cells) is enhanced under some autoimmune conditions.
Development of dual-κ B cells in a wild-type antibody repertoire
The discovery of dual-L chain-expressing B cells in the repertoire of Ig “knock-in” mice suggested that allelically included B cells might also arise within a wild-type antibody repertoire, an hypothesis supported by the rare observation of dual H chain [21] and κ+λ+ B cells in mice and humans [22,23]. Because the κ:λ ratio in mice is about 95:5, dual-κ B cells were expected to be the predominant allelically included B cell subset. However, their identification was not easily achieved until the Nussenzweig lab created the Igkm/h mouse strain in which one Igk allele bears the human instead of the mouse Ck gene region [14]. In Igkm/h mice, B cells that co-express two κ chains are identified by simultaneous staining with anti-mouse and anti-human Cκ antibodies. Studies from two groups used flow cytometric analysis as well as single cell and hybridoma analyses, respectively, to show that dual-κ B cells are generated within a wild-type B cell repertoire at frequencies of 2–10% [17*,24*], and demonstrating that dual antibody-expressing B cells are not an artifact of Ig transgenic animals. The high prevalence of allelically included B cells in autoreactive Ig transgenic mice suggested that these B cells might be more frequently autoreactive in wild-type animals as well. Indeed, the frequency of autoreactivity toward HEp-2 and DNA self-antigens is threefold higher in dual-κ B cells than in single-κ B cells of wild-type mice [17*,25**], indicating that autoreactive immature B cells are generally the progenitors of allelically included mature B cells.
Autoreactive L chains drive the development of allelically included B cells
A recent study that examined mechanisms of B cell selection in mice, also investigated the likelihood of generating allelically included B cells when cells are bestowed from the start with a κ chain that exhibits either a high (Vκ4-Jκ4) or low (αHELκ) tendency to contribute to autoreactivity [26**]. In these studies, the frequency of transitional B cells co-expressing the original κ chain and a newly rearranged one was more than twofold higher (50% versus 20%) in IgkVk4/h than in IgkαHEL/h mice. Reduced allelic inclusion in αHELκ B cells was achieved via greater frequency of RS recombination–a process that inactivates a previously rearranged Vκ-Jκ allele ([12,27], Box 1)–and possibly also as a result of increased out of frame secondary Vκ-Jκ recombination events. To explain the difference between Vκ4 and αHELκ B cell tolerance, the investigators suggested that some autoreactive κ chains (such as Vκ4) might have low affinity of pairing with H chains and thus be easily outcompeted by other κ chains for binding to the H chain and consequent surface expression. Alternatively, they might confer a level of autoreactivity that is easily diluted by other H+L chain combinations allowing the cell to resume differentiation without the need for receptor editing-mediated gene inactivation [26**]. The Vκ4-Jκ4 allele, moreover, had only one remaining Jκ for secondary recombination events while the αHELκ chain used Jκ2 and had, therefore, two remaining Jκs. It is plausible that the higher number of recombination substrates increases the chances of V-to-J recombination events, which are most often (2 out of 3) non-productive (Box 1). Although these options were not tested, results of this study suggests that the propensity to create dual antibody-expressing B cells varies depending on the number of unrearranged Jκ segments remaining on the “autoreactive” allele and/or the type of autoreactivity. Similar to the findings in 3-83Igi mice, most dual-κ B cells in both IgkVk4/h and IgkαHEL/h mice did not display detectable levels of the autoreactive κ chain on the cell surface–Vκ4 and αHELκ were detected by RT-PCR in single hCκ+ cells–suggesting a high avidity for the self-antigen or low affinity for the H chain. The former possibility was demonstrated in 3-83Ig+ allelically included B cells, which re-express the 3-83 autoreactive BCR on the cell surface upon removal of the self-antigen or pharmacological inhibition of receptor internalization [20].
