Abstract
Surgical options for cartilage resurfacing may be significantly improved by advances and application of biomaterials that direct tissue repair. A poly(ethylene glycol) diacrylate (PEGDA) hydrogel was designed to support cartilage matrix production, with easy surgical application. A model in vitro system demonstrated deposition of cartilage-specific extracellular matrix in the hydrogel biomaterial and stimulation of adjacent cartilage tissue development by mesenchymal stem cells. For translation to the joint environment, a chondroitin sulfate adhesive was applied to covalently bond and adhere the hydrogel to cartilage and bone tissue in articular defects. After preclinical testing in a caprine model, a pilot clinical study was initiated where the biomaterials system was combined with standard microfracture surgery in 15 patients with focal cartilage defects on the medial femoral condyle. Control patients were treated with microfracture alone. Magnetic resonance imaging showed that treated patients achieved significantly higher levels of tissue fill compared to controls. Magnetic resonance spin-spin relaxation times (T2) showed decreasing water content and increased tissue organization over time. Treated patients had less pain compared with controls, whereas knee function [International Knee Documentation Committee (IKDC)] scores increased to similar levels between the groups over the 6 months evaluated. No major adverse events were observed over the study period. With further clinical testing, this practical biomaterials strategy has the potential to improve the treatment of articular cartilage defects.
INTRODUCTION
Despite 2 decades of effort, cartilage resurfacing remains a challenge where new approaches and a paradigm shift in clinical treatment are required. Focal cartilage lesions seldom heal and frequently lead to degenerative changes in the joint. Cellular therapies, such as autologous chondrocyte transplantation, have some efficacy in generating new tissue; however, the surgical procedure requires two surgeries, and the cost and hurdles of delivering living cells prevent widespread use. In addition, clinical studies comparing chondrocyte transplantation with simple surgical strategies have not found significant improvements in clinical outcomes (1, 2). An effective cartilage repair strategy would not only alleviate pain and enhance patient activity levels but also mitigate or delay the onset of osteoarthritis. Previously, there was a desire to replace lost cartilage with a material or tissue that immediately replicates the native tissue structure. Unfortunately, implanting fully functional cartilage into defects, such as osteochondral auto- and allografts, often results in poor integration to the surrounding cartilage tissue (3). Furthermore, biomaterial implants designed to replicate the mechanical properties of native tissue have not succeeded in clinical and preclinical studies, owing to poor integration and the promotion of bone and fibrous tissue growth instead of hyaline cartilage, as is often seen with rigid materials in vivo (3, 4). A change in paradigm must be developed to successfully address clinical cartilage repair.
Clinicians apply the microfracture surgical procedure as a first line of therapy to treat focal cartilage defects. In the microfracture procedure, small holes are created in the subchondral bone underlying cartilage defects. The end result of microfracture is a blood clot that fills the defect and has access to progenitor cells in the bone marrow. Although a first-line treatment, the clinical outcomes of microfracture are still questionable and the longevity of tissue repair is not adequate, with about 50% of procedures failing. Ineffective repair from microfracture is due in part to insufficient volume of tissue to fill the defect space and the fibrous nature of the repair tissue that does not reproduce the native tissue function and longevity (5). Bone invasion into the cartilage repair tissue is also frequently observed and ultimately results in treatment failure (6). Good results are typically limited to young patients with small lesions (<2 cm2), low body mass indexes (BMIs), and acute onset of symptoms (7). Unfortunately, this does not encompass the demographic in need of cartilage repair.
Biomaterials have been proposed for application in defects in conjunction with microfracture surgery to improve efficacy, broaden the indications for use, and reduce complications or negative sequelae after the procedure. Several biomaterials have been proposed, including collagen membranes (8), fibrin glue (9), hyaluronic acid (HA) (10), and poly(ethylene glycol) (PEG)–based hydrogels (11). Here, we developed a PEG-based hydrogel biomaterial for use with microfracture to support cartilage development while discouraging scar formation (fibroblast activity) in articular cartilage defects. PEG hydrogels allow survival of nonadhesive cell types, such as chondrocytes, while discouraging adhesive cell (osteoblast, fibroblast) growth, and can promote chondrogenesis. To directly bond the hydrogel to tissue (bone or cartilage) surfaces, we also developed a chondroitin sulfate (CS) adhesive that physically immobilized the hydrogel and stimulated cartilage formation at the biomaterial interface (12). The bifunctional CS adhesive tissue primer reacts with amines on the tissue surface and with the PEG diacrylate (PEGDA) hydrogel.
We previously performed large-animal (caprine) studies to evaluate safety and performance of the adhesive-hydrogel technology with microfracture for treatment of articular cartilage defects (12). Here, we initiated a pilot clinical study in 18 patients to test safety and preliminary efficacy in humans (tissue formation and maturation) and to evaluate standard clinical and rehabilitation protocols that are not possible in animal models. This bench-to-bedside translation represents a potential change in paradigm for the clinical treatment of articular cartilage defects.
RESULTS
Cartilage growth in hydrogels in vitro
The overall design of the biomaterials and surgical technology to promote tissue repair in articular cartilage defects is presented in Fig. 1. First, the bifunctional CS-based adhesive was applied to the surface of a focal cartilage defect (Fig. 1, A and B) where it covalently bonded to amine groups in the tissue. Microfracture was then performed to create small holes in the subchondral bone (Fig. 1B). Finally, the liquid polymer solution was placed into the defect and cross-linked via a photopolymerization to form a solid hydrogel implant and also react with the CS tissue primer (Fig. 1C). The hydrogel implant was infiltrated with the blood and marrow components (Fig. 1D). Because the hydrogel-adhesive technology gels in situ, it can be applied to a variety of cartilage defect geometries without the need for removal of healthy tissue. Two clinical examples demonstrate defect geometries, and gels before and after bleeding from the microfracture holes have infiltrated the biomaterial (Fig. 1, C and D).
Fig. 1.
Clinical procedure for adhesive-hydrogel implantation into a cartilage defect. Both schematics and actual patient images are shown for the final steps. (A) A mini-incision approach was created to expose the cartilage defect. The defect edges were debrided to remove any dead tissue at the cartilage edge. (B) The adhesive was then applied to the base and walls of the defect followed by surgical microfracture. (C) Last, the hydrogel solution was injected into the defect and photopolymerized in situ with light. (D) Bleeding from the microfracture holes was trapped in and around the hydrogel.
