Abstract
Sleep is universal in animals, but its specific functions remain elusive. We propose that sleep’s primary function is to allow individual neurons to perform prophylactic cellular maintenance. Just as muscle cells must rest after strenuous exercise to prevent long-term damage, brain cells must rest after intense synaptic activity. We suggest that periods of reduced synaptic input (‘off periods’ or ‘down states’) are necessary for such maintenance. This in turn requires a state of globally synchronized neuronal activity, reduced sensory input and behavioural immobility — the well-known manifestations of sleep.
Sleep has been found in all animal species carefully studied to date1,2. Multiple studies, as well as our own experience, clearly show that sleep is necessary for normal functioning during waking. The effects of sleep loss are especially apparent after total sleep deprivation, which has serious consequences on subjective alertness, psychomotor vigilance, sustained attention and many other cognitive functions3–6. However, the biological function of sleep remains unclear1,7,8. There are two types of sleep: rapid eye movement (REM) sleep, which occurs in relatively short episodes usually associated with dreaming, and non-REM (NREM) sleep, which makes up the bulk of sleeping time. This Opinion article proposes a hypothesis for the function of adult NREM sleep.
Sleep can be defined on at least two distinct levels: the behaviour of the whole organism and the spatiotemporal patterns of neuronal activity in the brain. At the behavioural level, sleep is associated with immobility and sensory disconnection from the environment. At the level of brain activity, cortical neuronal firing patterns in NREM sleep are characteristically different from those in active wakefulness9–12. Specifically, upon falling asleep, cortical networks alternate between periods of generalized population firing and depolarization, and periods of relative silence and hyperpolarization9,12–17. This pattern of neuronal activity gives rise to electroencephalogram (EEG) and local field potential (LFP) oscillations at frequencies approximately between 1–4 Hz, which are termed slow waves12,18–20. For the purposes of this article, we refer to periods of enhanced and reduced population spiking activity as ‘on periods’ and ‘off periods’, respectively (FIG. 1). On and off periods in a local population are strongly associated both with LFP waves and with depolarized and hyperpolarized membrane potentials of individual neurons (‘up’ and ‘down’ states, respectively)21–24. Cortical neurons receive most of their inputs from nearby cells and are thus hyperpolarized when their neighbours do not spike. In physiological conditions, the occurrence of global neuronal off periods and high-amplitude, spatially synchronous slow waves in the neocortex generally coincides with behavioural manifestations of sleep.
Figure 1. Active and inactive states at the network and neuronal level.
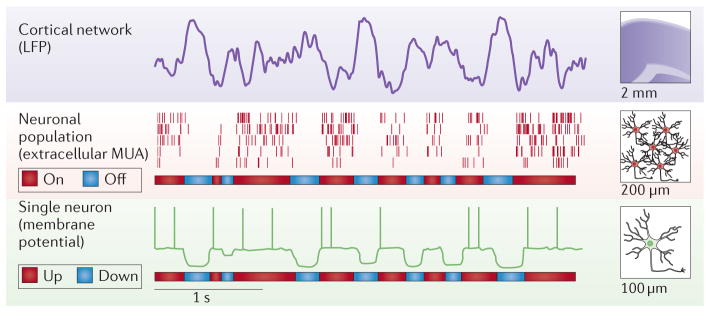
A schematic depiction of the local field potential (LFP) recorded from deep cortical layers during spontaneous non-rapid eye movement sleep in a rat (top row), and a raster plot of the corresponding neuronal spiking activity of five individual neurons (middle row) are shown. Note that LFP slow waves are associated with generalized population silence (off periods), which alternates with periods of raised spiking activity (on periods), each of which lasts several hundred milliseconds. The bottom row shows a schematic representation of a membrane potential expected in one individual neuron within this network. Note that LFP slow waves and extracellular off periods are associated with a prominent membrane hyperpolarization (down state), which alternate regularly with periods of depolarization, when spiking propensity is increased (up state). MUA, multi-unit activity.
Sleep probably has several roles, allowing an organism or the brain to perform multiple functions that are necessary for continued survival and performance but which cannot be performed during active behaviour. The idea that sleep has a restorative role fits well with our subjective experience. In general, ‘restorative’ theories suggest that sleep could provide the opportunity for processes such as synthesis of macromolecules25, recuperation from oxidative stress or toxins that accumulate during waking26,27 or replenishment of energy stores such as glycogen28,29. Although there is substantial evidence for metabolic functions of sleep, how restorative changes occurring at the level of individual cells are related to the complex spatiotemporal patterns of brain activity characteristic of sleep and why global, continuous sleep is necessary to perform them is still unknown.
Another proposed role for sleep is in information processing and synaptic plasticity30–38. Some of these theories ascribe a particular role in memory consolidation to sleep and are supported by evidence of similarities in the fine structure of neuronal activity patterns in waking during learning and subsequent sleep39,40. In this view, the slow oscillation could serve as a ‘carrier wave’ that allows or drives the transfer of information between structures such as the hippocampus and neocortex, thereby serving consolidation of memory traces41–44. Another view holds that sleep is associated with a net decrease of synaptic strength that would lead to the elimination of weak connections while preserving strong ones, thereby renormalizing neuronal firing rates and network excitability37. Although there is substantial evidence that sleep may be involved in neuronal plasticity at the level of fine cortical microcircuits33,45 and in the consolidation of certain memories46, the question remains as to why sleep deprivation has such serious consequences, including impaired subjective alertness, reduced sustained attention, impaired performance in executive tasks, state instability and decreases in many other cognitive functions4–6,47–49, rather than merely memory loss or a decreased ability to learn.
Thus, any conceptual framework that addresses the function of sleep should integrate the molecular, behavioural and electrophysiological correlates of sleep states and explain how these phenomena relate to sleep’s restorative role. Here we propose one such framework. We propose that the most important role of NREM sleep is to provide individual neurons with a period of time in which to perform processes of cellular maintenance, which are required after periods of high synaptic activity. In this view, sleep allows neurons to rest and recuperate after intense activity in the same way that skeletal muscles must rest after a period of strenuous physical exercise. We propose that just as a complex machine cannot be easily repaired and maintained while it is running, such periods of rest are required for the maintenance of neurons and synapses. And, as with the maintenance of a complex machine, we propose that the maintenance of neurons is not simply a reactive process that fixes problems that already affect brain function, but a prophylactic or preventive process by which minor cellular wear and tear is repaired before it has a major impact on neuronal function. In other words, fixing minor cellular problems early can prevent the catastrophic build-up of problems that lead to permanent damage.
The main question, therefore, is why maintenance functions at a single-cell level require behavioural sleep and its associated synchronous, high-amplitude slow-wave activity (SWA) in the neocortex. We argue that although most cells in the body can presumably undergo rest relatively independently, neurons must rest together, owing to their extensive interconnectivity50. Thus, the only strategy to provide rest for an individual neuron in the neocortex is to synchronously reduce activity of the entire network that directly or indirectly projects to that neuron. Last, we propose that although localized off periods can and indeed do occur during waking51, they are much less efficient in promoting cellular maintenance because they are too rare and too short owing to both high levels of arousal-promoting neuromodulators and high network activity that prevents the occurrence of sustained down states52–54. We also propose that because off periods during waking have a deleterious effect on behavioural performance49,51,55,56, the animal’s survival would be endangered if the brain’s need for rest was fulfilled entirely during waking. We thus posit that rest at the single-cell level requires both synchronized network activity and withdrawal from the environment — that is, the well-known manifestations of sleep.
Sleep homeostasis and slow waves
The idea that sleep has a restorative function is supported by its homeostatic regulation. The need for sleep (‘sleep pressure’) increases in proportion to the duration and quality of the preceding waking episode and dissipates during subsequent sleep in proportion to its duration and intensity57,58. The main feature of deep, intense sleep after prolonged waking is an increased arousal threshold, which is manifested in a reduced ability to waken upon stimulation59, and the best characterized physiological indicator of sleep–wake history is the level of cortical EEG SWA — that is, EEG power between 0.5 and 4.0 Hz — during NREM sleep57,58,60,61. In mammals, sleep SWA is correlated with sleep pressure: it is high in early sleep and after sleep deprivation and decreases progressively to reach low levels in late sleep62–64. It has therefore been proposed that SWA is a direct signature of the restorative processes occurring during sleep37,65. Sleep homeostasis has been found in all animal species that have been carefully studied, including invertebrates, suggesting that sleep is required for an essential restorative process throughout the animal kingdom2.
The idea that sleep has a restorative role is supported by the local regulation and homeostasis of SWA66–68. Although a prevailing view is that brain states are regulated in a global fashion, research over the past few decades has revealed that spontaneous brain activity during sleep can be locally modulated19,55. The most extreme example of this is the unihemispheric sleep of seals and dolphins69,70. In adults of terrestrial species, SWA is more intense in frontal areas compared with more posterior areas, especially in early sleep or after sleep deprivation71–74, and shows interhemispheric asymmetries75,76. Regional differences are also apparent at the level of individual sleep slow waves: although the alternation of periods of increased neuronal activity and silence is usually correlated across cortical regions and individual neurons19,77,78, an up state can sometimes be seen in one region of the cortex while another region is in a down state, with these states often spreading as travelling waves18,19,51,79–82. Importantly, topographic differences in brain activity during sleep are functional, as peripheral stimulation or the spontaneous use of circumscribed cortical areas leads to more intense local EEG SWA65,83–87. Such data demonstrate that not only waking duration per se but also strong activity in specific regions affects the ‘intensity’ of subsequent sleep. Notably, during sleep, increased SWA is associated with decreased regional cerebral blood flow, which suggests lower metabolic demand88,89. Together, these data indicate that SWA is associated with the restorative processes of sleep.