Rearrangements at Igλ occur subsequent to those at Igk and can salvage a developing B cell after failed Vκ-Jκ recombination attempts. Mice possess only four possible Vλ-Jλ rearrangements referred to as λX, and λ1, λ2, and λ3 (abbreviated into λ1,2,3). λX is one of the most negatively-charged L chains and can reduce DNA binding, while λ1,2,3 are positively-charged L chains that promote DNA binding [11**]. The murine Igλ locus does not support secondary recombination or RS-like inactivation events like those occurring at Igk and, thus, receptor editing at Igλ might lead to cells co-expressing multiple L chains. By using flow cytometric analyses with anti-λX- and anti-λ1,2,3-specific antibodies and also single cell genetic analyses, Weigert and colleagues have recently identified the presence of dual-λ+ cells co-expressing λ1, λ2, or λ3 together with λX [11**]. These cells, most of which express both L chains on the cell surface, are 2–4% of all (λ+) B cells in κ-deficient mice with a germline H chain locus, but markedly increase to 35% in mice with an anti-DNA H chain transgene. Thus, although rare (about 0.2%) within the normal murine B cell population, dual-λ B cells contribute to the allelically/isotypically-included autoreactive B cell repertoire of wild-type mice.
Receptor editing as the source of dual L chain B cells
Increasing effort has been spent to demonstrate that allelically included B cells arise via receptor editing. BrdU incorporation studies in Igkm/h mice demonstrated that the development of dual-κ B cells is delayed relative to that of single-κ B cells [17*], suggesting a requirement for extended Ig gene recombination (i.e., receptor editing). Moreover, if receptor editing is a pre-requisite for the development of L chain-included B cells, one of the H+L chain combinations should be autoreactive while the other should not. Wilson and colleagues tested this predicament by analyzing the reactivity toward HEp-2 and DNA of each H+κ antibody expressed by a sample of dual-κ B cells in IgkVk4/h mice [26**]. Remarkably, the reactivity with these self-antigens was consistently lower for antibodies containing the κ chain acquired via editing compared to those containing the original Vκ4. With a similar goal, Weigert and colleagues analyzed the CDR3’s arginine content of the H chains, indicative of DNA affinity, expressed by single or dual λ B cells of κ-deficient mice [11**]. They found that the arginine content of the H chains in dual λ1,2,3/λX B cells was significantly higher than in single λ1,2,3 cells suggesting that the dual cells had expressed a λ1,2,3 chain to reduce the overall autoreactivity. These sets of data indicate that receptor editing, rather than synchronous bi-allelic recombination, leads to allelic inclusion and the expression of a new L chain and that this latter reduces the level of autoreactivity relative to the original H+L antibody.
Dual antibody-expressing B cells in autoimmunity
Allelically included B cells have been shown to successfully compete with allelically excluded cells in immune responses [24*] and to enter the IgG+ memory B cell compartment in proportion to their frequency in the naive B cell population [17*] (Figure 1). As dual antibody-expressing B cells most often (if not always) express autoantibodies, this suggests included B cells might be more abundant in autoimmune-prone animals. To address this question, we recently evaluated dual-κ B cells in Igkm/h mice bred onto the MRL and MRL-Faslpr/lpr (MRL/lpr) genetic backgrounds [25**]. Notably, the frequency of dual-κ cells within the immature and mature B cell subsets was significantly increased in autoimmune MRL and MRL/lpr over control mice. Onset of disease was required for increased output of dual-κ B cells, indicating that the disease process modulates the extent of receptor editing and/or the efficiency of positive selection. Results from short-term kinetic studies of BrdU incorporation suggest that dual-κ B cells are generated more frequently in autoimmune than healthy mice because of either aborted editing or increased cell proliferation [25**]. Interestingly, the frequency of dual-κ/λ B cells was not similarly increased in MRL/lpr mice [25**] and also in anti-DNA 3H9/56R-MRL/lpr mice [28], supporting the idea that in this genetic background, dual-κ B cells develop because of aborted editing.
Many low avidity autoreactive B cells, such as anergic B cells, do not undergo receptor editing in the bone marrow but are instead removed from the repertoire at a checkpoint existing between the transitional and the mature B cell stages [6,29,30], a selection step apparent also in wild-type repertoires [2,26**]. B cells at this stage are apparently unable to induce receptor editing [26**,31], but remain susceptible to clonal deletion [32]. A comparison of dual-κ B cell frequencies in the transitional and follicular B cell subsets indicated that about half of these cells undergo clonal deletion at the transitional checkpoint in both healthy [26**] and autoimmune [25**] mice (Figure 1). Moreover, the majority of allelically included cells that bypass this checkpoint preferentially differentiate into marginal zone, instead of follicular, mature B cells [17*,19,25**,33*] thus reducing their participation in a germinal center reaction. Collectively these findings indicate that peripheral tolerance plays an important role in diminishing the burden of B cells that react with both foreign and self-antigens.