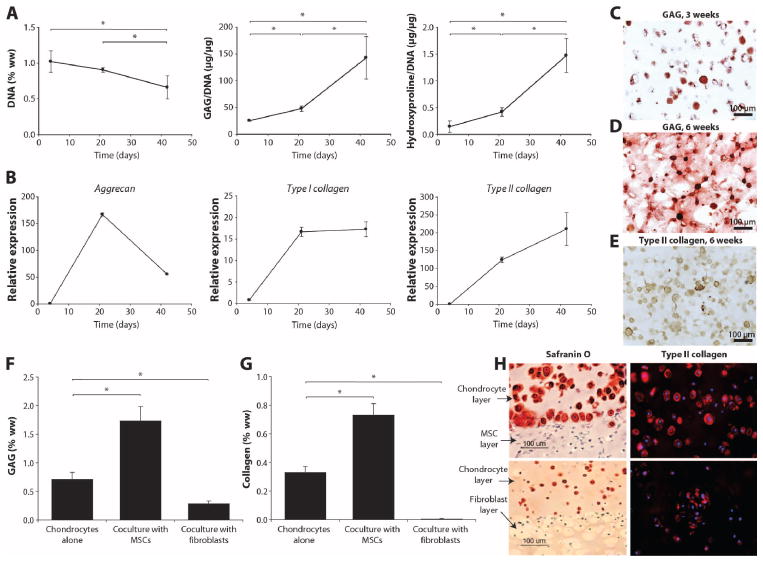
The composition of our material was designed for ease in clinical application in addition to promoting tissue growth. Linear, high–molecular weight HA was incorporated into the PEG hydrogel to increase the viscosity of the prepolymerized solution and to reduce gravity-related spillage into the joint space before cross-linking. After photopolymerization in vitro, the resulting hydrogel demonstrated a Young’s modulus of 68.7 ± 6.5 kPa under dynamic compression and 44.3 ± 2.2 kPa (mean ± SD; n = 4) under static compression. The ability of goat bone marrow–derived mesenchymal stem cells (gMSCs) to secrete cartilage matrix in this specific hydrogel formulation was evaluated. Over 6 weeks of in vitro culture, both proteoglycan [glycosaminoglycan (GAG)] and collagen secreted into the hydrogel increased, whereas DNA content (cell number) initially increased and then decreased over culture time (42 days) (Fig. 2A). GAG production by gMSCs was comparable to that of mature chondrocytes (40.8 ± 1.1 μg of GAG/μg of DNA at 3 weeks). Collagen production by the gMSCs was lower: gMSCs in culture for 6 weeks produced similar amounts of collagen to mature chondrocytes cultured for 3 weeks (1.04 ± 0.06 μg of hydroxyproline/μg of DNA at 3 weeks). Gene expression supported the matrix production results (Fig. 2B), with aggrecan expression reaching a maximum at 3 weeks, followed by type II collagen at 6 weeks. Type I collagen expression increased at 3 weeks and remained constant at a level 10-fold lower than aggrecan and type II collagen. Morphological analysis and histology also confirmed increasing matrix production over 3 weeks (Fig. 2C) and 6 weeks (Fig. 2D) of culture. The majority of cells [71.6 ± 3.0% (mean ± SD)] stained positive for type II collagen (Fig. 2E).
Fig. 2.
In vitro cartilage growth in hydrogels. MSCs encapsulated in the PEG hydrogel underwent chondrogenesis and secreted cartilage matrix in culture. (A) DNA, GAG, and hydroxyproline contents were measured over 6 weeks. Data are presented as means ± SD; n = 4. *P < 0.05. (B) Expression of aggrecan, type II collagen, and type I collagen over 6 weeks. (C to E) Safranin-O staining for GAGs at 3 weeks (C) and 6 weeks (D) and type II collagen at 6 weeks (E) in MSC cultures. (F and G) GAG (F) and total collagen (G) production by chondrocytes cultured alone in hydrogel compared with chondrocytes in bilayer coculture with MSCs and fibroblasts in vitro. (H) Histological staining of cocultures. Safranin-O staining at the interface of the chondrocyte-MSC coculture layers and the control chondrocyte-fibroblast layers; type II collagen in chondrocyte layer of chondrocyte-MSC bilayer (top) compared with chondrocytes cultured alone (bottom).
Recent evidence suggests that trophic factors secreted by MSCs stimulate tissue development and repair (13, 14). To model and evaluate the potential stimulatory role of bone marrow–derived gMSCs on local chondrocytes and cartilage tissue formation in vitro, a bilayered hydrogel coculture system was used. Bovine chondrocytes encapsulated in a PEG hydrogel adjacent to gMSCs more than doubled GAG and collagen production compared to when cultured alone (Fig. 2, F and G). Conversely, bovine chondrocytes cocultured adjacent to human fibroblasts were not stimulated to produce cartilage extracellular matrix (ECM) and, in fact, demonstrated a significant decrease in GAG and collagen production compared to chondrocytes cultured alone. Safranin-O staining and type II collagen immunostaining (Fig. 2H) supported the biochemical results that demonstrated increased cartilage matrix production in the chondrocytes cocultured with MSCs. The fibroblasts cultured in the hydrogel produced minimal levels of collagen (0.03 ± 0.05% of wet weight), and adjacent chondrocytes decreased GAG and collagen production significantly (Fig. 2, F to H), suggesting the importance of reducing the number of fibroblasts in the repair environment. Ultimately, the nonadhesive hydrogel discouraged fibroblast growth and matrix production. This simplified in vitro model system supports the concept of a potential stimulatory role of MSCs in local cartilage repair in a defect region and highlights the potential deleterious effects of fibroblasts in not only producing fibrocartilage and scar in a defect but also inhibiting adjacent chondrocytes and cartilage tissue growth.