In fact, we suggest that SWA is necessary for the restorative process to occur. Specifically, we propose that restorative maintenance of individual neurons requires a reduction in the activity of its synaptic inputs and outputs. Our central argument is that because cortical cells receive extensive synaptic input from other neurons, this restorative maintenance can only be achieved by a coordinated reduction in the spiking of all neurons in a functionally interconnected network: that is, by an off period. (The types of maintenance that may be performed and the reasons they require reduced synaptic activity are discussed later in the article.) As increased SWA reflects the synchronous occurrence of down states among large cortical populations12,20, SWA can be seen both as a marker of the increased need for cellular rest and as a necessary condition for achieving rest most efficiently at a single-cell level. Indeed, sleep with high SWA, such as under high sleep pressure, is characterized by increased local and global synchronization of cortical activity12,19,51,77,90,91. The fact that cortical areas that were highly active during waking exhibit additional SWA during subsequent sleep is thus an indication of their increased need for compensatory cellular maintenance.
Cellular stress and neuronal inactivity
Neurons — like all cells — have a limited metabolic and biosynthetic capacity. Running close to these limits for extended periods of time leads to multiple forms of ‘cellular stress’92,93. Intense metabolic rates lead to mitochondrial production of reactive oxygen species (ROS), which can cause damage to DNA, proteins, lipids and other biomolecules (‘oxidative stress’) owing to their high and relatively unspecific chemical reactivity94. Increased temperature or strong molecular motions can lead to unfolding, entanglement and aggregation of proteins (‘heat shock’)95. Prolonged and intense protein synthesis can in turn lead to an accumulation of misfolded proteins in the endoplasmic reticulum (ER), which causes ‘ER stress’96. Cells mount coordinated responses to these stresses, which restore cellular homeostasis. For example, heat shock leads to the expression of cytoplasmic chaperones (heat shock proteins) that bind to exposed hydrophobic residues of unfolded proteins, preventing their aggregation and leading to either their refolding or degradation. ER stress leads to an analogous unfolded protein response (UPR) — which includes expression of ER chaperones (such as binding immunoglobulin protein) and a general reduction in protein translation — that reduces the load on the ER96. Cellular stress has been implicated in many pathological conditions, including cardiac diseases97 and neurodegenerative disorders98. It is important to note, however, that cellular stress does not necessarily cause long-term damage. Although extreme, prolonged stress can lead to apoptosis, milder stress leads to upregulation of survival pathways that protect against future insults — a phenomenon known as stress hardening or hormesis92,93.
The role of cellular rest in the recovery from cellular stress can be seen in skeletal muscle. Repeated, intense use of muscles leads to a temporary decline in performance known as muscle fatigue99. Although this arises in part from adaptation in spinal motor neurons100, in large part it occurs in the muscle itself, with multiple factors such as decreased myofibrillar calcium sensitivity and calcium release from the sarcoplasmic reticulum playing a part99,101–105. These fatigue processes are triggered by stressors such as ROS and by the increased temperature of the muscle during exercise106. Importantly, however, muscle fatigue does not occur because cellular damage has compromised the ability of the muscles to contract. Rather, it is a prophylactic response by which the activity of the muscles is capped before the level of accumulated damage reaches a critical threshold that would cause irreversible dysfunction107. Recent research suggests that one of the key mediators of skeletal muscle repair is the UPR: it has been shown that acute exercise causes the expression of transcription factors involved in the UPR, and in transgenic mice in which the ATF6α UPR pathway is compromised, repetitive exercise causes long-lasting exercise intolerance and muscle damage108.
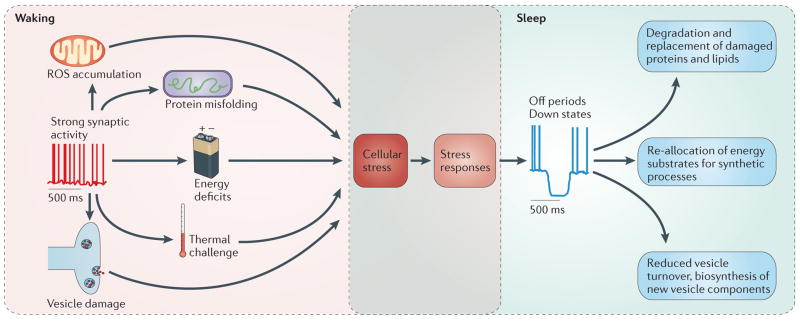
The central tenet of our hypothesis is that the off periods of sleep provide neurons with an analogous opportunity to rest and repair minor cellular damage before it becomes irreversible. Indeed, prolonged wakefulness or sleep deprivation activates the UPR, as indicated by increased expression of mRNA for heat shock proteins and ER chaperones after acute short-term sleep deprivation in the brains of rats109, mice110,111 and Drosophila melanogaster112. Notably, although wild-type flies survive sleep deprivation with no long-term effects, flies carrying a mutation for the heat shock protein Hsp83 die after sleep deprivation112. It is likely that waking activity causes multiple types of cellular stress, which require multiple solutions at the cellular level (FIG. 2). For example, in rodents, waking is associated with increased brain temperature113 and intensified mitochondrial metabolism114, which could lead to thermal and oxidative stress, respectively. Furthermore, the high synaptic activity that occurs during waking probably results in a growing need for the biosynthesis of new vesicle components (both proteins and lipids) to replace those damaged by constant cycling115–118. However, the synthetic capacity of the ER is limited, and it is reduced still further when the cell is metabolically highly active, recharging ionic gradients dissipated by synaptic activity119. We propose that homeostasis can only be restored by a temporary reduction in synaptic activity, allowing the cell to clear its backlog of biosynthetic work, which is made easier without intense respiration and the associated oxidative stress. Consistent with this hypothesis, a recent study found that lysolipids — chemically compromised membrane components — are increased both in vivo in the cortex of sleep-deprived mice and in vitro in an organotypical cortical neuronal culture after stimulation that promotes intense wake-like activity120. Moreover, many of the approximately 100 known genes whose expression increases during sleep are involved in protein synthesis, membrane trafficking and cellular maintenance109.
Figure 2. Transient network silences (off periods) allow cellular rest at a single-neuron level.
We propose that the intense synaptic activity that is typical of active waking eventually leads to irreversible cellular damage if it is not compensated by intermittent periods of rest. This damage could occur through multiple pathways, including the accumulation of reactive oxygen species (ROS), repeated vesicle turnover, insufficient energy substrates for synthetic processes, increased brain temperature and accumulation of misfolded proteins in the endoplasmic reticulum lumen. These challenges trigger various stress responses that allow the cell to restore homeostasis. We suggest that a key effect of these stress responses is to produce ‘neuronal fatigue’ that reduces electrical excitability, promoting local or global off periods of generalized neuronal silence. These in turn reduce neuronal energy expenditure and lower the need to replace structures such as synaptic vesicles, allowing cells to perform essential maintenance before damage becomes irreversible.
We emphasize that the proposed role of off periods is not to repair major cellular damage that has already occurred. Instead, the occurrence of down states is a prophylactic or preventive process in which cells ‘shut down’ before relatively minor and easily fixed problems become irreparable. Because neurons in most brain regions cannot be replaced, we suggest that this prophylactic process must be very strong (BOX 1). Consistent with this theory, even prolonged sleep deprivation for up to several hours or days does not cause obvious signs of neuronal degeneration121 or oxidative damage122 in the brains of rats. We suggest this is because sleep deprivation presents neurons with a difficult choice: either continue at high levels of synaptic activity and face long-term cellular damage; or shut down activity sufficiently to prevent permanent damage but at a cost to behavioural performance. For the long-term good of the organism, neurons almost always choose to reduce synaptic activity: even relatively short-term sleep deprivation is invariably associated with an occurrence of sleep-like activity at the level of the EEG and neuronal activity in behaviourally awake animals1,51,123. In other words, changes incurred at an individual cell level after a certain amount of activity are incompatible with a continuation of their activity; this ensures that the neuron is never damaged permanently.
Box 1. Does waking induce ‘wear and tear’ on neurons?
It is commonly held that sleep has a restorative function that serves to repair ‘wear and tear’ produced by waking168. In support of this view, several studies reported that wakefulness and sleep deprivation are associated with an upregulation of molecules, such as molecular chaperones, that are involved in the response to cellular stress109–112,169. Moreover, voluntary wheel running in mice, which is known to prolong spontaneous waking170,171, results in increased levels of markers of the unfolded protein response in several brain regions172. Molecules that are released during the high synaptic activity of waking, such as tumour necrosis factor-α173 and amyloid-β152, can activate cellular stress responses174,175. Nevertheless, substantial neuronal damage has not been reported in wild-type animals after spontaneous physiological waking or sleep deprivation121,122. Although it is impossible to keep an animal permanently and fully awake for more than a few hours or days1, these results suggest that even without extended sleep, protective responses — perhaps including waking off periods — may be sufficient to prevent rapid and permanent damage to neural circuits. Studies in genetically compromised animals or pathological models, however, suggest that sleep deprivation or excessive synaptic activity can have major consequences. In a mouse model of early-onset Alzheimer’s disease, chronic sleep restriction led to a marked increase in amyloid-β plaque deposition152. Sleep deprivation after experimental ischaemia led to increased neurodegeneration in rats176. In flies mutant for the chaperone protein Hsp83, prolonged sleep deprivation rapidly led to death of the whole organism112. Moreover, intense synaptic activity generated by experimental induction of seizures triggers neuronal degeneration in the cortex, hippocampus and parahippocampal regions177–180. Notably, prolonged seizure-induced neuronal loss is attenuated in transgenic mice overexpressing the molecular chaperone 14-3-3ζ181, whereas its depletion increases kainic acid-induced damage to neurons182. Thus, existing evidence suggests that in non-pathological conditions, physiological defence mechanisms are probably sufficient to prevent immediate and permanent damage to neurons. Whether chronic sleep deprivation can lead to an increased probability of later developing neurodegenerative disorders (which are often associated with disturbed sleep183) remains to be firmly established.