An important remaining question regarding alIelically/isotypically included B cells is whether they have the ability to participate in autoimmune processes. Our studies indicate this may indeed be the case as dual-κ B cells were strongly favored for entry into the effector B cell subsets over single-κ B cells in both MRL and MRL/lpr mice (Figure 1), resulting in about 40% of plasmablasts and memory B cells that were allelically included [25**]. Furthermore, dual-κ plasmablasts, which were clonally diverse, secreted autoantibodies at levels significantly above those of single-κ cells, and Ig gene sequencing demonstrated the presence of somatic hypermutations in some of these cells. Whether the mutations were in the autoreactive or the nonautoreactive κ chain was not investigated. Interestingly, Fas inactivation did not play an important role in the selection of dual-κ B cells in MRL/lpr mice besides that of accelerating the kinetics of their generation and further increasing their number in the memory B cell subset. Overall, dual-κ B cell numbers correlated with the level of serum autoantibodies and disease progression in MRL mice. Since these cells are most often autoreactive and abundant in effector B cell populations, this strongly suggests allelically included B cells play a major role in disease.
In similar studies by the Eilat lab, dual-κ B cells were evaluated in NZB/NZW autoimmune mice, and results were significantly different from those in MRL mice [33*]. Generation of dual-κ B cells was not increased in autoimmune NZB/NZW mice relative to control animals, and the frequency of autoreactive dual-κ B cells was similar to that of single-κ cells [33*]. These differences among autoimmune-prone mouse strains indicate that a genetic factor associated with the development of lupus-like disease alters the efficiency and mechanism of central tolerance but is not required for the development of lupus.
Potential mechanisms driving the development of included B cells
Allelically included B cells develop despite the presence of established mechanisms at the immature B cell stage that promote tolerance of autoreactive cells and allelic exclusion of Ig-positive cells. Distinct types of BCR signals mediate these mechanisms (Figure 2). On the one hand, binding self-antigen promotes the BCR to transduce a signal that leads to both arrest in cell differentiation and receptor editing [34]. On the other, a ligand-independent BCR signal referred to as tonic inhibits V(D)J recombination and promotes cell differentiation [35,36,37*,38*]. It is important to note that in allelically included cells, both (autoreactive and nonautoreactive) antibodies compete for binding to Igα/Igβ and, thus, for surface expression [20]. Thus, at least two events must occur to promote development of allelically included autoreactive B cells: 1) the autoreactive BCR must be sufficiently diluted to decrease any “negative” signal below the level required for the continuation of receptor editing, and 2) the nonautoreactive BCR and its tonic signal must attain levels sufficient to inhibit receptor editing and mediate differentiation (Figure 2). Behrens and colleagues have suggested that antigen-induced BCR signaling might not be the actual cause of receptor editing. They proposed instead that this signal might just induce receptor downmodulation, with the consequence of reducing tonic signaling and its ability to inhibit V(DJ) recombination [39*] (Figure 2). Studies of mice with subnormal levels of BCR give support to this model [38*,40,41], which implies that the maturation of allelically included B cells could depend solely on whether surface IgM and tonic BCR signaling reach the threshold level for positive selection. In this case, the activation level of molecular mediators of the tonic BCR signaling cascade such as PI3K and Erk [37*,38*,42] could play a primary role in the development of B cells that co-express autoreactive and nonautoreactive antibodies.
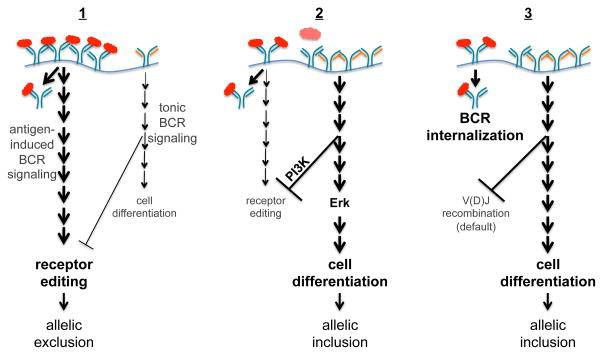
Figure 2.