Implantation in a large-animal model
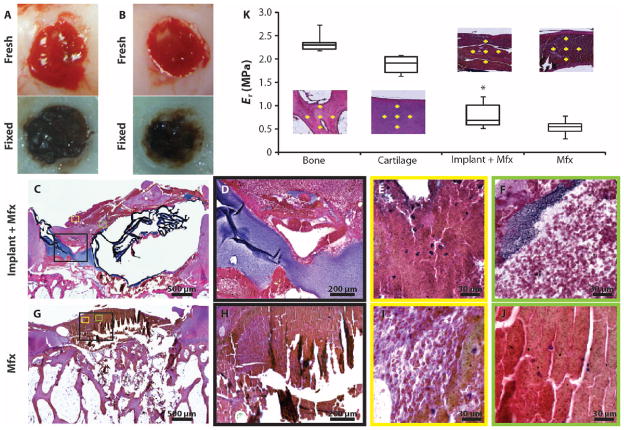
We previously performed a long-term large-animal (caprine) study to establish safety and evaluate efficacy of the adhesive-hydrogel system in conjunction with microfracture (12). However, we did not evaluate how the implant interacted with the marrow and bleeding in the defect after microfracture, making potential mechanisms of action impossible to establish. A better understanding of the biomaterial immediately after implantation (bleeding and clot formation) was important for establishing clinical guidelines for the surgical application of the hydrogel. Therefore, we investigated the immediate and short-term (minutes to hours) interactions of the hydrogel in combination with microfracture. Caprine chondral defects were treated with the adhesive-hydrogel (n = 6) (Fig. 3A) or microfracture alone (n = 6) (Fig. 3B). Bleeding from the microfracture holes was observed in both defects. However, upon visual inspection during surgery, the microfracture defect contained a thin layer of blood clot that did not fill the entire defect, whereas defects implanted with the hydrogel in combination with microfracture contained hydrogel–blood clot composites that filled most of the defect. Histological evaluation after 6 hours showed greater defect fill in the presence of the biomaterial compared to microfracture alone (Fig. 3, C and G), despite shrinkage artifacts from the hydrogel processing.
Fig. 3.
Hydrogel implantation in conjunction with microfracture in a goat model. (A) Six hours after microfracture and hydrogel implantation, blood components are visible throughout the defect and hydrogel, which filled the defect to the level of the articular surface. Image is representative of n = 6. (B) Six hours after microfracture alone, blood clot fills the defect below the level of the articular defect. Image is representative of n = 6. (C to J) Hematoxylin and eosin (H&E) staining was performed to evaluate the morphology of defect contents in both groups. (D and H) Higher-magnification images of the areas boxed in black in (C) and (G), respectively. (E, F, I, and J) Higher-magnification images of the respective areas boxed in color in (C) and (G). (K) Nanoindentation mechanical testing was performed on the histological sections. Yellow crosses define where the mechanical testing was performed for representative sections. Data are standard median box plots (n = 3 sections per group with 10 to 15 indentations per represented area).
The heterogeneity of the defect fill was also different in the presence of the hydrogel compared with microfracture alone. Multiple layers of clot components (cells, proteins) and hydrogel were visible (Fig. 3, C to F) compared with a more homogeneous-appearing microfracture-alone clot (Fig. 3, G to J). Dense layers of hydrogel (purple) can be observed in the hydrogel-clot (Fig. 3D). Regions of blood clot can be seen (Fig. 3E) as well as marrow cells (white) distributed within the hydrogel (Fig. 3F). In the microfracture alone group (Fig. 3H), there were regions of loose clot (Fig. 3I) and dense clot (Fig. 3J).
Nanoindentation of the histological sections confirmed a difference in mechanical properties between the hydrogel-clot and the microfracture-clot with 1.56-fold greater strength (P < 0.05, Student’s t test) observed when the biomaterial was present (Fig. 3K). Although the tissue was fixed in the histological sections, the relative properties of the two implants could be compared. Internal control testing of cartilage and bone in the surrounding tissue provided a reference for comparison.
Human cadaveric implantation
The large-animal studies provided some evidence of the behavior of the material in combination with microfracture in an active environment. However, clinically relevant defects in humans are substantially larger than those that can be created in the caprine model. Therefore, the application of the hydrogel and the equivalence of material properties in larger defects were evaluated in human cadaveric joints. First, chondral defects of various sizes were created in the articular cartilage on the femoral surface (fig. S1A). Then, the CS-adhesive was applied to the defect surface, the defect was filled with the viscous polymer solution, and the polymer was exposed to light to induce cross-linking and hydrogel formation (fig. S1, B to D). A cone was designed to apply the light at a constant distance from the implant to achieve a consistent light intensity.
Physical properties of the hydrogels were determined by the cross-linking density or pore size, which can be extrapolated from the gel’s equilibrium swelling volume ratio (wet to dry weights) (fig. S1E). The water content of the hydrogels after removal and the equilibrium swelling of the hydrogels created in the various defects were similar to control hydrogels cross-linked in an external mold (P = 0.44, Student’s t test), suggesting that the cross-linking density and physical properties of the hydrogels were similar. In addition to varying defect size, the accessibility of defects on various locations on the condyle was also evaluated to determine guidelines for knee positioning during surgery (Supplementary Methods) and to confirm that the implant did not dislodge during movement. These cadaveric studies were used to establish the surgical protocol for hydrogel and adhesive application in the pilot clinical study.
Pilot clinical study design and tissue imaging
A pilot clinical study of the hydrogel-directed cartilage repair was initiated in 18 patients to evaluate human safety and clinical feasibility. Patients without general osteoarthritis who had a single, symptomatic cartilage defect 2 to 4 cm2 in size on the medial femoral condyle in a stable knee were enrolled in the study (table S1). Fifteen patients were treated with the adhesive-hydrogel combination after microfracture, and three patients were treated with microfracture alone.
After standard debridement to remove dead/damaged cartilage at the edge of the defect as well as calcified cartilage, the CS adhesive was “painted” on the defect surface (Fig. 1B). Subsequently, standard microfracture technique (15) was performed. For patients receiving the biomaterial implant, the PEGDA-HA solution was introduced into the defect and photopolymerized with light for 4 min. Little bleeding in the microfracture holes was observed before closure (Fig. 1C). In one case, the tourniquet was released before closure, and blood infiltrated the hydrogel intraoperatively (Fig. 1D). The implant remained robust and elastic and firmly adhered within the defect when probed by the surgeon (N.M.). The defects varied in shape, ranging from oblong to irregular (Fig. 1, C and D), but because the hydrogel formed (cross-linked) in situ, the material conformed to the shape of the defect. No major adverse events related to implant were observed 6 months after surgery. One minor event deemed related to the procedure included a mild hemarthrosis in the implant plus microfracture group, which resolved without treatment.