Thus, we suggest that there are two key aspects to the function of off periods at the single-neuron level. First, the periods of neuronal inactivity are signs of neuronal fatigue and serve the prophylactic purpose of reducing activity in those neurons that are primarily affected before damage becomes permanent. Second, cessation of synaptic activity could contribute actively to the processes of cellular restoration by re-allocating energy substrates to protein synthesis and membrane repair and by placing less demand on synaptic vesicles, which in turn would lead to lower biosynthetic requirements (FIG. 2). Of course, the processes of cellular maintenance enabled by off periods do not have to be completed within a single off period, which has a duration of a few hundred milliseconds and therefore is far too short for processes such as protein synthesis. Rather, we suggest that regular brief pauses in neuronal spiking can reduce cellular energy consumption and synaptic activity sufficiently to allow cellular maintenance processes that occur over a much longer timescale. Now we can turn to the questions that we raised at the beginning: why can maintenance functions at a single-cell level not be fulfilled during waking, and why do they require global sleep?
Single-cell rest during waking
Why could periods of metabolic ‘rest’ not occur during waking, allowing an animal to continue performing in the world while its neurons take turns to rest? Indeed, the fact that animals do exhibit off periods during quiet waking22,51,124 suggests that, in principle, they could get away without global sleep by doing this all the time. There are two main arguments against this possibility.
First, the occurrence of neuronal off periods or slow waves during waking compromises behavioural and cognitive performance49,51,56. Sleep deprivation is associated with increased low-frequency EEG activity during waking in both animals and humans51,86,113,123,125–129. Recordings in rats suggested that this EEG pattern reflects local neuronal off periods51. Importantly, the occurrence of these off periods in the primary motor cortex is associated with reduced performance in a fine-skilled motor task51. The mechanisms by which off periods affect cortical function have not yet been clarified but are probably related to the difference in neuronal responses to incoming inputs between the up and down state, as has been shown in experiments using peripheral sensory or cortical electrical stimulation130–134. These experiments suggest that networks undergoing spontaneous slow oscillations are less able to faithfully process sensory stimuli or inputs from other brain areas16,135–137, which could in turn compromise information processing that is necessary for successful behaviour.
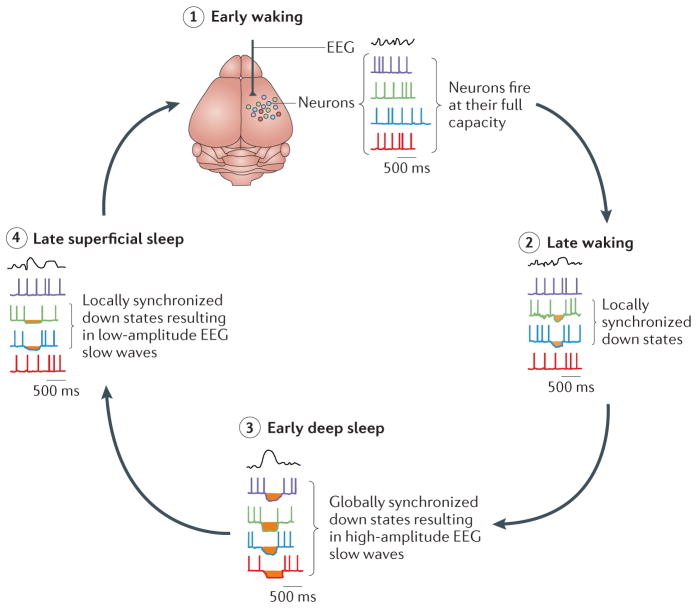
Second, off periods occurring during waking may be less effective at restoration than those occurring during deep sleep. Indeed, owing to the extensive lateral connectivity of the cortex, prolonged periods of zero synaptic activity can occur only when large neuronal populations engage in down states at the same time. Although sleep deprivation produces slow waves during waking, they are typically faster (4–7 Hz) and less globally coherent than those found during deep NREM sleep early in a sleep session; they show a closer homology to the light NREM sleep seen towards the end of the night51,123 (FIG. 3). We propose that this early deep sleep reflects a highly restorative state that is enabled by prolonged off periods encompassing large distributed cortical networks (hence the requirement for global sleep rather than merely local sleep). Conversely, the shorter and more localized off periods during waking51,123,126,128,129,138,139 are likely to have less restorative power, at most serving a prophylactic function for smaller cortical networks.
Figure 3. Global behavioural sleep provides conditions for single-cell rest by allowing sustained uninterrupted down states.
In early waking (stage 1), neurons can fire at their full capacity. As waking time progresses (stage 2), the need for cellular maintenance builds up as a result of prolonged synaptic and spiking activity. The resulting down states are short, localized and easily interrupted by inputs arising from distal neurons during waking. Although they may fulfil the prophylactic function of preventing permanent damage, this comes at a cost of reduced cognitive performance. During early deep sleep (stage 3), sustained uninterrupted down states occur across large cortical neuronal populations, efficiently providing cellular rest to a large number of neurons. Synchronized occurrence of down states results in the high-amplitude electroencephalogram (EEG) slow waves that are typical of early non-rapid eye movement sleep. As individual neurons obtain the necessary amount of rest (stage 4) they resume firing, and progressively smaller neuronal populations engage in synchronized down states. This results in the low-amplitude EEG slow waves that are typical of later sleep.
A related question is why ‘deep’ sleep is not even deeper. To rest most efficiently, why would the brain not simply cease all electrical activity rather than continuing to exhibit sporadic on periods? We propose that this again reflects a compromise. As well as restorative functions, sleep has been suggested to serve information processing functions related to memory consolidation and synaptic plasticity, which presumably require electrical activity. In addition, even while asleep, an animal must maintain the ability to discriminate background sensory stimuli from those that indicate danger, which again presumably requires some degree of neural activity. We propose that the alternation of on and off periods balances these competing priorities and that the stronger and more coherent off periods of early deep sleep reflect a prioritization of essential cellular maintenance, whereas lighter NREM sleep and REM sleep seen later in the night reflects performance of less critical information processing tasks.
In summary, we propose that slow waves during waking, as seen after sleep deprivation, are sufficient to prevent long-term neuronal damage but come with a large cost. First, the impaired behavioural performance caused by this state has serious ethological drawbacks, such as a higher risk of predation. Second, the lower restorative efficiency of waking off periods means that in order to be an effective substitute for sleep, they would need to occur for a much longer period or even continuously. Between continuous 24-hour waking activity but with substantial cognitive impairment or a shorter active day followed by a relatively brief period of intense sleep, evolution has favoured the latter. Aquatic mammals provide an intriguing example of a compromise between neuronal rest and performance impairment. Because these animals must continue swimming in order to breathe, they cannot show global sleep accompanied by complete immobility but instead enter a state in which their cerebral hemispheres alternately exhibit a sleep-like slow-wave EEG69,140. Interestingly, seals exhibit prominent unihemispheric sleep when they are in the water70, but on land global bihemispheric sleep is more abundant141. This suggests that unihemispheric sleep is a suboptimal solution — tolerated only when unavoidable.
From local to global sleep
As discussed above, sleep homeostasis is not just a global phenomenon but also a local phenomenon, with brain areas that were intensely active during waking exhibiting stronger SWA during subsequent sleep. We have hypothesized that this serves the increased restorative need of the local neurons. But what mechanisms could cause an increase in SWA specifically in those areas that need it most?
The mechanisms underpinning cortical slow oscillations are still uncertain. One of the leading theories is the ‘excitable system’ model. This model postulates that the on period is maintained by recurrent synaptic activity, but as the on period progresses, a build-up of processes such as synaptic depression and intrinsic hyperpolarizing conductances reduce network excitability or alter the excitation–inhibition balance, until activity can no longer be sustained16,142,143. This causes an off period during which synapses and cells recover from depression until a new on period can be triggered. A change in the propensity of the network to generate slow waves could therefore be caused by a change in the excitability or amount of adaptation of individual neurons. One can hypothesize that a build-up in the need for cellular maintenance could cause individual neurons to show lower excitability and stronger adaptation — analogous to how signalling pathways such as those involving ROS cause a decrease in myocyte excitability during muscle fatigue99,144 or to increased potassium conductances in the suprachiasmatic nucleus145. In this way, down states would automatically occur where they are most needed, providing a potential explanation for local sleep patterns (FIG. 3). Similarly, it can be suggested that as soon as individual neurons and their neighbours have obtained necessary restoration from cellular stress, their excitability is restored, leading to a more wake-like pattern of activity.
Although the above mechanism in principle provides a sufficient explanation for how local sleep is regulated, considerable evidence also suggests a role for local paracrine signalling pathways and for global neuromodulation. For example, it is known that the level of sleep pressure correlates with the levels of adenosine, tumour necrosis factor-α, amyloid-β and other molecules, which are synthesized locally in those cells that were metabolically or synaptically active and are released in a local (that is, paracrine) manner29,55,146–153. Local neuromodulators could increase the propensity for local down states by reducing single-cell excitability or synaptic transmission149,154. The occurrence of isolated local off periods in waking could subsequently lead to global sleep through the involvement of neuromodulatory systems responsible for the generation of NREM sleep153,155 and/or through ‘behavioural feedback’, whereby the impaired performance caused by local off periods triggers withdrawal from active behaviours and initiates consummatory behaviours directed towards satisfying sleep need.
In this article, we have taken a mostly ‘corticocentric’ view. However, it is reasonable to assume that the need to rest is shared by a great many neurons throughout the brain. Recordings in sleeping animals and during anaesthesia-induced sleep-like states revealed alternations between more active and more quiet periods of neuronal activity in many structures — including the hippocampus156– 158, cerebellum159, thalamus160 and basal ganglia161 — that are at least partly correlated with cortical on and off periods. Notably, a generalized increase of SWA after sleep deprivation in cats was found in several subcortical structures, such as the hippocampus, amygdala, hypothalamus, nucleus centralis lateralis of the thalamus, septum, nucleus caudatus and substantia nigra162. This suggests that SWA may reflect a restorative process in both cortical and subcortical structures.
Conclusions
In this article, we proposed that a key function of NREM sleep is to allow individual neurons to experience a substantial lowering of synaptic and metabolic activity, preventing cellular damage and favouring processes of cellular maintenance after sustained synaptic activity during waking. Because of the extensive recurrent circuitry of the cortex, this requires off periods of coordinated silence in a local population. Although off periods can occur in waking as well as in sleep, we suggest that waking off periods are not only less efficient at cellular restoration but are also incompatible with efficient sensory, cognitive and behavioural function. Efficient and safe neuronal restoration therefore requires a state of functional sensory disconnection from the environment and behavioural immobility. Thus, to allow individual neurons to obtain a sufficient amount of ‘rest’, the organism must be globally and behaviourally asleep. The electrographic manifestation of sleep — high-amplitude EEG slow waves — arises from such synchronized alternation between on and off periods across large cortical neuronal populations.
