Abstract
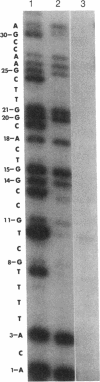
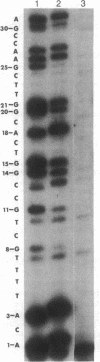
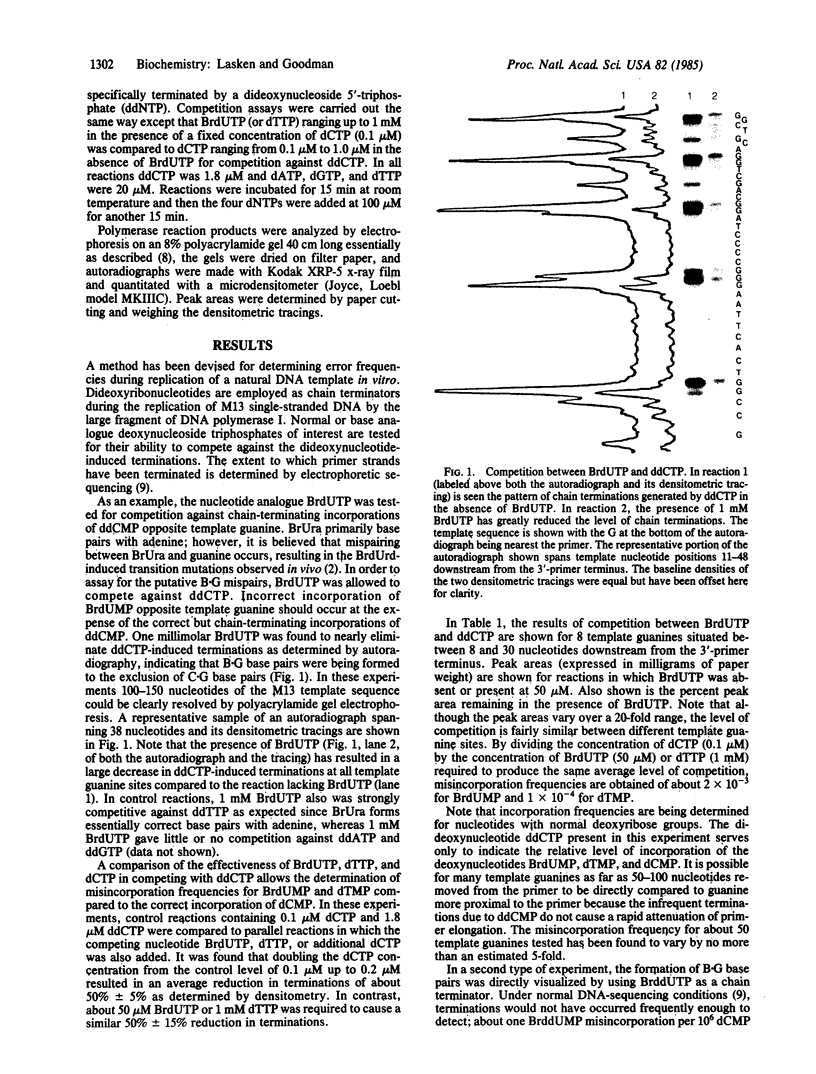
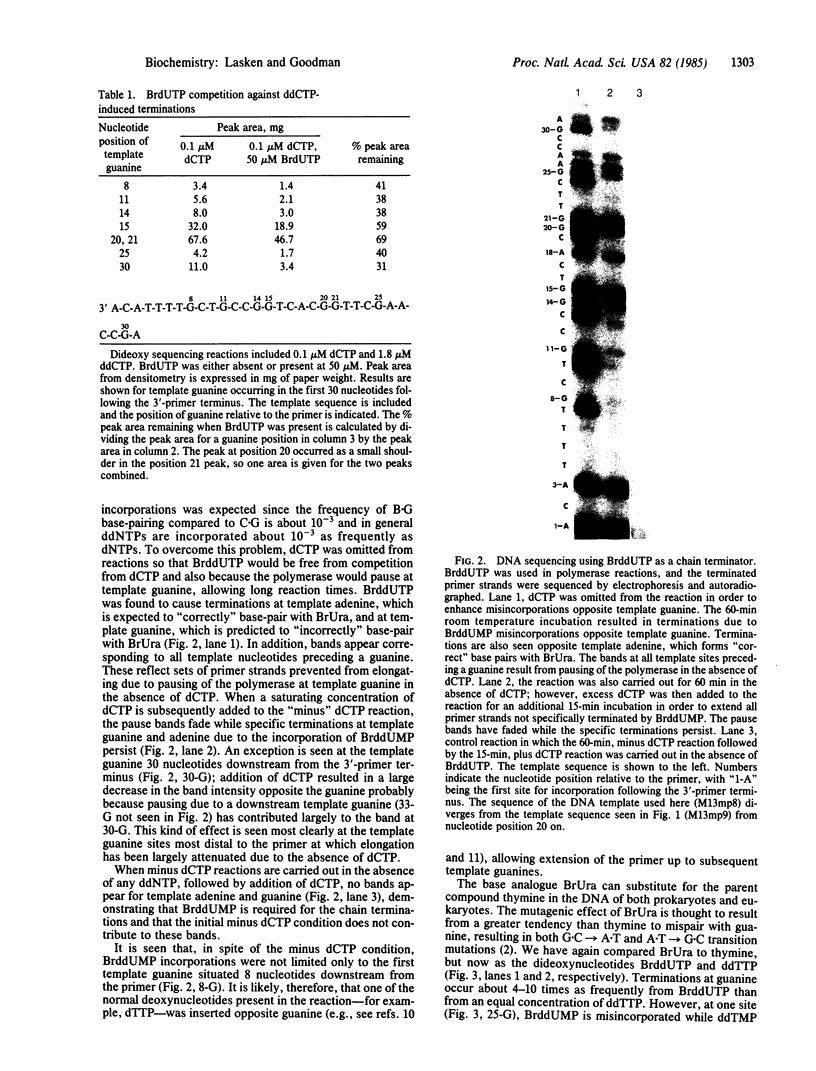
DNA replication fidelity has been assayed by using a modified DNA sequencing reaction. In one experimental approach, dideoxycytidine 5'-triphosphate (ddCTP) was used as a chain terminator during replication of M13 phage DNA by the large fragment of DNA polymerase I. The deoxyribonucleotide analogue BrdUTP was used to compete against ddCTP-induced chain terminations as an assay for B X G base mispairing (B represents bromodeoxyuridine when the analogue is present as a base pair or base mispair). By comparing BrdUTP to dCTP for competition against ddCTP, an average misincorporation frequency for BrdUMP of 0.2% was found. A similar average misincorporation frequency has been measured previously for the incorporation of radioactively labeled BrdUMP and dCMP into the synthetic template-primer poly-[d(G,T)] X oligo(dA). The advantage of the sequencing method is that an error frequency is determined for each template guanine in a defined DNA sequence, thus providing information on the effect of neighboring base sequences on fidelity. Misincorporation frequencies varied no more than 5-fold among 50 template guanines tested. The approach used here is not limited for use with nucleotide analogues but is generally applicable in determining misincorporation frequencies and sequence specificities for any deoxynucleoside triphosphate substrate. In a second experimental approach, base mispairing between bromouracil and guanine was demonstrated directly by using 5-bromodideoxyuridine 5'-triphosphate (BrddUTP). A comparison of chain terminations attributable to BrddUTP and to dideoxythymidine 5'-triphosphate (ddTTP) revealed that B X A and T X A base pairs formed at about the same rate, whereas B X G mispairs occurred 4-10 times more frequently than T X G. The elevation in the frequency of B X G over T X G mispairs is consistent with the mutagenic behavior of the base analogue.