Imaging of the articular surface as well as pain and function evaluation were performed to evaluate the safety and efficacy of the hydrogel implantation. Magnetic resonance imaging (MRI) confirmed repair tissue fill, maturation, and integration with the surrounding cartilage over time (Fig. 4 and table S2). Baseline MRIs were taken 3 weeks after implantation when postoperative swelling had subsided and defect boundaries/size could be determined. At this stage, the defects contained a mixture of clot material, hydrogel, and synovial fluid that could be distinguished from surrounding native tissue. MRIs were subsequently taken after 3 and 6 months to monitor new tissue development (Fig. 4A). Repair tissue filled 86% of the defect in patients treated with the hydrogel implant compared with 64% in the microfractured patients (Fig. 4B and table S2). Overall, 12 of the 14 patients evaluated by MRI in the implant group had a defect fill that was greater than 75% (table S2). In the microfracture group, only one of the three patients had a defect fill greater than 75% at 6 months.
Fig. 4.
Imaging and clinical evaluation of cartilage repair. (A) MR images show tissue repair over 6 months after treatment with implant + microfracture (Mfx) or Mfx alone. Sample sagittal MR images are of the tibiofemoral joint acquired using T1-weighted (T1W), fat-suppressed gradient echo and proton density–weighted (PDW), fat-suppressed fast spin echo at baseline and at 6 months. Arrows highlight the defects. (B) Quantification of defect fill from MR images in (A), using both the fast spin-echo and gradient-echo images; the average of the two measurements was used. Data are means ± SD (n = 15 at baseline, n = 12 at 3 months, and n = 14 at 6 months). *P < 0.05 versus microfracture control. (C) T2 relaxation times measured from four regions of interest on two images (n = 8 for each patient). Data are means ± SD (n = 9 patients). *P < 0.05 versus respective baseline measurement.
T2 relaxation times indicate tissue organization and water content (16, 17). The T2 relaxation time in the original defect space decreased significantly over time in the implant group and progressively approached that of the surrounding native cartilage by 6 months (Fig. 4C). In the adjacent cartilage, the T2 relaxation time was consistent among patients and time points, with an average value of 49.5 ± 4.1 (SD) ms. The T2 relaxation times in the microfracture control defects were highly variable and did not demonstrate a significant decline over time (P = 0.18, Student’s t test). In general, the T2 relaxation times were higher in the microfracture group than in the implant with microfracture group; however, statistical significance was not observed (Fig. 4C).
Additional imaging parameters, including integration with surrounding cartilage tissue and osseous overgrowth, were also evaluated from the MRI (table S2). Analysis at 6 months of integration of repair tissue with host tissue in the hydrogel implant group demonstrated 100% integration in 7 of the 14 patients evaluated, whereas the remaining patients had small (<2 mm) gaps. No implant patients at 6 months had large gaps (>2 mm) between the repair and native tissues. In the microfracture group, two of the three patients demonstrated 100% integration and one patient had a small gap, albeit with significantly less new tissue filling the defect. No osseous overgrowth within the defect was observed in any patients in the implant group. In the microfracture control group, one patient demonstrated osseous overgrowth at 6 months, as seen by areas of no signal on all MR sequences corresponding to osseous tissue projecting from the cortical bone into the expected area of the repair (Fig. 4A and fig. S2).
Patient pain evaluation
Patients treated with the hydrogel implant in conjunction with microfracture also demonstrated significant improvements in the severity and frequency of pain (Fig. 5 and table S2). Overall pain severity and frequency decreased significantly over time, with the largest decrease observed between baseline and 3 months. The majority of patients (14 of 15) in the implant group experienced significant reduction in the frequency and severity of pain compared to baseline (Fig. 5, A and B). The one implant patient that continued to experience pain after 6 months was later determined to have misalignment in the treated knee, which may have affected the pain self-evaluation. This patient’s defect fill was more than 75%, indicating that lack of repair tissue formation was not contributing to the pain.
Fig. 5.

Patient self-evaluated clinical outcomes. (A to C) Patients evaluated their frequency of pain (A), severity of pain (B), and IKDC scores (knee function) (C) over 6 months. For the implant + microfracture (Mfx) group (n = 15), the box represents values between the 25th and 75th percentiles, the line indicates median value, and the whiskers are the 10th and 90th percentiles. For the Mfx group (n = 3), the box bounds the upper and lower data points, with the line representing the median value. *P < 0.05 compared to baseline value.
In the microfracture control group, there was no significant decrease in severity or frequency of pain at 6 months compared to baseline. There was an initial decrease in pain at 3 months; however, only one of the three control patients continued to experience a decrease in pain by 6 months, whereas the remaining two patients experienced an increase in pain to levels at or close to their baseline pain (Fig. 5B). The microfracture control patient who experienced the greatest pain reduction also had a high defect fill (84%), whereas the other two patients had fill values less than 75% (table S2). Despite differences in the pain profile, the International Knee Documentation Committee (IKDC) scores (a composite of pain and disability) for the implant and microfracture controls were similar at each time point and increased over time (Fig. 5C), indicating improved knee function.
DISCUSSION
When considering clinical translation, delivering cells to patients is an expensive and often burdensome task, which is likely why widespread use of autologous chondrocyte transplantation has not been achieved (18). Therefore, we designed a strategy where we could deliver an acellular biomaterial to a cartilage defect to work in conjunction with standard microfracture, which often suffers from poor defect fill and osseous ingrowth. Use of a biomaterial could reduce variability in clinical outcome, help fill the defects faster, and possibly extend the application of this technique to a broader patient population, for example, patients who are more than 40 years of age, with chronic lesions, and with higher BMIs who would typically have poor microfracture outcomes.
Hydrogels are a class of materials that have the appropriate mechanical properties to provide an environment supportive for cartilage formation. Several hydrogel formulations are being tested preclinically and clinically for cartilage repair, including the thermosensitive chitosan hydrogel BST-CarGel (Piramal Life Sciences) (19) and a PEG-fibrinogen hydrogel, GelrinC (Regentis Biomaterials) (20). The primary barrier to application of hydrogels, or any material, to cartilage repair in the joint is adhesion and integration with the surrounding tissue. Surrounding cartilage is “slippery” and difficult to bond with a material. Hydrogels are soft materials that are generally implanted only in a chondral defect, so integration with surrounding tissue is critical for retention of the hydrogel and new tissue development. Both BST-CarGel and GelrinC depend on blood clot or components of a clot (for example, fibrinogen) to integrate the hydrogel with surrounding bone and cartilage. Unfortunately, blood clots and related reactions have weak mechanical properties.