This hypothesis proposes a novel ‘umbrella’ framework that can reconcile the existing theories of sleep regulation and sleep function. It is fully compatible with ‘metabolic’ theories, inasmuch as we suggest that neuronal down states occur in response to the need for cellular restoration and contribute actively to the processes of prophylactic cellular maintenance. However, we propose here that the most crucial requirement for the cellular maintenance processes is that large distributed neuronal populations enter down states near-synchronously. Our view is also compatible with theories that link sleep and synaptic plasticity. It is reasonable to assume that synchronous recruitment of specific neuronal populations in up states that recur at about 1 Hz frequency may facilitate efficient information processing and transfer within a specific circuit, thereby providing ideal conditions for neural plasticity. Thus, although the slow oscillation ultimately serves single-cell rest, it could have other functions, such as selective strengthening or weakening of synaptic connections, thereby enabling memory consolidation and forgetting.
To test and strengthen our hypothesis, several specific questions have to be answered in future research. First, what specific stresses resulting from prolonged synaptic activity necessitate rest at a single-cell level? Second, what mechanisms translate the need for single- cell rest into the occurrence of an individual off period? Third, what specific functions do off periods allow for cellular maintenance at the molecular, structural and functional level? Fourth, how do local and globally distributed neuronal assemblies, which consist of thousands and millions of neurons, self-organize in order to allow the occurrence of uninterrupted off periods? Fifth, how is the restorative function of sleep at a single-cell level associated with the alternation of sleep stages? Sixth, can neurons in brain structures with different connectivity patterns (such as the cerebellum or the hippocampus) obtain rest relatively independently from each other and from the global behavioural state? Seventh, can this framework be expanded to include animal species with smaller and simpler brains, such as Caenorhabditis elegans or D. melanogaster? Last, can other brain states characterized by global slow-wave patterns — such as sleep in early perinatal age163 (BOX 2), anaesthesia164,165 and experimentally induced coma166 — or artificially induced slow waves167 fulfil the restorative function of physiological sleep? Different experimental models are required for addressing these specific questions: in some cases, simple preparations capable of generating spontaneous down states, such as acute slices or neuronal cultures suffice, whereas in other cases, a ‘whole-brain’ preparation with intact functional connectivity is the only option.
Box 2. Sleep during early development and in adulthood.
Sleep shows pronounced changes across ontogeny, raising the question of whether it serves the same function in early perinatal age as in adulthood. As the definition of sleep and waking rely heavily on behavioural criteria and the electroencephalogram (EEG), studying sleep at early ages is a significant challenge, because behaviour is not yet well developed and it is often difficult to obtain continuous stable EEG recordings184–186. Nevertheless, studies have shown that sleep in early development has a different character to that in adults. A typical feature of sleep in neonate rodents (postnatal days 1–8) is the occurrence of prolonged periods of complete neuronal silence in the neocortex, interrupted regularly with bursts of spindle-like EEG activity187. This pattern of brain activity remarkably resembles the ‘delta-brush’ pattern typical of human premature neonates188,189. Thus, sleep-like activity in early perinatal age seems to resemble the bursting pattern observed under certain anaesthetics or in coma more than the slow waves during adult sleep187,190. The function of this neonatal brain activity is unclear, although a role in organizing neuronal connections has been suggested191. After this early stage, sleep in children and adolescents is more similar to that seen in adults192–196. However, the total amount of sleep decreases continuously throughout life, with children and adolescents needing more sleep than adults197. The reasons for greater sleep need during development are still unclear. However, it is tempting to speculate that it is related to two proposed primary functions of sleep: synaptic plasticity and metabolic rest. Childhood and adolescence are times of intense learning, which could be expected to require greater sleep-related consolidation and synaptic plasticity198. In addition, several studies show that protein synthesis and turnover are substantially higher during early postnatal age as compared with adulthood199–202. This high rate of biosynthesis might place a heavy load on cellular machinery such as the endoplasmic reticulum, and the unfolded protein response is indeed expressed as early as during the embryonic development of the CNS203. This might in turn limit the amount of prolonged and intense synaptic activity that can be safely produced, accounting, at least in part, for the characteristic pattern of brain activity in early age.
Our hypothesis integrates multiple levels at which the function of sleep can be considered. Earlier theories have explored various possibilities, from individual cells or even subcellular components to global behaviour. According to our hypothesis, sleep can be defined as a global dynamic process that accommodates the need for rest at the level of individual cells that are embedded in highly interconnected complex networks. We propose that understanding the function of sleep requires one to bridge the gap between these different levels, because the most important defining characteristic of sleep — global cortical synchrony — appears to be a crucial prerequisite for the expression of down states at a single-neuron level, allowing them to fulfil their manifold restorative functions.
Acknowledgments
We thank G. Buzsáki, A. A. Borbély and I. Tobler for valuable comments on the manuscript. The authors are supported by FP7-PEOPLE-CIG SleepNeed, PCIG11-GA-2012-322050 (to V.V.V.), US National Institutes of Health grant (R01DC009947), EPSRC (EP/I005102) and a Wellcome Trust Investigator award (to K.D.H.).
Footnotes
Competing interests statement
The authors declare no competing financial interests.
Contributor Information
Vladyslav V. Vyazovskiy, Email: vyazovskiy@gmail.com, University of Surrey, Faculty of Health and Medical Sciences, Department of Biochemistry and Physiology, Guildford, GU2 7XH, UK
Kenneth D. Harris, Email: kenneth.harris@ucl.ac.uk, University College London (UCL) Institute of Neurology, UCL Department of Neuroscience, Physiology, and Pharmacology, London, WC1E 6DE, UK
References
- 1.Cirelli C, Tononi G. Is sleep essential? PLoS Biol. 2008;6:e216. doi: 10.1371/journal.pbio.0060216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tobler I. In: Principles and Practice of Sleep Medicine. Kryger MH, Roth T, Dement WC, editors. Saunders; 2005. pp. 77–90. [Google Scholar]
- 3.Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–126. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 4.Killgore WD. Effects of sleep deprivation on cognition. Prog Brain Res. 2010;185:105–129. doi: 10.1016/B978-0-444-53702-7.00007-5. [DOI] [PubMed] [Google Scholar]
- 5.McCoy JG, Strecker RE. The cognitive cost of sleep lost. Neurobiol Learn Mem. 2011;96:564–582. doi: 10.1016/j.nlm.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lo JC, et al. Effects of partial and acute total sleep deprivation on performance across cognitive domains, individuals and circadian phase. PLoS ONE. 2012;7:e45987. doi: 10.1371/journal.pone.0045987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siegel JM. Clues to the functions of mammalian sleep. Nature. 2005;437:1264–1271. doi: 10.1038/nature04285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vassalli A, Dijk DJ. Sleep function: current questions and new approaches. Eur J Neurosci. 2009;29:1830–1841. doi: 10.1111/j.1460-9568.2009.06767.x. [DOI] [PubMed] [Google Scholar]
- 9.Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: a view from inside neocortical neurons. J Neurophysiol. 2001;85:1969–1985. doi: 10.1152/jn.2001.85.5.1969. [DOI] [PubMed] [Google Scholar]
- 10.Hobson JA, McCarley RW. Cortical unit activity in sleep and waking. Electroencephalogr Clin Neurophysiol. 1971;30:97–112. doi: 10.1016/0013-4694(71)90271-9. [DOI] [PubMed] [Google Scholar]
- 11.Buzsáki G. Rhythms of the Brain. Oxford Univ. Press; 2006. [Google Scholar]
- 12.Vyazovskiy VV, et al. Cortical firing and sleep homeostasis. Neuron. 2009;63:865–878. doi: 10.1016/j.neuron.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verzeano M, Negishi K. Neuronal activity in cortical and thalamic networks. J Gen Physiol. 1960;43:177–195. doi: 10.1085/jgp.43.6.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noda H, Adey WR. Firing of neuron pairs in cat association cortex during sleep and wakefulness. J Neurophysiol. 1970;33:672–684. doi: 10.1152/jn.1970.33.5.672. [DOI] [PubMed] [Google Scholar]
- 15.Burns BD, Stean JP, Webb AC. The effects of sleep on neurons in isolated cerebral cortex. Proc R Soc Lond B. 1979;206:281–291. doi: 10.1098/rspb.1979.0105. [DOI] [PubMed] [Google Scholar]