Proposed models for the development of allelically included B cells. Models “1” and “2”: in immature B cells, self-antigen binding by BCR, signals receptor editing in addition to receptor internalization. Tonic BCR signaling, on the other hand, inhibits receptor editing (via PI3K) and induces cell differentiation (via Erk). Note that in low avidity cells, tonic BCR signaling may also be induced by BCRs that are not currently binding self-antigen (as shown in model “2”). If the majority of BCR is engaged with self-antigen, the cell continues the editing process to delete the autoreactive antibody resulting in allelically excluded cells (“1”). In contrast, if most BCRs are not engaged and thus transduce tonic signals, the cell stops editing and differentiates in spite of expressing an autoantibody (“2”). Model “3” postulates that antigen-induced BCR signaling mediates only receptor internalization, and the extent of both receptor editing and cell differentiation depends solely on the level of tonic BCR signaling.
Conclusions
Receptor editing is considered a major mechanism of central B cell tolerance and is also noteworthy for repertoire diversification. Studies presented herein provide demonstration that receptor editing is also responsible for increasing the self-reactive potential of the mature B cell repertoire, by generating B cells that co-express autoreactive and nonautoreactive antibodies. In depth evaluation of dual-κ B cells have revealed that in spite of being autoreactive in both healthy and autoimmune mice, these cells contribute to disease only when fostered by autoimmune genetic predisposition. Therefore, the role of dual-κ B cells in autoimmunity is most likely not in the initiation, but in the progression of disease. Studies of two distinct autoimmune-prone mice, moreover, demonstrate that not every autoimmune genetic background fosters the development and positive selection of allelically included B cells suggesting that their relevance in human autoimmunity, if any, may not be widespread. A challenge for the future will be to identify the molecular mechanisms that drive the selection of allelically included autoreactive B cells in the bone marrow to determine whether the tonic BCR signaling cascade can promote the development of autoreactive B cells independent of the level of antigen-mediated signaling. Moreover, with respect to their potential contribution to disease it will be of foremost importance to identify the signals that drive the activation of allelically included B cells and their participation in immune responses, whether these cells require binding to a foreign antigen or they are just driven by self-antigen in the context of autoimmunity. Finally, a better definition of their role in murine autoimmunity and their association with human autoimmunity will clarify whether allelically included B cell represent a relevant therapeutic target.
Highlights.
Receptor editing can lead to the development of dual Ig light chain B cells.
These cells are referred to as allelically included B cells.
Allelically included B cells coexpress autoreactive and nonautoreactive antibodies.
Preferential activation of these cells is enhanced in some autoimmune mice.
Acknowledgments
The author acknowledges the support of the National Institute of Health through grants RO1 AI052310 and PO1 AI022295. Dr. Pelanda is particularly grateful to Drs. Sucai Liu, Maria-Gabriela Velez, Sarah Rowland, and Emilie Fournier for research performed in her lab and that is presented herein. She is also thankful to Drs. Raul Torres, Julie Lang and Chiara Babolin for proof reading the article and providing useful suggestions that increased its clarity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
* of special interest
** of outstanding interest
- 1.Grandien A, Fucs R, Nobrega A, Andersson J, Coutinho A. Negative selection of multireactive B cell clones in normal adult mice. Eur J Immunol. 1994;24:1345–1352. doi: 10.1002/eji.1830240616. [DOI] [PubMed] [Google Scholar]
- 2.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 3.Shlomchik MJ. Sites and stages of autoreactive B cell activation and regulation. Immunity. 2008;28:18–28. doi: 10.1016/j.immuni.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Goodnow CC, Vinuesa CG, Randall KL, Mackay F, Brink R. Control systems and decision making for antibody production. Nat Immunol. 2010;11:681–688. doi: 10.1038/ni.1900. [DOI] [PubMed] [Google Scholar]