To address this challenge, we developed a CS adhesive to covalently bond the PEG hydrogels to a cartilage (or other joint tissue) surface (12). When the adhesive bonds a hydrogel to a cartilage explant, the mechanical integrity of the tissue-hydrogel interface is greater than the bulk hydrogel, and cartilage tissue growth is promoted in vivo (12). Ultimately, the cartilage tissue priming concept can be applied to any hydrogel system to improve cartilage tissue integration.
PEG is generally considered a biocompatible material. However, despite its frequent use as an in vitro culture system for cartilage, it has not been evaluated in a clinical setting until this study. Hydrogel formulation and physical and mechanical properties were optimized for chondrocyte and stem cell viability and matrix secretion. PEG-based hydrogels promote entrapped cells to acquire a spherical morphology, which is important for initiating chondrogenesis and maintaining a chondrocytic phenotype (21, 22). Of equal importance, this feature also discourages differentiation into fibroblast or osteogenic lineages unless adhesion sites are incorporated into the material (23, 24). This is particularly important in microfracture surgery where fibrocartilage is generally produced and osseous overgrowth is problematic. In a previous study, collagen membranes applied to microfracture demonstrated improvements in repair, but intralesional osteophytes were observed (25), which may be related to high cell adhesion to collagen and its ability to support fibroblast and osteoblast growth.
For clinical application of cartilage material, fast and efficient implant formation is required. A light-initiated reaction was chosen to cross-link the liquid polymer solution such that application and gelation could be performed in situ within minutes. The use of a liquid solution allows filling of irregular-shaped defects so that the maximal amount of healthy cartilage surrounding the defect can be preserved. This is an important challenge when working on a concave/convex surface with irregular lesion patterns, such as the knee joint. Final gelation and “locking” of the implant is physician-controlled with light exposure. To facilitate retention in defects before polymerization, the liquid “pre-gel” polymer solution was thickened with high–molecular weight HA to increase viscosity from 10 to 956 cP. Although this clinical protocol used a mini-incision for implantation, with the appropriate equipment a purely injectable system can be envisioned with this biomaterial technology.
We previously performed long-term large-animal studies to evaluate cartilage tissue formation in articular defects treated with the adhesive-hydrogel system (12). However, the short-term interactions of the adhesive-hydrogel in the bleeding microfracture environment were unknown. We hypothesized that incorporating the hydrogel into a defect with microfracture would enhance the physical and biological properties of the resulting blood clot compared with microfracture alone by increasing its mechanical strength and integration and by providing an environment conducive to chondrogenesis. Indeed, the resulting clot-material volume in the defect space was larger than that with clot alone (Fig. 3, A and B), which potentially can increase the volume of repair tissue. Other than the hydrogel itself, this larger defect-fill volume may be due in part to the lack in clot contraction of platelets in the PEG (26). In addition to the increased volume, the hydrogel improved the mechanical properties of the clot (Fig. 3K). Although these mechanical studies were performed on preserved histological sections, the differences in properties between the biomaterial and clot could be observed and compared to regions of bone and cartilage tissue in the same section. The increase in clot mechanical strength with the addition of hydrogel is expected from the literature, where a blood clot is on the order of a few pascals and hydrogels are tens of kilopascals (27, 28). Finally, the composition of the clot is different between the groups. The greater hydrogel-implant volume in the defect after treatment suggests that the biomaterial may be able to retain more proteins and cells in the defect space compared to microfracture, further augmenting the biological factors that can promote new tissue growth. Chitosan hydrogels implanted in rabbit and ovine articular cartilage defects were also found to increase clot volume, cellularity, and mechanical properties compared with clots alone (29, 30).
Critical to clinical success, the hydrogel discourages osseous overgrowth commonly associated with microfracture (31) and reduces the contribution of fibrous scar tissue during new tissue formation. Bone growth can occur immediately or later after microfracture is performed. The pilot clinical data here did not capture long-term bone abnormalities, but early events over the course of 6 months suggest that the hydrogel prevents bone growth better than microfracture alone. The preliminary evaluation of the hydrogel implants found no instance of osseous overgrowth with the biomaterial implants.
This pilot study demonstrated the human safety and clinical applicability of hydrogel biomaterials to articular cartilage repair. Although there were only a small number of control microfracture patients, the results may also be compared to historical data of microfracture. One of the most comprehensive studies of microfracture outcomes reported that only 54% of patients had greater than 67% tissue fill on MRI, and 92% of patients had gaps between the repair and native tissues [50% of the gaps were small (<2 mm), 40% were large (> 2 mm)] after 24 months (7). Another study evaluating MRI parameters 6 to 18 months after microfracture surgery reported that 47% of patients had defect fill between 67 and 100%, and 53% had large fissures (>2 mm) (6).
We also compared pain scores at 6 months to historical data. Differences in control patient pain (Fig. 5) may have been due to osseous overgrowth, which has been reported in 25 to 49% of cases (6, 7). Whereas the pain profile differed between the implant and controls, the IKDC scores increased over 6 months for both groups. The IKDC scoring system is a comprehensive methodology that includes pain, function, and return to sporting activities. It is based on 18 components, and it is therefore possible that, in some circumstances, the rating of pain is masked by an increased emphasis placed on other components of the score such as sporting activities (32). In this small cohort of microfracture controls, the increase in pain observed from 3 to 6 months was not captured by the IKDC score.
This pilot study was designed as a proof of concept for a new approach to cartilage repair using an adhesive-hydrogel technology to augment a surgical microfracture treatment. However, a significant improvement in defect tissue fill by MRI imaging and patient pain scores in the treated group was observed compared to a small number of internal controls as well as historical microfracture outcomes (6, 7). Further evaluation of these patients and a larger patient population are needed to fully validate this new approach to cartilage repair. In sum, this biomaterials-directed cartilage repair provides a simple strategy that works in combination with current surgical options. Preclinical studies provide evidence that a mechanically soft material can promote tissue remodeling after cartilage loss and microfracture-mediated repair. Pilot clinical results demonstrated a significant increase in tissue fill with defects when using the biomaterial with microfractures versus microfracture alone. Further studies are continuing to follow long-term tissue repair in a larger number of patients, working toward a shift in clinical treatment of articular cartilage defects.