- 16.Harris KD, Thiele A. Cortical state and attention. Nature Rev Neurosci. 2011;12:509–523. doi: 10.1038/nrn3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crunelli V, Hughes SW. The slow (<1 Hz) rhythm of non-REM sleep: a dialogue between three cardinal oscillators. Nature Neurosci. 2009;13:9–17. doi: 10.1038/nn.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Massimini M, Huber R, Ferrarelli F, Hill S, Tononi G. The sleep slow oscillation as a traveling wave. J Neurosci. 2004;24:6862–6870. doi: 10.1523/JNEUROSCI.1318-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nir Y, et al. Regional slow waves and spindles in human sleep. Neuron. 2011;70:153–169. doi: 10.1016/j.neuron.2011.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buzsaki G, Anastassiou CA, Koch C. The origin of extracellular fields and currents — EEG, ECoG, LFP and spikes. Nature Rev Neurosci. 2012;13:407–420. doi: 10.1038/nrn3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chauvette S, Volgushev M, Timofeev I. Origin of active states in local neocortical networks during slow sleep oscillation. Cereb Cortex. 2010;20:2660–2674. doi: 10.1093/cercor/bhq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poulet JF, Petersen CC. Internal brain state regulates membrane potential synchrony in barrel cortex of behaving mice. Nature. 2008;454:881–885. doi: 10.1038/nature07150. [DOI] [PubMed] [Google Scholar]
- 23.Saleem AB, Chadderton P, Apergis-Schoute J, Harris KD, Schultz SR. Methods for predicting cortical UP and DOWN states from the phase of deep layer local field potentials. J Comput Neurosci. 2010;29:49–62. doi: 10.1007/s10827-010-0228-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okun M, Lampl I. Instantaneous correlation of excitation and inhibition during ongoing and sensory-evoked activities. Nature Neurosci. 2008;11:535–537. doi: 10.1038/nn.2105. [DOI] [PubMed] [Google Scholar]
- 25.Mackiewicz M, et al. Macromolecule biosynthesis: a key function of sleep. Physiol Genomics. 2007;31:441–457. doi: 10.1152/physiolgenomics.00275.2006. [DOI] [PubMed] [Google Scholar]
- 26.Reimund E. The free radical flux theory of sleep. Med Hypotheses. 1994;43:231–233. doi: 10.1016/0306-9877(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 27.Inoue S, Honda K, Komoda Y. Sleep as neuronal detoxification and restitution. Behav Brain Res. 1995;69:91–96. doi: 10.1016/0166-4328(95)00014-k. [DOI] [PubMed] [Google Scholar]
- 28.Benington JH, Heller HC. Restoration of brain energy metabolism as the function of sleep. Prog Neurobiol. 1995;45:347–360. doi: 10.1016/0301-0082(94)00057-o. [DOI] [PubMed] [Google Scholar]
- 29.Scharf MT, Naidoo N, Zimmerman JE, Pack AI. The energy hypothesis of sleep revisited. Prog Neurobiol. 2008;86:264–280. doi: 10.1016/j.pneurobio.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buzsaki G. Memory consolidation during sleep: a neurophysiological perspective. J Sleep Res. 1998;7:17–23. doi: 10.1046/j.1365-2869.7.s1.3.x. [DOI] [PubMed] [Google Scholar]
- 31.Sejnowski TJ, Destexhe A. Why do we sleep? Brain Res. 2000;886:208–223. doi: 10.1016/s0006-8993(00)03007-9. [DOI] [PubMed] [Google Scholar]
- 32.Born J, Rasch B, Gais S. Sleep to remember. Neuroscientist. 2006;12:410–424. doi: 10.1177/1073858406292647. [DOI] [PubMed] [Google Scholar]
- 33.Tononi G, Cirelli C. Time to be SHY? Some comments on sleep and synaptic homeostasis. Neural Plast. 2012;2012:415250. doi: 10.1155/2012/415250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hernandez PJ, Abel T. A molecular basis for interactions between sleep and memory. Sleep Med Clin. 2011;6:71–84. doi: 10.1016/j.jsmc.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoo SS, Hu PT, Gujar N, Jolesz FA, Walker MP. A deficit in the ability to form new human memories without sleep. Nature Neurosci. 2007;10:385–392. doi: 10.1038/nn1851. [DOI] [PubMed] [Google Scholar]
- 36.Krueger JM, Tononi G. Local use-dependent sleep; synthesis of the new paradigm. Curr Top Med Chem. 2011;11:2490–2492. doi: 10.2174/156802611797470330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 38.Frank MG. The mystery of sleep function: current perspectives and future directions. Rev Neurosci. 2006;17:375–392. doi: 10.1515/revneuro.2006.17.4.375. [DOI] [PubMed] [Google Scholar]
- 39.Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- 40.Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nature Neurosci. 2007;10:100–107. doi: 10.1038/nn1825. [DOI] [PubMed] [Google Scholar]
- 41.Fujisawa S, Buzsaki GA. 4 Hz oscillation adaptively synchronizes prefrontal, VTA, and hippocampal activities. Neuron. 2011;72:153–165. doi: 10.1016/j.neuron.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tononi G, Massimini M, Riedner BA. Sleepy dialogues between cortex and hippocampus: who talks to whom? Neuron. 2006;52:748–749. doi: 10.1016/j.neuron.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 43.Molle M, Born J. Slow oscillations orchestrating fast oscillations and memory consolidation. Prog Brain Res. 2011;193:93–110. doi: 10.1016/B978-0-444-53839-0.00007-7. [DOI] [PubMed] [Google Scholar]
- 44.Logothetis NK, et al. Hippocampal–cortical interaction during periods of subcortical silence. Nature. 2012;491:547–553. doi: 10.1038/nature11618. [DOI] [PubMed] [Google Scholar]
- 45.Frank MG. Erasing synapses in sleep: is it time to be SHY? Neural Plast. 2012;2012:264378. doi: 10.1155/2012/264378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diekelmann S, Born J. The memory function of sleep. Nature Rev Neurosci. 2010;11:114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 47.Goel N, Rao H, Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2009;29:320–339. doi: 10.1055/s-0029-1237117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Doran SM, Van Dongen HP, Dinges DF. Sustained attention performance during sleep deprivation: evidence of state instability. Arch Ital Biol. 2001;139:253–267. [PubMed] [Google Scholar]
- 49.Van Dongen HP, Belenky G, Krueger JM. A local, bottom-up perspective on sleep deprivation and neurobehavioral performance. Curr Top Med Chem. 2011;11:2414–2422. doi: 10.2174/156802611797470286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Piantoni G, et al. Individual differences in white matter diffusion affect sleep oscillations. J Neurosci. 2013;33:227–233. doi: 10.1523/JNEUROSCI.2030-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vyazovskiy VV, et al. Local sleep in awake rats. Nature. 2011;472:443–447. doi: 10.1038/nature10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leger JF, Stern EA, Aertsen A, Heck D. Synaptic integration in rat frontal cortex shaped by network activity. J Neurophysiol. 2005;93:281–293. doi: 10.1152/jn.00067.2003. [DOI] [PubMed] [Google Scholar]
- 53.Petersen CC, Hahn TT, Mehta M, Grinvald A, Sakmann B. Interaction of sensory responses with spontaneous depolarization in layer 2/3 barrel cortex. Proc Natl Acad Sci USA. 2003;100:13638–13643. doi: 10.1073/pnas.2235811100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Constantinople CM, Bruno RM. Effects and mechanisms of wakefulness on local cortical networks. Neuron. 2011;69:1061–1068. doi: 10.1016/j.neuron.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krueger JM, et al. Sleep as a fundamental property of neuronal assemblies. Nature Rev Neurosci. 2008;9:910–919. doi: 10.1038/nrn2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rattenborg NC, Lima SL, Lesku JA. Sleep locally, act globally. Neuroscientist. 2012;18:533–546. doi: 10.1177/1073858412441086. [DOI] [PubMed] [Google Scholar]
- 57.Borbely AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 58.Borbély AA, Achermann P. In: Principles and Practice of Sleep Medicine. Kryger MH, Roth T, Dement WC, editors. Saunders; 2005. pp. 405–417. [Google Scholar]
- 59.Ferrara M, De Gennaro L, Casagrande M, Bertini M. Auditory arousal thresholds after selective slow-wave sleep deprivation. Clin Neurophysiol. 1999;110:2148–2152. doi: 10.1016/s1388-2457(99)00171-6. [DOI] [PubMed] [Google Scholar]
- 60.Daan S, Beersma DG, Borbely AA. Timing of human sleep: recovery process gated by a circadian pacemaker. Am J Physiol. 1984;246:R161–R183. doi: 10.1152/ajpregu.1984.246.2.R161. [DOI] [PubMed] [Google Scholar]
- 61.Achermann P, Dijk DJ, Brunner DP, Borbely AA. A model of human sleep homeostasis based on EEG slow-wave activity: quantitative comparison of data and simulations. Brain Res Bull. 1993;31:97–113. doi: 10.1016/0361-9230(93)90016-5. [DOI] [PubMed] [Google Scholar]
- 62.Franken P, Chollet D, Tafti M. The homeostatic regulation of sleep need is under genetic control. J Neurosci. 2001;21:2610–2621. doi: 10.1523/JNEUROSCI.21-08-02610.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tobler I, Borbely AA. Sleep EEG in the rat as a function of prior waking. Electroencephalogr Clin Neurophysiol. 1986;64:74–76. doi: 10.1016/0013-4694(86)90044-1. [DOI] [PubMed] [Google Scholar]
- 64.Vyazovskiy VV, Achermann P, Tobler I. Sleep homeostasis in the rat in the light and dark period. Brain Res Bull. 2007;74:37–44. doi: 10.1016/j.brainresbull.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 65.Vyazovskiy V, Borbely AA, Tobler I. Unilateral vibrissae stimulation during waking induces interhemispheric EEG asymmetry during subsequent sleep in the rat. J Sleep Res. 2000;9:367–371. doi: 10.1046/j.1365-2869.2000.00230.x. [DOI] [PubMed] [Google Scholar]
- 66.Kristiansen K, Courtois G. Rhythmic electrical activity from isolated cerebral cortex. Electroencephalogr Clin Neurophysiol. 1949;1:265–272. [PubMed] [Google Scholar]
- 67.Krueger JM, Obal F. A neuronal group theory of sleep function. J Sleep Res. 1993;2:63–69. doi: 10.1111/j.1365-2869.1993.tb00064.x. [DOI] [PubMed] [Google Scholar]