- 5.Halverson R, Torres RM, Pelanda R. Receptor editing is the main mechanism of B cell tolerance toward membrane antigens. Nat Immunol. 2004;5:645–650. doi: 10.1038/ni1076. [DOI] [PubMed] [Google Scholar]
- 6.Hippen KL, Schram BR, Tze LE, Pape KA, Jenkins MK, Behrens TW. In vivo assessment of the relative contributions of deletion, anergy, and editing to B cell self-tolerance. J Immunol. 2005;175:909–916. doi: 10.4049/jimmunol.175.2.909. [DOI] [PubMed] [Google Scholar]
- 7.Huang H, Kearney JF, Grusby MJ, Benoist C, Mathis D. Induction of tolerance in arthritogenic B cells with receptors of differing affinity for self-antigen. Proc Natl Acad Sci U S A. 2006;103:3734–3739. doi: 10.1073/pnas.0600214103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tiegs SL, Russell DM, Nemazee D. Receptor editing in self-reactive bone marrow B cells. Journal of Experimental Medicine. 1993;177:1009–1020. doi: 10.1084/jem.177.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gay D, Saunders T, Camper S, Weigert M. Receptor editing: an approach by autoreactive B cells to escape tolerance. Journal of Experimental Medicine. 1993;177:999–1008. doi: 10.1084/jem.177.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Radic MZ, Erikson J, Litwin S, Weigert M. B lymphocytes may escape tolerance by revising their antigen receptors. J Exp Med. 1993;177:1165–1173. doi: 10.1084/jem.177.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **11.Kalinina O, Doyle-Cooper CM, Miksanek J, Meng W, Prak EL, Weigert MG. Alternative mechanisms of receptor editing in autoreactive B cells. Proc Natl Acad Sci U S A. 2011;108:7125–7130. doi: 10.1073/pnas.1019389108. Using Ig gene sequencing in single cells sorted from Igκ-deficient mice, the authors discover that receptor editing can result in allelic inclusion at the Igλ locus and the co-expression of two λ chains. They also show that, within the repertoire of κ-deficient mice, successful H chain replacements occur in about 1% of cells that already harbored a functional H chain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pelanda R, Schwers S, Sonoda E, Torres RM, Nemazee D, Rajewsky K. Receptor editing in a transgenic mouse model: site, efficiency, and role in B cell tolerance and antibody diversification. Immunity. 1997;7:765–775. doi: 10.1016/s1074-7613(00)80395-7. [DOI] [PubMed] [Google Scholar]
- 13.Ait-Azzouzene D, Verkoczy L, Peters J, Gavin A, Skog P, Vela JL, Nemazee D. An immunoglobulin C kappa-reactive single chain antibody fusion protein induces tolerance through receptor editing in a normal polyclonal immune system. J Exp Med. 2005;201:817–828. doi: 10.1084/jem.20041854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casellas R, Shih TA, Kleinewietfeld M, Rakonjac J, Nemazee D, Rajewsky K, Nussenzweig MC. Contribution of receptor editing to the antibody repertoire. Science. 2001;291:1541–1544. doi: 10.1126/science.1056600. [DOI] [PubMed] [Google Scholar]
- 15.Duong BH, Ota T, Aoki-Ota M, Cooper AB, Ait-Azzouzene D, Vela JL, Gavin AL, Nemazee D. Negative selection by IgM superantigen defines a B cell central tolerance compartment and reveals mutations allowing escape. J Immunol. 2011;187:5596–5605. doi: 10.4049/jimmunol.1102479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartley SB, Cooke MP, Fulcher DA, Harris AW, Cory S, Basten A, Goodnow CC. Elimination of self-reactive B lymphocytes proceeds in two stages: arrested development and cell death. Cell. 1993;72:325–335. doi: 10.1016/0092-8674(93)90111-3. [DOI] [PubMed] [Google Scholar]
- *17.Casellas R, Zhang Q, Zheng NY, Mathias MD, Smith K, Wilson PC. Igkappa allelic inclusion is a consequence of receptor editing. J Exp Med. 2007;204:153–160. doi: 10.1084/jem.20061918. This study, along with ref. #24, uses Igkm/h mice to show for the first time the existence of allelically included dual-κ B cells within a wild-type antibody repertoire. It also indicates that dual-κ B cells are delayed in development relative to single κ B cells and that receptor editing targets each Igk allele, whether rearranged or not, with equal probability. The authors also show that dual-κ B cells are more frequently autoreactive than single κ B cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li H, Jiang Y, Prak EL, Radic M, Weigert M. Editors and editing of anti-DNA receptors. Immunity. 2001;15:947–957. doi: 10.1016/s1074-7613(01)00251-5. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Li H, Weigert M. Autoreactive B cells in the marginal zone that express dual receptors. Journal of Experimental Medicine. 2002;195:181–188. doi: 10.1084/jem.20011453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu S, Velez MG, Humann J, Rowland S, Conrad FJ, Halverson R, Torres RM, Pelanda R. Receptor editing can lead to allelic inclusion and development of B cells that retain antibodies reacting with high avidity autoantigens. J Immunol. 2005;175:5067–5076. doi: 10.4049/jimmunol.175.8.5067. [DOI] [PubMed] [Google Scholar]
