Abstract
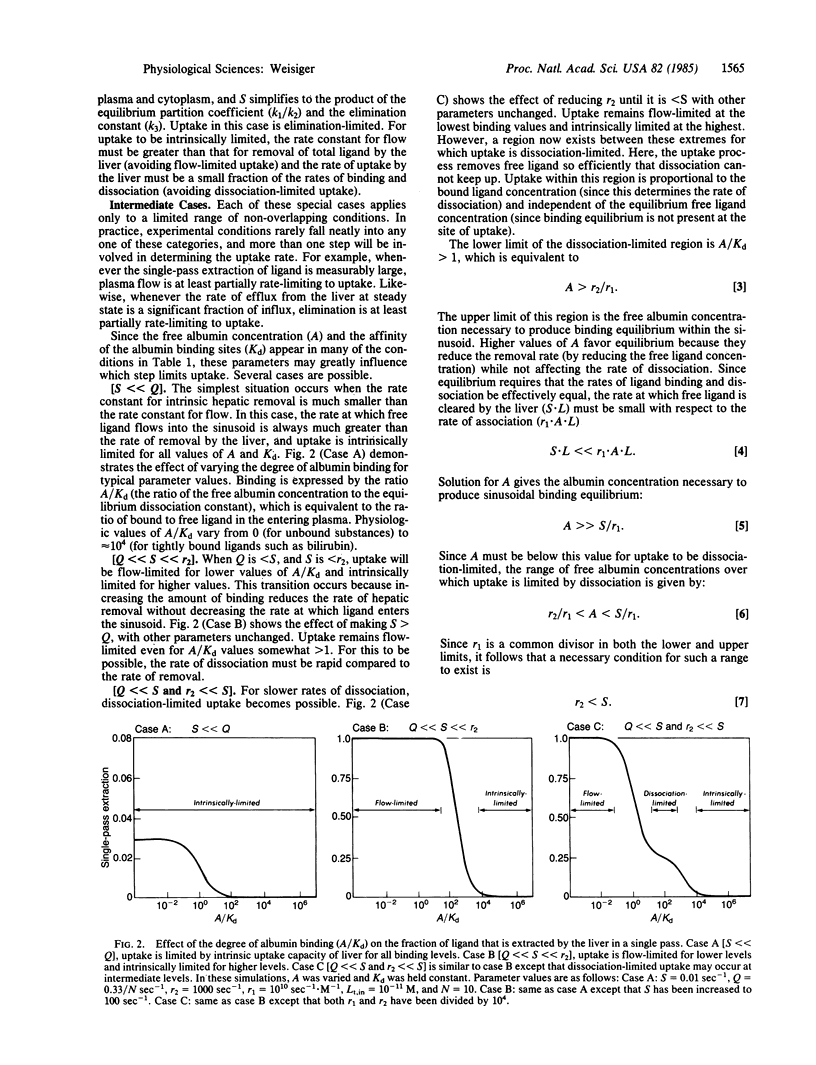
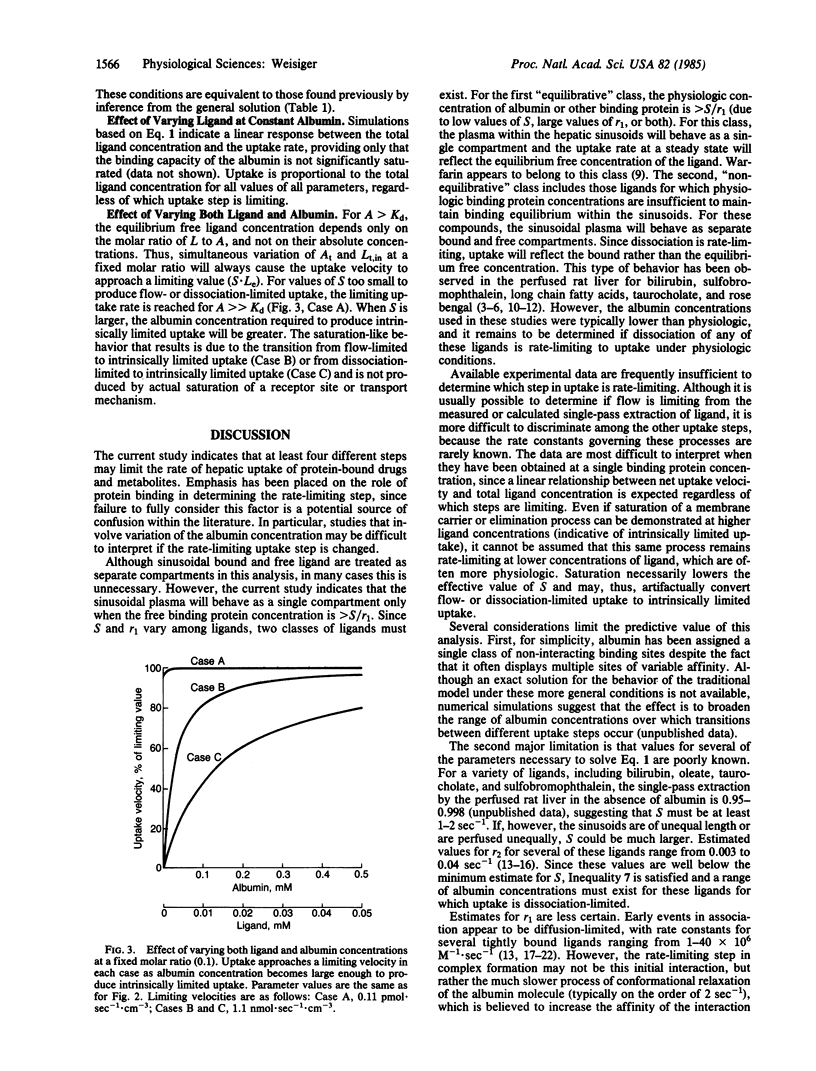
The hepatic uptake rate for certain albumin-bound drugs and metabolites correlates poorly with their equilibrium unbound concentration in the plasma, suggesting that binding equilibrium may not always exist within the hepatic sinusoids. Currently available models for the uptake process assume binding equilibrium and, thus, cannot be used to investigate this possibility. This report presents a more general model that treats plasma-bound and free concentrations separately. A solution is provided that specifies the hepatic uptake rate as a function of the total plasma concentrations of the transported substance and of binding protein and the rate constants for influx, efflux, elimination, association, dissociation, and flow. Analysis of this solution indicates that hepatic uptake may be limited by the rate of plasma flow, dissociation from the binding protein, influx into the liver, cellular elimination, or any combination of these processes. The affinity and concentration of the binding protein strongly influence which of these steps are rate-limiting in any given case, and binding equilibrium exists within the hepatic sinusoids only for binding protein concentrations greater than a specified value (the ratio of the uptake and association rate constants). The precise conditions under which each step is rate-limiting and the kinetic behavior expected when two or more steps mutually limit uptake are provided. The results are compatible with previously reported data for the uptake of certain albumin-bound ligands such as bilirubin, and they offer an alternative to attributing these kinetics to the presence of an albumin receptor.