- 68.Pigarev IN, Nothdurft HC, Kastner S. Evidence for asynchronous development of sleep in cortical areas. Neuroreport. 1997;8:2557–2560. doi: 10.1097/00001756-199707280-00027. [DOI] [PubMed] [Google Scholar]
- 69.Mukhametov LM, Supin AY, Polyakova IG. Interhemispheric asymmetry of the electroencephalographic sleep patterns in dolphins. Brain Res. 1977;134:581–584. doi: 10.1016/0006-8993(77)90835-6. [DOI] [PubMed] [Google Scholar]
- 70.Lyamin OI, Pavlova IF, Kosenko PO, Mukhametov LM, Siegel JM. Regional differences in cortical electroencephalogram (EEG) slow wave activity and interhemispheric EEG asymmetry in the fur seal. J Sleep Res. 2012;21:603–611. doi: 10.1111/j.1365-2869.2012.01023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Werth E, Achermann P, Borbely AA. Brain topography of the human sleep EEG: antero-posterior shifts of spectral power. Neuroreport. 1996;8:123–127. doi: 10.1097/00001756-199612200-00025. [DOI] [PubMed] [Google Scholar]
- 72.Cajochen C, Foy R, Dijk DJ. Frontal predominance of a relative increase in sleep delta and theta EEG activity after sleep loss in humans. Sleep Res Online. 1999;2:65–69. [PubMed] [Google Scholar]
- 73.Huber R, Deboer T, Tobler I. Topography of EEG dynamics after sleep deprivation in mice. J Neurophysiol. 2000;84:1888–1893. doi: 10.1152/jn.2000.84.4.1888. [DOI] [PubMed] [Google Scholar]
- 74.Vyazovskiy VV, Tobler I. Regional differences in NREM sleep slow-wave activity in mice with congenital callosal dysgenesis. J Sleep Res. 2005;14:299–304. doi: 10.1111/j.1365-2869.2005.00456.x. [DOI] [PubMed] [Google Scholar]
- 75.Vyazovskiy VV, Borbely AA, Tobler I. Interhemispheric sleep EEG asymmetry in the rat is enhanced by sleep deprivation. J Neurophysiol. 2002;88:2280–2286. doi: 10.1152/jn.00304.2002. [DOI] [PubMed] [Google Scholar]
- 76.Achermann P, Finelli LA, Borbely AA. Unihemispheric enhancement of delta power in human frontal sleep EEG by prolonged wakefulness. Brain Res. 2001;913:220–223. doi: 10.1016/s0006-8993(01)02796-2. [DOI] [PubMed] [Google Scholar]
- 77.Destexhe A, Contreras D, Steriade M. Spatiotemporal analysis of local field potentials and unit discharges in cat cerebral cortex during natural wake and sleep states. J Neurosci. 1999;19:4595–4608. doi: 10.1523/JNEUROSCI.19-11-04595.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Volgushev M, Chauvette S, Mukovski M, Timofeev I. Precise long-range synchronization of activity and silence in neocortical neurons during slow-wave oscillations [corrected] J Neurosci. 2006;26:5665–5672. doi: 10.1523/JNEUROSCI.0279-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Luczak A, Bartho P, Marguet SL, Buzsaki G, Harris KD. Sequential structure of neocortical spontaneous activity in vivo. Proc Natl Acad Sci USA. 2007;104:347–352. doi: 10.1073/pnas.0605643104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mohajerani MH, McVea DA, Fingas M, Murphy TH. Mirrored bilateral slow-wave cortical activity within local circuits revealed by fast bihemispheric voltage-sensitive dye imaging in anesthetized and awake mice. J Neurosci. 2010;30:3745–3751. doi: 10.1523/JNEUROSCI.6437-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sirota A, Buzsaki G. Interaction between neocortical and hippocampal networks via slow oscillations. Thalamus Relat Syst. 2005;3:245–259. doi: 10.1017/S1472928807000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vyazovskiy VV, Faraguna U, Cirelli C, Tononi G. Triggering slow waves during NREM sleep in the rat by intracortical electrical stimulation: effects of sleep/wake history and background activity. J Neurophysiol. 2009;101:1921–1931. doi: 10.1152/jn.91157.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kattler H, Dijk DJ, Borbely AA. Effect of unilateral somatosensory stimulation prior to sleep on the sleep EEG in humans. J Sleep Res. 1994;3:159–164. doi: 10.1111/j.1365-2869.1994.tb00123.x. [DOI] [PubMed] [Google Scholar]
- 84.Vyazovskiy VV, Welker E, Fritschy JM, Tobler I. Regional pattern of metabolic activation is reflected in the sleep EEG after sleep deprivation combined with unilateral whisker stimulation in mice. Eur J Neurosci. 2004;20:1363–1370. doi: 10.1111/j.1460-9568.2004.03583.x. [DOI] [PubMed] [Google Scholar]
- 85.Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- 86.Vyazovskiy VV, Tobler I. Handedness leads to interhemispheric EEG asymmetry during sleep in the rat. J Neurophysiol. 2008;99:969–975. doi: 10.1152/jn.01154.2007. [DOI] [PubMed] [Google Scholar]
- 87.Miyamoto H, Katagiri H, Hensch T. Experience-dependent slow-wave sleep development. Nature Neurosci. 2003;6:553–554. doi: 10.1038/nn1064. [DOI] [PubMed] [Google Scholar]
- 88.Dang-Vu TT, et al. Neuroimaging in sleep medicine. Sleep Med. 2007;8:349–372. doi: 10.1016/j.sleep.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 89.Hofle N, et al. Regional cerebral blood flow changes as a function of delta and spindle activity during slow wave sleep in humans. J Neurosci. 1997;17:4800–4808. doi: 10.1523/JNEUROSCI.17-12-04800.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vyazovskiy V, Achermann P, Borbely AA, Tobler I. Interhemispheric coherence of the sleep electroencephalogram in mice with congenital callosal dysgenesis. Neuroscience. 2004;124:481–488. doi: 10.1016/j.neuroscience.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 91.Riedner BA, et al. Sleep homeostasis and cortical synchronization: III. A high-density EEG study of sleep slow waves in humans. Sleep. 2007;30:1643–1657. doi: 10.1093/sleep/30.12.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kultz D. Molecular and evolutionary basis of the cellular stress response. Annu Rev Physiol. 2005;67:225–257. doi: 10.1146/annurev.physiol.67.040403.103635. [DOI] [PubMed] [Google Scholar]
- 93.Kourtis N, Tavernarakis N. Cellular stress response pathways and ageing: intricate molecular relationships. EMBO J. 2011;30:2520–2531. doi: 10.1038/emboj.2011.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Martindale JL, Holbrook NJ. Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol. 2002;192:1–15. doi: 10.1002/jcp.10119. [DOI] [PubMed] [Google Scholar]
- 95.Richter K, Haslbeck M, Buchner J. The heat shock response: life on the verge of death. Mol Cell. 2010;40:253–266. doi: 10.1016/j.molcel.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 96.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 97.Groenendyk J, Sreenivasaiah PK, Kim do H, Agellon LB, Michalak M. Biology of endoplasmic reticulum stress in the heart. Circ Res. 2010;107:1185–1197. doi: 10.1161/CIRCRESAHA.110.227033. [DOI] [PubMed] [Google Scholar]
- 98.Doyle KM, et al. Unfolded proteins and endoplasmic reticulum stress in neurodegenerative disorders. J Cell Mol Med. 2011;15:2025–2039. doi: 10.1111/j.1582-4934.2011.01374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 2008;88:287–332. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- 100.Garland SJ, Gossen ER. The muscular wisdom hypothesis in human muscle fatigue. Exerc Sport Sci Rev. 2002;30:45–49. doi: 10.1097/00003677-200201000-00009. [DOI] [PubMed] [Google Scholar]
- 101.Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88:1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Westerblad H, Allen DG. Emerging roles of ROS/RNS in muscle function and fatigue. Antioxid Redox Signal. 2011;15:2487–2499. doi: 10.1089/ars.2011.3909. [DOI] [PubMed] [Google Scholar]
- 103.Keyser RE. Peripheral fatigue: high-energy phosphates and hydrogen ions. PMR. 2010;2:347–358. doi: 10.1016/j.pmrj.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 104.Fitts RH. The cross-bridge cycle and skeletal muscle fatigue. J Appl Physiol. 2008;104:551–558. doi: 10.1152/japplphysiol.01200.2007. [DOI] [PubMed] [Google Scholar]
- 105.Debold EP. Recent insights into muscle fatigue at the cross-bridge level. Front Physiol. 2012;3:151. doi: 10.3389/fphys.2012.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Salo DC, Donovan CM, Davies KJ. HSP70 and other possible heat shock or oxidative stress proteins are induced in skeletal muscle, heart, and liver during exercise. Free Radic Biol Med. 1991;11:239–246. doi: 10.1016/0891-5849(91)90119-n. [DOI] [PubMed] [Google Scholar]
- 107.MacIntosh BR, Holash RJ, Renaud JM. Skeletal muscle fatigue-regulation of excitation-contraction coupling to avoid metabolic catastrophe. J Cell Sci. 2012;125:2105–2114. doi: 10.1242/jcs.093674. [DOI] [PubMed] [Google Scholar]
- 108.Wu J, et al. The unfolded protein response mediates adaptation to exercise in skeletal muscle through a PGC-1α/ATF6α complex. Cell Metab. 2011;13:160–169. doi: 10.1016/j.cmet.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cirelli C, Gutierrez CM, Tononi G. Extensive and divergent effects of sleep and wakefulness on brain gene expression. Neuron. 2004;41:35–43. doi: 10.1016/s0896-6273(03)00814-6. [DOI] [PubMed] [Google Scholar]
- 110.Naidoo N, Giang W, Galante RJ, Pack AI. Sleep deprivation induces the unfolded protein response in mouse cerebral cortex. J Neurochem. 2005;92:1150–1157. doi: 10.1111/j.1471-4159.2004.02952.x. [DOI] [PubMed] [Google Scholar]
- 111.Terao A, et al. Differential increase in the expression of heat shock protein family members during sleep deprivation and during sleep. Neuroscience. 2003;116:187–200. doi: 10.1016/s0306-4522(02)00695-4. [DOI] [PubMed] [Google Scholar]
- 112.Shaw PJ, Tononi G, Greenspan RJ, Robinson DF. Stress response genes protect against lethal effects of sleep deprivation in Drosophila. Nature. 2002;417:287–291. doi: 10.1038/417287a. [DOI] [PubMed] [Google Scholar]