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benzer S., Freese E. INDUCTION OF SPECIFIC MUTATIONS WITH 5-BROMOURACIL. Proc Natl Acad Sci U S A. 1958 Feb;44(2):112–119. doi: 10.1073/pnas.44.2.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessman M. J., Lehman I. R., Adler J., Zimmerman S. B., Simms E. S., Kornberg A. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEIC ACID. III. THE INCORPORATION OF PYRIMIDINE AND PURINE ANALOGUES INTO DEOXYRIBONUCLEIC ACID. Proc Natl Acad Sci U S A. 1958 Jul 15;44(7):633–640. doi: 10.1073/pnas.44.7.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brutlag D., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. 36. A proofreading function for the 3' leads to 5' exonuclease activity in deoxyribonucleic acid polymerases. J Biol Chem. 1972 Jan 10;247(1):241–248. [PubMed] [Google Scholar]
- Fersht A. R. Fidelity of replication of phage phi X174 DNA by DNA polymerase III holoenzyme: spontaneous mutation by misincorporation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4946–4950. doi: 10.1073/pnas.76.10.4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese E. THE DIFFERENCE BETWEEN SPONTANEOUS AND BASE-ANALOGUE INDUCED MUTATIONS OF PHAGE T4. Proc Natl Acad Sci U S A. 1959 Apr;45(4):622–633. doi: 10.1073/pnas.45.4.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillebrand G. G., McCluskey A. H., Abbott K. A., Revich G. G., Beattie K. L. Misincorporation during DNA synthesis, analyzed by gel electrophoresis. Nucleic Acids Res. 1984 Apr 11;12(7):3155–3171. doi: 10.1093/nar/12.7.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins R. L., Goodman M. F. Deoxyribonucleotide pools, base pairing, and sequence configuration affecting bromodeoxyuridine- and 2-aminopurine-induced mutagenesis. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1801–1805. doi: 10.1073/pnas.77.4.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INMAN R. B., BALDWIN R. L. HELIX--RANDOM COIL TRANSITIONS IN DNA HOMOPOLYMER PAIRS. J Mol Biol. 1964 Apr;8:452–469. doi: 10.1016/s0022-2836(64)80003-6. [DOI] [PubMed] [Google Scholar]