MATERIALS AND METHODS
Chondrogenic differentiation of gMSCs
gMSCs were isolated and expanded as described previously (21). After four passages, gMSCs were trypsinized, centrifuged, and re-suspended in a PEGDA-HA macromer solution containing 10% (w/v) PEGDA (3.4 kD, Sunbio) with 0.5% (w/v) HA (975 kD, Lifecore) and 0.05% photoinitiator (Irgacure 2959; Ciba) in phosphate-buffered saline (PBS; Invitrogen). Cells were suspended at 20 × 106/ml, and hydrogels were polymerized in 100-μl molds (8 mm diameter × 6 mm height) by exposure to ultraviolet light at 365 nm (5 mW/cm2) for 5 min. Hydrogel constructs were transferred to 24-well plates containing chondrogenic differentiation medium [100 nM dexamethasone (Sigma), proline (40 mg/liter; Sigma), ascorbic acid-2-phosphate (50 mg/liter; Sigma), sodium pyruvate (100 mg/liter; Invitrogen), ITS Premix (insulin, transferrin, selenous acid; 50 mg/ml; BD Biosciences), 1% penicillin/streptomycin (Invitrogen), and transforming growth factor–β (10 ng/ml; Fitzgerald)]. Constructs were cultured at 37C, 5% CO2, for up to 6 weeks.
Characterization of in vitro chondrogenesis
Hydrogel constructs were harvested at 3, 21, and 42 days for analysis. Biochemical, polymerase chain reaction, and histological evaluations are described in Supplementary Methods.
Coculture model studies
Articular chondrocytes were isolated from the femoropatellar groove and medial and lateral condyles of juvenile bovine calves by enzymatic digestion as described previously (33). Chondrocytes were cocultured with gMSCs or human fibroblasts (Hs27, American Type Culture Collection) in bilayered hydrogels such that each cell population was confined in one layer (34). Chondrocytes, gMSCs, and fibroblasts were cultured alone in single-layer hydrogels as controls by photoencapsulation. Cell-hydrogel constructs were cultured for 3 weeks in medium containing Dulbecco’s modified Eagle’s medium (Invitrogen) supplemented with 10% fetal bovine serum (HyClone), 100 nM dexamethasone (Sigma), 50 mM ascorbic acid-2-phosphate (Sigma), and 10 mM β-glycerolphosphate (Sigma). Constructs were then analyzed for ECM composition by biochemical analysis and immunohistochemistry, as described in Supplementary Methods. The layers of the bilayered hydrogels were separated before biochemical characterization to isolate GAG and collagen production by chondrocytes in response to coculture with MSCs and fibroblasts.
Biomaterial formulation
The hydrogel solution and adhesive used in clinical studies were manufactured according to Good Manufacturing Practices (GMP). The hydrogel solution of PEGDA (100 mg/ml; 3400 MW, Sunbio), HA (5 mg/ml; 1.2 MD, Lifecore), and photoinitiator (0.5 mg/ml; Irgacure 2959, Ciba/Sigma) was prepared in PBS and filter-sterilized. Mechanical characterization of the hydrogel is described in Supplementary Methods. The CS-based adhesive was prepared as described previously (12) and filter-sterilized.
Short-term caprine studies
Animal studies were performed at T.D. Morris Inc. in accordance with the guidelines for the care and use of laboratory animals, and protocols were approved by the Institutional Animal Care and Use Committee. Multiple chondral defects (n = 6; diameter = 1 cm each) were created on the medial and lateral condyles of one caprine femur (2 years old). Microfracture was performed with (n = 6) and without (n = 6) implantation of the adhesive and hydrogel. These surgeries were conducted without a tourniquet to observe the rate and extent of bleeding through the microfracture holes. Gross observation of the biomaterials in conjunction with the bleeding process was observed over time. Six hours after surgery, the defects were explanted, preserved in 10% buffered formalin, and decalcified, followed by standard histological processing. Histology slides were stained with H&E. Nanoindentation experiments were performed as described in Supplementary Methods.
Pilot clinical trial
This study was approved by the respective National and Institutional Ethics Review Boards in the Netherlands, Germany, and Italy, and all patients gave informed consent before joining the study. Clinical protocols were approved by the respective ethics committees, and informed consent was obtained from all patients before entry into the study. Patient enrollment, surgical implant procedure, and postoperative rehabilitation are described in Supplementary Methods.
MRI (1.5 T) was performed, and images were evaluated at 3 weeks, 3 months, and 6 months after surgery. The 3-week time point was used as a baseline for determining defect volume because the contents of the defect (hydrogel, blood clot, and/or early repair tissue) could be distinguished from the surrounding cartilage tissue, and any postoperative swelling and/or inflammation that could interfere with the MRI signal would be minimal. Two MRI methods were used on the basis of clinical site location (Supplemental Methods). T2 relaxation times were measured using a multiecho fast spin-echo sequence, and signal levels were fitted to a monoexponential decay (35).
Knee pain was assessed preoperatively (baseline) and at 3 and 6 months postoperatively. Pain scores were determined with a visual analog scale and converted to a score between 0 and 100, with 0 representing no pain and 100 representing the worst pain. The IKDC form was completed by patients preoperatively (baseline) and at 3 and 6 months postoperatively as a measure of knee function. Scores were calculated according to the IKDC guidelines (32).
Statistical analysis
All data are reported as means ± SD. All statistical analysis was performed with Student’s t test, unless otherwise noted. Statistical significance was determined as P ≤ 0.05.
Supplementary Material
Acknowledgments
We thank clinical investigators G. H. Albers and L. Taminiau (Tergooi Hospital, the Netherlands), A. B. Stibbe (Meander Medical Center, the Netherlands), U. Pietzner and L. Völker (Dietrich-Bonhöffer Hospital, Germany), A. Lüth and H. Schmitz (Orthopedic Clinic Zähringen, Germany), and E. Kon and M. Marcacci (Rizzoli Orthopaedic Institute, Italy). We thank T. G. Kim for critical review of the manuscript.
Funding: Funding for the biomaterials work was provided to J.H.E. by the Arthritis Foundation and the NIH (grants R01AR054005 and 5R01EB005517), and to G.E.G. by the NIH (grants 2R01EB002524-08, 1K24AR062068-01, and 1P01159992-01) and the Arthritis Foundation. Large-animal studies were supported by the State of Maryland Technology Development Corporation and Cartilix Inc. Clinical studies were supported by Cartilix Inc. and Biomet.