- 21.Barreto V, Cumano A. Frequency and characterization of phenotypic Ig heavy chain allelically included IgM-expressing B cells in mice. J Immunol. 2000;164:893–899. doi: 10.4049/jimmunol.164.2.893. [DOI] [PubMed] [Google Scholar]
- 22.Rezanka LJ, Kenny JJ, Longo DL. Dual isotype expressing B cells [kappa(+)/lambda(+)] arise during the ontogeny of B cells in the bone marrow of normal nontransgenic mice. Cell Immunol. 2005;238:38–48. doi: 10.1016/j.cellimm.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Giachino C, Padovan E, Lanzavecchia A. kappa+lambda+ dual receptor B cells are present in the human peripheral repertoire. J Exp Med. 1995;181:1245–1250. doi: 10.1084/jem.181.3.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *24.Velez MG, Kane M, Liu S, Gauld SB, Cambier JC, Torres RM, Pelanda R. Ig allotypic inclusion does not prevent B cell development or response. J Immunol. 2007;179:1049–1057. doi: 10.4049/jimmunol.179.2.1049. Similar to ref. #17, this study uses Igkm/h mice to show for the first time the existence of allelically included dual-κ B cells within a wild-type antibody repertoire. It also demonstrates that dual-κ B cells respond functionally to BCR stimulation in vitro and participate in antigen-specific antibody responses in vivo. [DOI] [PubMed] [Google Scholar]
- **25.Fournier EM, Velez MG, Leahy K, Swanson CL, Rubtsov AV, Torres RM, Pelanda R. Dual-reactive B cells are autoreactive and highly enriched in the plasmablast and memory B cell subsets of autoimmune mice. J Exp Med. 2012;209:1797–1812. doi: 10.1084/jem.20120332. By studying Igkm/h mice on disease-prone MRL and MRL/lpr genetic backgrounds, the authors demonstrate that dual-κ B cells are generated more frequently in autoimmune mice, they are partly eliminated at the transitional checkpoint of selection, but undergo extensive positive selection into the plasmablast and memory B cell subsets. Dual-κ cells, moreover, secrete autoantibodies in larger quantities than single-κ cells. This study, therefore, strongly supports a role of allelically included B cells in autoimmune disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **26.Andrews SF, Zhang Q, Lim S, Li L, Lee JH, Zheng NY, Huang M, Taylor WM, Farris AD, Ni D, et al. Global analysis of B cell selection using an immunoglobulin light chain-mediated model of autoreactivity. J Exp Med. 2013;210:125–142. doi: 10.1084/jem.20120525. The authors analyze in great detail the selection of Igkm/h B cells that harbor a κ chain with either a high or low probabily of generating autoantibodies. By cloning the Ig genes from individual single and dual-κ B cells, re-expressing each H+κ pair, and testing their reactivity toward self-antigens, the authors demonstrate the role of receptor editing in the development and tolerance of single and dual-κ B cells. They also show that about half of allelically included B cells are removed at the transitional tolerance checkpoint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore MW, Durdik J, Persiani DM, Selsing E. Deletions of kappa chain constant region genes in mouse lambda chain-producing B cells involve intrachromosomal DNA recombinations similar to V-J joining. Proc Natl Acad Sci U S A. 1985;82:6211–6215. doi: 10.1073/pnas.82.18.6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Li H, Ni D, Weigert M. Anti-DNA B cells in MRL/lpr mice show altered differentiation and editing pattern. J Exp Med. 2002;196:1543–1552. doi: 10.1084/jem.20021560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cyster JG, Goodnow CC. Antigen-induced exclusion from follicles and anergy are separate and complementary processes that influence peripheral B cell fate. Immunity. 1995;3:691–701. doi: 10.1016/1074-7613(95)90059-4. [DOI] [PubMed] [Google Scholar]
- 30.Merrell KT, Benschop RJ, Gauld SB, Aviszus K, Decote-Ricardo D, Wysocki LJ, Cambier JC. Identification of anergic B cells within a wild-type repertoire. Immunity. 2006;25:953–962. doi: 10.1016/j.immuni.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 31.Tussiwand R, Rauch M, Fluck LA, Rolink AG. BAFF-R expression correlates with positive selection of immature B cells. Eur J Immunol. 2012;42:206–216. doi: 10.1002/eji.201141957. [DOI] [PubMed] [Google Scholar]