- Lasken R. S., Goodman M. F. The biochemical basis of 5-bromouracil-induced mutagenesis. Heteroduplex base mispairs involving bromouracil in G x C----A x T and A x T----G x C mutational pathways. J Biol Chem. 1984 Sep 25;259(18):11491–11495. [PubMed] [Google Scholar]
- Liu C. C., Burke R. L., Hibner U., Barry J., Alberts B. Probing DNA replication mechanisms with the T4 bacteriophage in vitro system. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):469–487. doi: 10.1101/sqb.1979.043.01.053. [DOI] [PubMed] [Google Scholar]
- Loeb L. A., Kunkel T. A. Fidelity of DNA synthesis. Annu Rev Biochem. 1982;51:429–457. doi: 10.1146/annurev.bi.51.070182.002241. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzyczka N., Poland R. L., Bessman M. J. Studies on the biochemical basis of spontaneous mutation. I. A comparison of the deoxyribonucleic acid polymerases of mutator, antimutator, and wild type strains of bacteriophage T4. J Biol Chem. 1972 Nov 25;247(22):7116–7122. [PubMed] [Google Scholar]
- Pless R. C., Bessman M. J. Influence of local nucleotide sequence on substitution of 2-aminopurine for adenine during deoxyribonucleic acid synthesis in vitro. Biochemistry. 1983 Oct 11;22(21):4905–4915. doi: 10.1021/bi00290a006. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortle D., Grisafi P., Benkovic S. J., Botstein D. Gap misrepair mutagenesis: efficient site-directed induction of transition, transversion, and frameshift mutations in vitro. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1588–1592. doi: 10.1073/pnas.79.5.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRAUTNER T. A., SWARTZ M. N., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. X. Influence of bromouracil substitutions on replication. Proc Natl Acad Sci U S A. 1962 Mar 15;48:449–455. doi: 10.1073/pnas.48.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver D. T., DePamphilis M. L. Specific sequences in native DNA that arrest synthesis by DNA polymerase alpha. J Biol Chem. 1982 Feb 25;257(4):2075–2086. [PubMed] [Google Scholar]
- Weymouth L. A., Loeb L. A. Mutagenesis during in vitro DNA synthesis. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1924–1928. doi: 10.1073/pnas.75.4.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]