Footnotes
www.sciencetranslationalmedicine.org/cgi/content/full/5/167/167ra6/DC1
Methods
Fig. S1. Cadaveric implantation of the adhesive-hydrogel system.
Fig. S2. Magnified MR images.
Table S1. Patient demographics and clinicopathology.
Table S2. Clinical outcomes after treatment with hydrogel implant in combination with microfracture and microfracture alone.
Table S3. Study subject imaging protocol.
Author contributions: B.S., G.E.G., N.M., and J.H.E. wrote the manuscript. B.S., S.F., and N.M. performed the cadaver study. S.F. and B.S. conducted GMP manufacturing of biomaterials. B.S., S.F., N.M., B.C., and J.H.E. participated in clinic study design, execution, and analysis. S.U., B.S., and A.Y.H. performed the in vitro studies. M.G. and J.C. performed the animal study and related data analysis. G.E.G. managed and reviewed the MRI. D.A.H. reviewed the MRI.
Competing interests: Intellectual property related to the hydrogel and adhesive technology is owned by Johns Hopkins University (patent application nos. 20070098675, 20090324722, and 20100003329) and Biomet (patent application no. 7897165).
Data and materials availability: All biomaterials described can be synthesized from commercially available materials as described in Materials and Methods.
REFERENCES AND NOTES
- 1.Knutsen G, Drogset JO, Engebretsen L, Grøntvedt T, Isaksen V, Ludvigsen TC, Roberts S, Solheim E, Strand T, Johansen O. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am. 2007;89:2105–2112. doi: 10.2106/JBJS.G.00003. [DOI] [PubMed] [Google Scholar]
- 2.Van Assche D, Staes F, Van Caspel D, Vanlauwe J, Bellemans J, Saris DB, Luyten FP. Autologous chondrocyte implantation versus microfracture for knee cartilage injury: A prospective randomized trial, with 2-year follow-up. Knee Surg Sports Traumatol Arthrosc. 2010;18:486–495. doi: 10.1007/s00167-009-0955-1. [DOI] [PubMed] [Google Scholar]
- 3.Hunziker EB. Articular cartilage repair: Basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10:432–463. doi: 10.1053/joca.2002.0801. [DOI] [PubMed] [Google Scholar]
- 4.Custers RJ, Saris DB, Dhert WJ, Verbout AJ, van Rijen MH, Mastbergen SC, Lafeber FP, Creemers LB. Articular cartilage degeneration following the treatment of focal cartilage defects with ceramic metal implants and compared with microfracture. J Bone Joint Surg Am. 2009;91:900–910. doi: 10.2106/JBJS.H.00668. [DOI] [PubMed] [Google Scholar]
- 5.Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: An evidence-based systematic analysis. Am J Sports Med. 2009;37:2053–2063. doi: 10.1177/0363546508328414. [DOI] [PubMed] [Google Scholar]
- 6.Brown WE, Potter HG, Marx RG, Wickiewicz TL, Warren RF. Magnetic resonance imaging appearance of cartilage repair in the knee. Clin Orthop Relat Res. 2004;422:214–223. doi: 10.1097/01.blo.0000129162.36302.4f. [DOI] [PubMed] [Google Scholar]
- 7.Mithoefer K, Williams RJ, III, Warren RF, Potter HG, Spock CR, Jones EC, Wickiewicz TL, Marx RG. The microfracture technique for the treatment of articular cartilage lesions in the knee. A prospective cohort study. J Bone Joint Surg Am. 2005;87:1911–1920. doi: 10.2106/JBJS.D.02846. [DOI] [PubMed] [Google Scholar]
- 8.Benthien JP, Behrens P. The treatment of chondral and osteochondral defects of the knee with autologous matrix-induced chondrogenesis (AMIC): Method description and recent developments. Knee Surg Sports Traumatol Arthrosc. 2011;19:1316–1319. doi: 10.1007/s00167-010-1356-1. [DOI] [PubMed] [Google Scholar]
- 9.Marmotti A, Bruzzone M, Bonasia DE, Castoldi F, Rossi R, Piras L, Maiello A, Realmuto C, Peretti GM. One-step osteochondral repair with cartilage fragments in a composite scaffold. Knee Surg Sports Traumatol Arthrosc. 2012;20:2590–2601. doi: 10.1007/s00167-012-1920-y. [DOI] [PubMed] [Google Scholar]
- 10.Kang SW, Bada LP, Kang CS, Lee JS, Kim CH, Park JH, Kim BS. Articular cartilage regeneration with microfracture and hyaluronic acid. Biotechnol Lett. 2008;30:435–439. doi: 10.1007/s10529-007-9576-2. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt O, Mizrahi J, Elisseeff J, Seliktar D. Immobilized fibrinogen in PEG hydrogels does not improve chondrocyte-mediated matrix deposition in response to mechanical stimulation. Biotechnol Bioeng. 2006;95:1061–1069. doi: 10.1002/bit.21072. [DOI] [PubMed] [Google Scholar]
- 12.Wang DA, Varghese S, Sharma B, Strehin I, Fermanian S, Gorham J, Fairbrother DH, Cascio B, Elisseeff JH. Multifunctional chondroitin sulphate for cartilage tissue–biomaterial integration. Nat Mater. 2007;6:385–392. doi: 10.1038/nmat1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 14.Rothenberg AR, Ouyang L, Elisseeff JH. Mesenchymal stem cell stimulation of tissue growth depends on differentiation state. Stem Cells Dev. 2011;20:405–414. doi: 10.1089/scd.2010.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Outcomes of microfracture for traumatic chondral defects of the knee: Average 11-year follow-up. Arthroscopy. 2003;19:477–484. doi: 10.1053/jars.2003.50112. [DOI] [PubMed] [Google Scholar]
- 16.Mosher TJ, Liu Y, Torok CM. Functional cartilage MRI T2 mapping: Evaluating the effect of age and training on knee cartilage response to running. Osteoarthritis Cartilage. 2010;18:358–364. doi: 10.1016/j.joca.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mosher TJ, Smith H, Dardzinski BJ, Schmithorst VJ, Smith MB. MR imaging and T2 mapping of femoral cartilage: In vivo determination of the magic angle effect. AJR Am J Roentgenol. 2001;177:665–669. doi: 10.2214/ajr.177.3.1770665. [DOI] [PubMed] [Google Scholar]