- 32.Norvell A, Monroe JG. Acquisition of surface IgD fails to protect from tolerance-induction. Both surface IgM- and surface IgD-mediated signals induce apoptosis of immature murine B lymphocytes. J Immunol. 1996;156:1328–1332. [PubMed] [Google Scholar]
- *33.Makdasi E, Eilat D. L chain allelic inclusion does not increase autoreactivity in lupus-prone New Zealand Black/New Zealand White mice. J Immunol. 2013;190:1472–1480. doi: 10.4049/jimmunol.1202331. The authors study dual-κ B cells in Igkm/h NZB/NZW autoimmune mice that harbor or not an anti-DNA H chain. They find that NZB/NZW mice generate dualκ B cells at a frequency similar to that of control mice, and that these cells are not more frequently autoreactive relative to single-κ B cells. However, allelically included B cells are generated more commonly within the developing B cells of anti-DNA H chain transgenic mice, which more frequently utilize receptor editing to silence autoreactivity. [DOI] [PubMed] [Google Scholar]
- 34.Melamed D, Nemazee D. Self-antigen does not accelerate immature B cell apoptosis, but stimulates receptor editing as a consequence of developmental arrest. Proc Natl Acad Sci U S A. 1997;94:9267–9272. doi: 10.1073/pnas.94.17.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bannish G, Fuentes-Panana EM, Cambier JC, Pear WS, Monroe JG. Ligand-independent signaling functions for the B lymphocyte antigen receptor and their role in positive selection during B lymphopoiesis. J Exp Med. 2001;194:1583–1596. doi: 10.1084/jem.194.11.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tze LE, Schram BR, Lam KP, Hogquist KA, Hippen KL, Liu J, Shinton SA, Otipoby KL, Rodine PR, Vegoe AL, et al. Basal immunoglobulin signaling actively maintains developmental stage in immature B cells. PLoS Biology. 2005;3:e82. doi: 10.1371/journal.pbio.0030082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *37.Verkoczy L, Duong B, Skog P, Ait-Azzouzene D, Puri K, Vela JL, Nemazee D. Basal B cell receptor-directed phosphatidylinositol 3-kinase signaling turns off RAGs and promotes B cell-positive selection. J Immunol. 2007;178:6332–6341. doi: 10.4049/jimmunol.178.10.6332. The authors demonstrate that immature B cells with an innocuous BCR have sustained elevation of PIP3 levels and that the activity of PI3K is required to inhibit Rag expression and, therefore, receptor editing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *38.Rowland SL, DePersis CL, Torres RM, Pelanda R. Ras activation of Erk restores impaired tonic BCR signaling and rescues immature B cell differentiation. Journal of Experimental Medicine. 2010;207:607–621. doi: 10.1084/jem.20091673. This study shows that threshold levels of tonic BCR signaling lead to basal activation of Erk, an event required for the differentiation of immature B cells into transitional B cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *39.Schram BR, Tze LE, Ramsey LB, Liu J, Najera L, Vegoe AL, Hardy RR, Hippen KL, Farrar MA, Behrens TW. B cell receptor basal signaling regulates antigen-induced Ig light chain rearrangements. Journal of Immunology. 2008;180:4728–4741. doi: 10.4049/jimmunol.180.7.4728. The authors perform in vitro studies of immature B cells to show that Rag expression and receptor editing correlate more closely with BCR internalization than with BCR signaling. They propose that receptor editing is promoted by lack of tonic BCR signaling rather than antigen-induced BCR signaling. [DOI] [PubMed] [Google Scholar]
- 40.Brenner S, Drewel D, Steinbart T, Weisel F, Hartel E, Potzsch S, Welzel H, Brandl A, Yu P, Mudde GC, et al. A hypomorphic IgH-chain allele affects development of B-cell subsets and favours receptor editing. EMBO Journal. 2011;30:2705–2718. doi: 10.1038/emboj.2011.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen S, Manser T. Direct reduction of antigen receptor expression in polyclonal B cell populations developing in vivo results in light chain receptor editing. Journal of Immunology. 2012;188:47–56. doi: 10.4049/jimmunol.1102109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Llorian M, Stamataki Z, Hill S, Turner M, Martensson IL. The PI3K p110delta is required for down-regulation of RAG expression in immature B cells. J Immunol. 2007;178:1981–1985. doi: 10.4049/jimmunol.178.4.1981. [DOI] [PubMed] [Google Scholar]