- 113.Franken P, Dijk DJ, Tobler I, Borbely AA. Sleep deprivation in rats: effects on EEG power spectra, vigilance states, and cortical temperature. Am J Physiol. 1991;261:R198–R208. doi: 10.1152/ajpregu.1991.261.1.R198. [DOI] [PubMed] [Google Scholar]
- 114.Nikonova EV, et al. Changes in components of energy regulation in mouse cortex with increases in wakefulness. Sleep. 2010;33:889–900. doi: 10.1093/sleep/33.7.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Denker A, Rizzoli SO. Synaptic vesicle pools: an update. Front Synaptic Neurosci. 2010;2:135. doi: 10.3389/fnsyn.2010.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Esposito G, Ana Clara F, Verstreken P. Synaptic vesicle trafficking and Parkinson’s disease. Dev Neurobiol. 2011;72:134–144. doi: 10.1002/dneu.20916. [DOI] [PubMed] [Google Scholar]
- 117.Vanden Berghe P, Klingauf J. Synaptic vesicles in rat hippocampal boutons recycle to different pools in a use-dependent fashion. J Physiol. 2006;572:707–720. doi: 10.1113/jphysiol.2005.100842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Burre J, et al. α-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329:1663–1667. doi: 10.1126/science.1195227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Attwell D, Gibb A. Neuroenergetics and the kinetic design of excitatory synapses. Nature Rev Neurosci. 2005;6:841–849. doi: 10.1038/nrn1784. [DOI] [PubMed] [Google Scholar]
- 120.Hinard V, et al. Key electrophysiological, molecular, and metabolic signatures of sleep and wakefulness revealed in primary cortical cultures. J Neurosci. 2012;32:12506–12517. doi: 10.1523/JNEUROSCI.2306-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cirelli C, Shaw PJ, Rechtschaffen A, Tononi G. No evidence of brain cell degeneration after long-term sleep deprivation in rats. Brain Res. 1999;840:184–193. doi: 10.1016/s0006-8993(99)01768-0. [DOI] [PubMed] [Google Scholar]
- 122.Gopalakrishnan A, Ji LL, Cirelli C. Sleep deprivation and cellular responses to oxidative stress. Sleep. 2004;27:27–35. doi: 10.1093/sleep/27.1.27. [DOI] [PubMed] [Google Scholar]
- 123.Vyazovskiy VV, Tobler I. Theta activity in the waking EEG is a marker of sleep propensity in the rat. Brain Res. 2005;1050:64–71. doi: 10.1016/j.brainres.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 124.Okun M, Naim A, Lampl I. The subthreshold relation between cortical local field potential and neuronal firing unveiled by intracellular recordings in awake rats. J Neurosci. 2010;30:4440–4448. doi: 10.1523/JNEUROSCI.5062-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cajochen C, Wyatt JK, Czeisler CA, Dijk DJ. Separation of circadian and wake duration-dependent modulation of EEG activation during wakefulness. Neuroscience. 2002;114:1047–1060. doi: 10.1016/s0306-4522(02)00209-9. [DOI] [PubMed] [Google Scholar]
- 126.Finelli LA, Baumann H, Borbely AA, Achermann P. Dual electroencephalogram markers of human sleep homeostasis: correlation between theta activity in waking and slow-wave activity in sleep. Neuroscience. 2000;101:523–529. doi: 10.1016/s0306-4522(00)00409-7. [DOI] [PubMed] [Google Scholar]
- 127.Landolt HP, et al. Caffeine attenuates waking and sleep electroencephalographic markers of sleep homeostasis in humans. Neuropsychopharmacology. 2004;29:1933–1939. doi: 10.1038/sj.npp.1300526. [DOI] [PubMed] [Google Scholar]
- 128.Leemburg S, et al. Sleep homeostasis in the rat is preserved during chronic sleep restriction. Proc Natl Acad Sci USA. 2010;107:15939–15944. doi: 10.1073/pnas.1002570107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hung CS, et al. Local experience-dependent changes in the wake EEG after prolonged wakefulness. Sleep. 2013;36:59–72. doi: 10.5665/sleep.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Crochet S, Petersen CC. Correlating whisker behavior with membrane potential in barrel cortex of awake mice. Nature Neurosci. 2006;9:608–610. doi: 10.1038/nn1690. [DOI] [PubMed] [Google Scholar]
- 131.Haider B, Duque A, Hasenstaub AR, Yu Y, McCormick DA. Enhancement of visual responsiveness by spontaneous local network activity in vivo. J Neurophysiol. 2007;97:4186–4202. doi: 10.1152/jn.01114.2006. [DOI] [PubMed] [Google Scholar]
- 132.Hasenstaub A, Sachdev RN, McCormick DA. State changes rapidly modulate cortical neuronal responsiveness. J Neurosci. 2007;27:9607–9622. doi: 10.1523/JNEUROSCI.2184-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sachdev RN, Ebner FF, Wilson CJ. Effect of subthreshold up and down states on the whisker-evoked response in somatosensory cortex. J Neurophysiol. 2004;92:3511–3521. doi: 10.1152/jn.00347.2004. [DOI] [PubMed] [Google Scholar]
- 134.Vyazovskiy VV, Olcese U, Cirelli C, Tononi G. Prolonged wakefulness alters neuronal responsiveness to local electrical stimulation of the neocortex in awake rats. J Sleep Res. 2012 Nov 21; doi: 10.1111/jsr.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Luczak A, Bartho P, Harris KD. Gating of sensory input by spontaneous cortical activity. J Neurosci. 2013;33:1684–1695. doi: 10.1523/JNEUROSCI.2928-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Goard M, Dan Y. Basal forebrain activation enhances cortical coding of natural scenes. Nature Neurosci. 2009;12:1444–1449. doi: 10.1038/nn.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Marguet SL, Harris KD. State-dependent representation of amplitude-modulated noise stimuli in rat auditory cortex. J Neurosci. 2011;31:6414–6420. doi: 10.1523/JNEUROSCI.5773-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Aeschbach D, et al. Dynamics of the human EEG during prolonged wakefulness: evidence for frequency-specific circadian and homeostatic influences. Neurosci Lett. 1997;239:121–124. doi: 10.1016/s0304-3940(97)00904-x. [DOI] [PubMed] [Google Scholar]
- 139.Cajochen C, Brunner DP, Krauchi K, Graw P, Wirz-Justice A. Power density in theta/α frequencies of the waking EEG progressively increases during sustained wakefulness. Sleep. 1995;18:890–894. doi: 10.1093/sleep/18.10.890. [DOI] [PubMed] [Google Scholar]
- 140.Oleksenko AI, Mukhametov LM, Polyakova IG, Supin AY, Kovalzon VM. Unihemispheric sleep deprivation in bottlenose dolphins. J Sleep Res. 1992;1:40–44. doi: 10.1111/j.1365-2869.1992.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 141.Lyamin OI, Kosenko PO, Lapierre JL, Mukhametov LM, Siegel JM. Fur seals display a strong drive for bilateral slow-wave sleep while on land. J Neurosci. 2008;28:12614–12621. doi: 10.1523/JNEUROSCI.2306-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sanchez-Vives MV, et al. Inhibitory modulation of cortical up states. J Neurophysiol. 2010;104:1314–1324. doi: 10.1152/jn.00178.2010. [DOI] [PubMed] [Google Scholar]
- 143.Chen JY, Chauvette S, Skorheim S, Timofeev I, Bazhenov M. Interneuron-mediated inhibition synchronizes neuronal activity during slow oscillation. J Physiol. 2012;590:3987–4010. doi: 10.1113/jphysiol.2012.227462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lamb GD, Westerblad H. Acute effects of reactive oxygen and nitrogen species on the contractile function of skeletal muscle. J Physiol. 2011;589:2119–2127. doi: 10.1113/jphysiol.2010.199059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang TA, et al. Circadian rhythm of redox state regulates excitability in suprachiasmatic nucleus neurons. Science. 2012;337:839–842. doi: 10.1126/science.1222826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kapas L, Obal F, Jr, Krueger JM. Humoral regulation of sleep. Int Rev Neurobiol. 1993;35:131–160. doi: 10.1016/s0074-7742(08)60570-x. [DOI] [PubMed] [Google Scholar]
- 147.Obal F, Jr, Krueger JM. Biochemical regulation of non-rapid-eye-movement sleep. Front Biosci. 2003;8:d520–550. doi: 10.2741/1033. [DOI] [PubMed] [Google Scholar]
- 148.Porkka-Heiskanen T, et al. Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science. 1997;276:1265–1268. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Latini S, Pedata F. Adenosine in the central nervous system: release mechanisms and extracellular concentrations. J Neurochem. 2001;79:463–484. doi: 10.1046/j.1471-4159.2001.00607.x. [DOI] [PubMed] [Google Scholar]
- 150.Krueger JM, Obal FJ, Fang J, Kubota T, Taishi P. The role of cytokines in physiological sleep regulation. Ann NY Acad Sci. 2001;933:211–221. doi: 10.1111/j.1749-6632.2001.tb05826.x. [DOI] [PubMed] [Google Scholar]
- 151.Kilduff TS, Cauli B, Gerashchenko D. Activation of cortical interneurons during sleep: an anatomical link to homeostatic sleep regulation? Trends Neurosci. 2011;34:10–19. doi: 10.1016/j.tins.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kang JE, et al. Amyloid-β dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326:1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of sleep and wakefulness. Physiol Rev. 2012;92:1087–1187. doi: 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kamenetz F, et al. APP processing and synaptic function. Neuron. 2003;37:925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 155.Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep state switching. Neuron. 2010;68:1023–1042. doi: 10.1016/j.neuron.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Battaglia FP, Sutherland GR, McNaughton BL. Hippocampal sharp wave bursts coincide with neocortical “up-state” transitions. Learn Mem. 2004;11:697–704. doi: 10.1101/lm.73504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sirota A, Csicsvari J, Buhl D, Buzsaki G. Communication between neocortex and hippocampus during sleep in rodents. Proc Natl Acad Sci USA. 2003;100:2065–2069. doi: 10.1073/pnas.0437938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Isomura Y, et al. Integration and segregation of activity in entorhinal-hippocampal subregions by neocortical slow oscillations. Neuron. 2006;52:871–882. doi: 10.1016/j.neuron.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 159.Ros H, Sachdev RN, Yu Y, Sestan N, McCormick DA. Neocortical networks entrain neuronal circuits in cerebellar cortex. J Neurosci. 2009;29:10309–10320. doi: 10.1523/JNEUROSCI.2327-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Timofeev I, Steriade M. Low-frequency rhythms in the thalamus of intact-cortex and decorticated cats. J Neurophysiol. 1996;76:4152–4168. doi: 10.1152/jn.1996.76.6.4152. [DOI] [PubMed] [Google Scholar]