- 18.Martin I, Baldomero H, Bocelli-Tyndall C, Passweg J, Saris D, Tyndall A. The survey on cellular and engineered tissue therapies in Europe in 2010. Tissue Eng Part A. 2012;18:2268–2279. doi: 10.1089/ten.TEA.2012.0169. [DOI] [PubMed] [Google Scholar]
- 19.Méthot S, Hoemann CD, Rossomacha E, Restrepo A, Stanish WD, Macdonald P, Mohtadi N, Marks P, Malo M, McCormack R, Desnoyers J, Pelet S, Lopez-Olivia F, Vaquero J, Macule F, Shive MS, Buschmann MD. ICRS histology scores of biopsies from an interim analysis of a randomized controlled clinical trial show significant improvement in tissue quality at 13 months for BST-CarGel versus microfracture. paper presented at International Cartilage Repair Society; Barcelona, Spain. 2010. [Google Scholar]
- 20.Ahmed TA, Hincke MT. Strategies for articular cartilage lesion repair and functional restoration. Tissue Eng Part B Rev. 2010;16:305–329. doi: 10.1089/ten.TEB.2009.0590. [DOI] [PubMed] [Google Scholar]
- 21.Williams CG, Kim TK, Taboas A, Malik A, Manson P, Elisseeff J. In vitro chondrogenesis of bone marrow-derived mesenchymal stem cells in a photopolymerizing hydrogel. Tissue Eng. 2003;9:679–688. doi: 10.1089/107632703768247377. [DOI] [PubMed] [Google Scholar]
- 22.Musumeci G, Loreto C, Carnazza ML, Strehin I, Elisseeff J. OA cartilage derived chondrocytes encapsulated in poly(ethylene glycol) diacrylate (PEGDA) for the evaluation of cartilage restoration and apoptosis in an in vitro model. Histol Histopathol. 2011;26:1265–1278. doi: 10.14670/HH-26.1265. [DOI] [PubMed] [Google Scholar]
- 23.Yang F, Williams CG, Wang DA, Lee H, Manson PN, Elisseeff J. The effect of incorporating RGD adhesive peptide in polyethylene glycol diacrylate hydrogel on osteogenesis of bone marrow stromal cells. Biomaterials. 2005;26:5991–5998. doi: 10.1016/j.biomaterials.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 24.Benoit DS, Anseth KS. Heparin functionalized PEG gels that modulate protein adsorption for hMSC adhesion and differentiation. Acta Biomater. 2005;1:461–470. doi: 10.1016/j.actbio.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Dhollander AA, De Neve F, Almqvist KF, Verdonk R, Lambrecht S, Elewaut D, Verbruggen G, Verdonk PC. Autologous matrix-induced chondrogenesis combined with platelet-rich plasma gel: Technical description and a five pilot patients report. Knee Surg Sports Traumatol Arthrosc. 2011;19:536–542. doi: 10.1007/s00167-010-1337-4. [DOI] [PubMed] [Google Scholar]
- 26.Gersh KC, Nagaswami C, Weisel JW. Fibrin network structure and clot mechanical properties are altered by incorporation of erythrocytes. Thromb Haemost. 2009;102:1169–1175. doi: 10.1160/TH09-03-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCarty WJ, Luan A, Siddiqui M, Hansen BC, Masuda K, Sah RL. Biomechanical properties of mixtures of blood and synovial fluid. J Orthop Res. 2011;29:240–246. doi: 10.1002/jor.21209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lam WA, Chaudhuri O, Crow A, Webster KD, Li TD, Kita A, Huang J, Fletcher DA. Mechanics and contraction dynamics of single platelets and implications for clot stiffening. Nat Mater. 2011;10:61–66. doi: 10.1038/nmat2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoemann CD, Hurtig M, Rossomacha E, Sun J, Chevrier A, Shive MS, Buschmann MD. Chitosan-glycerol phosphate/blood implants improve hyaline cartilage repair in ovine microfracture defects. J Bone Joint Surg Am. 2005;87:2671–2686. doi: 10.2106/JBJS.D.02536. [DOI] [PubMed] [Google Scholar]
- 30.Hoemann CD, Sun J, McKee MD, Chevrier A, Rossomacha E, Rivard GE, Hurtig M, Buschmann MD. Chitosan-glycerol phosphate/blood implants elicit hyaline cartilage repair integrated with porous subchondral bone in microdrilled rabbit defects. Osteoarthritis Cartilage. 2007;15:78–89. doi: 10.1016/j.joca.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 31.Fortier LA, Cole BJ, McIlwraith CW. Science and animal models of marrow stimulation for cartilage repair. J Knee Surg. 2012;25:3–8. doi: 10.1055/s-0032-1310389. [DOI] [PubMed] [Google Scholar]
- 32.Hambly K, Griva K. IKDC or KOOS? Which measures symptoms and disabilities most important to postoperative articular cartilage repair patients? Am J Sports Med. 2008;36:1695–1704. doi: 10.1177/0363546508317718. [DOI] [PubMed] [Google Scholar]
- 33.Elisseeff J, McIntosh W, Anseth K, Riley S, Ragan P, Langer R. Photoencapsulation of chondrocytes in poly(ethylene oxide)-based semi-interpenetrating networks. J Biomed Mater Res. 2000;51:164–171. doi: 10.1002/(sici)1097-4636(200008)51:2<164::aid-jbm4>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 34.Sharma B, Williams CG, Kim TK, Sun D, Malik A, Khan M, Leong K, Elisseeff JH. Designing zonal organization into tissue-engineered cartilage. Tissue Eng. 2007;13:405–414. doi: 10.1089/ten.2006.0068. [DOI] [PubMed] [Google Scholar]
- 35.Mosher TJ, Smith HE, Collins C, Liu Y, Hancy J, Dardzinski BJ, Smith MB. Change in knee cartilage T2 at MR imaging after running: A feasibility study. Radiology. 2005;234:245–249. doi: 10.1148/radiol.2341040041. [DOI] [PubMed] [Google Scholar]
- 36.Varghese S, Hwang NS, Canver AC, Theprungsirikul P, Lin DW, Elisseeff J. Chondroitin sulfate based niches for chondrogenic differentiation of mesenchymal stem cells. Matrix Biol. 2008;27:12–21. doi: 10.1016/j.matbio.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 37.Oliver WC, Pharr GM. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J Mater Res. 1992;7:1564–1583. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.