- 161.Cowan RL, Wilson CJ. Spontaneous firing patterns and axonal projections of single corticostriatal neurons in the rat medial agranular cortex. J Neurophysiol. 1994;71:17–32. doi: 10.1152/jn.1994.71.1.17. [DOI] [PubMed] [Google Scholar]
- 162.Lancel M, van Riezen H, Glatt A. Enhanced slow-wave activity within NREM sleep in the cortical and subcortical EEG of the cat after sleep deprivation. Sleep. 1992;15:102–118. doi: 10.1093/sleep/15.2.102. [DOI] [PubMed] [Google Scholar]
- 163.Khazipov R, Luhmann HJ. Early patterns of electrical activity in the developing cerebral cortex of humans and rodents. Trends Neurosci. 2006;29:414–418. doi: 10.1016/j.tins.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 164.Nelson AB, Faraguna U, Tononi G, Cirelli C. Effects of anesthesia on the response to sleep deprivation. Sleep. 2010;33:1659–1667. doi: 10.1093/sleep/33.12.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Murphy M, et al. Propofol anesthesia and sleep: a high-density EEG study. Sleep. 2011;34:283–291. doi: 10.1093/sleep/34.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Amzica F, Kroeger D. Cellular mechanisms underlying EEG waveforms during coma. Epilepsia. 2011;52:25–27. doi: 10.1111/j.1528-1167.2011.03229.x. [DOI] [PubMed] [Google Scholar]
- 167.Marshall L, Helgadottir H, Molle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:610–613. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- 168.Moruzzi G. The sleep-waking cycle. Ergeb Physiol. 1972;64:1–165. doi: 10.1007/3-540-05462-6_1. [DOI] [PubMed] [Google Scholar]
- 169.Cirelli C, Tononi G. Gene expression in the brain across the sleep-waking cycle. Brain Res. 2000;885:303–321. doi: 10.1016/s0006-8993(00)03008-0. [DOI] [PubMed] [Google Scholar]
- 170.Vyazovskiy VV, Ruijgrok G, Deboer T, Tobler I. Running wheel accessibility affects the regional electroencephalogram during sleep in mice. Cereb Cortex. 2006;16:328–336. doi: 10.1093/cercor/bhi110. [DOI] [PubMed] [Google Scholar]
- 171.Vyazovskiy VV, Tobler I. The temporal structure of behaviour and sleep homeostasis. PLoS ONE. 2012;7:e50677. doi: 10.1371/journal.pone.0050677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kim Y, Park M, Boghossian S, York DA. Three weeks voluntary running wheel exercise increases endoplasmic reticulum stress in the brain of mice. Brain Res. 2010;1317:13–23. doi: 10.1016/j.brainres.2009.12.062. [DOI] [PubMed] [Google Scholar]
- 173.Churchill L, et al. Tumor necrosis factor α: activity dependent expression and promotion of cortical column sleep in rats. Neuroscience. 2008;156:71–80. doi: 10.1016/j.neuroscience.2008.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Xue X, et al. Tumor necrosis factor α (TNFα) induces the unfolded protein response (UPR) in a reactive oxygen species (ROS)-dependent fashion, and the UPR counteracts ROS accumulation by TNFα. J Biol Chem. 2005;280:33917–33925. doi: 10.1074/jbc.M505818200. [DOI] [PubMed] [Google Scholar]
- 175.Roussel BD, et al. Endoplasmic reticulum dysfunction in neurological disease. Lancet Neurol. 2013;12:105–118. doi: 10.1016/S1474-4422(12)70238-7. [DOI] [PubMed] [Google Scholar]
- 176.Gao B, et al. Sleep disruption aggravates focal cerebral ischemia in the rat. Sleep. 2010;33:879–887. doi: 10.1093/sleep/33.7.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Du F, Eid T, Lothman EW, Kohler C, Schwarcz R. Preferential neuronal loss in layer III of the medial entorhinal cortex in rat models of temporal lobe epilepsy. J Neurosci. 1995;15:6301–6313. doi: 10.1523/JNEUROSCI.15-10-06301.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Drexel M, Preidt AP, Sperk G. Sequel of spontaneous seizures after kainic acid-induced status epilepticus and associated neuropathological changes in the subiculum and entorhinal cortex. Neuropharmacology. 2012;63:806–817. doi: 10.1016/j.neuropharm.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Fritsch B, et al. Pathological alterations in GABAergic interneurons and reduced tonic inhibition in the basolateral amygdala during epileptogenesis. Neuroscience. 2009;163:415–429. doi: 10.1016/j.neuroscience.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Arundine M, Tymianski M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium. 2003;34:325–337. doi: 10.1016/s0143-4160(03)00141-6. [DOI] [PubMed] [Google Scholar]
- 181.Brennan GP, et al. Transgenic overexpression of 14-3-3 zeta protects hippocampus against endoplasmic reticulum stress and status epilepticus in vivo. PLoS ONE. 2013;8:e54491. doi: 10.1371/journal.pone.0054491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Murphy N, et al. Depletion of 14-3-3 zeta elicits endoplasmic reticulum stress and cell death, and increases vulnerability to kainate-induced injury in mouse hippocampal cultures. J Neurochem. 2008;106:978–988. doi: 10.1111/j.1471-4159.2008.05447.x. [DOI] [PubMed] [Google Scholar]
- 183.Dauvilliers Y. Insomnia in patients with neurodegenerative conditions. Sleep Med. 2007;8:S27–S34. doi: 10.1016/S1389-9457(08)70006-6. [DOI] [PubMed] [Google Scholar]
- 184.Frank MG, Heller HC. The ontogeny of mammalian sleep: a reappraisal of alternative hypotheses. J Sleep Res. 2003;12:25–34. doi: 10.1046/j.1365-2869.2003.00339.x. [DOI] [PubMed] [Google Scholar]
- 185.Corner M, van der Togt C. No phylogeny without ontogeny: a comparative and developmental search for the sources of sleep-like neural and behavioral rhythms. Neurosci Bull. 2012;28:25–38. doi: 10.1007/s12264-012-1062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Blumberg MS. Homology, correspondence, and continuity across development: the case of sleep. Dev Psychobiol. 2013;55:92–100. doi: 10.1002/dev.21024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Khazipov R, et al. Early motor activity drives spindle bursts in the developing somatosensory cortex. Nature. 2004;432:758–761. doi: 10.1038/nature03132. [DOI] [PubMed] [Google Scholar]
- 188.Dreyfus-Brisac C, Larroche JC. Discontinuous electroencephalograms in the premature newborn and at term. Electro-anatomo-clinical correlations. Rev Electroencephalogr Neurophysiol Clin. 1971;1:95–99. doi: 10.1016/s0370-4475(71)80022-9. (in French) [DOI] [PubMed] [Google Scholar]
- 189.Andre M, et al. Electroencephalography in premature and full-term infants. Developmental features and glossary. Neurophysiol Clin. 2010;40:59–124. doi: 10.1016/j.neucli.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 190.Kroeger D, Amzica F. Hypersensitivity of the anesthesia-induced comatose brain. J Neurosci. 2007;27:10597–10607. doi: 10.1523/JNEUROSCI.3440-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 192.Frank MG, Heller HC. Development of diurnal organization of EEG slow-wave activity and slow-wave sleep in the rat. Am J Physiol. 1997;273:R472–R478. doi: 10.1152/ajpregu.1997.273.2.R472. [DOI] [PubMed] [Google Scholar]
- 193.Alfoldi P, Tobler I, Borbely AA. Sleep regulation in rats during early development. Am J Physiol. 1990;258:R634–R644. doi: 10.1152/ajpregu.1990.258.3.R634. [DOI] [PubMed] [Google Scholar]
- 194.Kurth S, et al. Characteristics of sleep slow waves in children and adolescents. Sleep. 2010;33:475–480. doi: 10.1093/sleep/33.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kurth S, et al. Mapping of cortical activity in the first two decades of life: a high-density sleep electroencephalogram study. J Neurosci. 2010;30:13211–13219. doi: 10.1523/JNEUROSCI.2532-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Jenni OG, Carskadon MA. Spectral analysis of the sleep electroencephalogram during adolescence. Sleep. 2004;27:774–783. [PubMed] [Google Scholar]
- 197.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–1273. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 198.Maret S, Faraguna U, Nelson AB, Cirelli C, Tononi G. Sleep and waking modulate spine turnover in the adolescent mouse cortex. Nature Neurosci. 2011;14:1418–1420. doi: 10.1038/nn.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Shahbazian FM, Jacobs M, Lajtha A. Regional and cellular differences in rat brain protein synthesis in vivo and in slices during development. Int J Dev Neurosci. 1986;4:209–215. doi: 10.1016/0736-5748(86)90060-2. [DOI] [PubMed] [Google Scholar]
- 200.Sun Y, et al. Rates of local cerebral protein synthesis in the rat during normal postnatal development. Am J Physiol. 1995;268:R549–R561. doi: 10.1152/ajpregu.1995.268.2.R549. [DOI] [PubMed] [Google Scholar]
- 201.Giuffrida AM, et al. Mitochondrial DNA, RNA, and protein synthesis in different regions of developing rat brain. Neurochem Res. 1979;4:37–52. doi: 10.1007/BF00963830. [DOI] [PubMed] [Google Scholar]
- 202.Lerner MP, Johnson TC. Regulation of protein synthesis in developing mouse brain tissue. Alteration in ribosomal activity. J Biol Chem. 1970;245:1388–1393. [PubMed] [Google Scholar]
- 203.Zhang X, Szabo E, Michalak M, Opas M. Endoplasmic reticulum stress during the embryonic development of the central nervous system in the mouse. Int J Dev Neurosci. 2007;25:455–463. doi: 10.1016/j.ijdevneu.2007.08.007. [DOI] [PubMed] [Google Scholar]