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen R. F. Fluorescence stopped-flow study of relaxation processes in the binding of bilirubin to serum albumins. Arch Biochem Biophys. 1974 Jan;160(1):106–112. doi: 10.1016/s0003-9861(74)80014-7. [DOI] [PubMed] [Google Scholar]
- Faerch T., Jacobsen J. Kinetics of the binding of bilirubin to human serum albumin studied by stopped-flow technique. Arch Biochem Biophys. 1977 Nov;184(1):282–289. doi: 10.1016/0003-9861(77)90352-6. [DOI] [PubMed] [Google Scholar]
- Forker E. L., Luxon B. A. Albumin helps mediate removal of taurocholate by rat liver. J Clin Invest. 1981 May;67(5):1517–1522. doi: 10.1172/JCI110182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forker E. L., Luxon B. A., Snell M., Shurmantine W. O. Effect of albumin binding on the hepatic transport of rose bengal: surface-mediated dissociation of limited capacity. J Pharmacol Exp Ther. 1982 Nov;223(2):342–347. [PubMed] [Google Scholar]
- Gray R. D., Stroupe S. D. Kinetics and mechanism of bilirubin binding to human serum albumin. J Biol Chem. 1978 Jun 25;253(12):4370–4377. [PubMed] [Google Scholar]
- Jansen J. A. Influence of plasma protein binding kinetics on hepatic clearance assessed from a "tube" model and a "well-stirred" model. J Pharmacokinet Biopharm. 1981 Feb;9(1):15–26. doi: 10.1007/BF01059340. [DOI] [PubMed] [Google Scholar]
- Koren R., Nissani E., Perlmutter-Hayman B. The kinetics of the reaction between bovine serum albumin and bilirubin. A second look. Biochim Biophys Acta. 1982 Apr 21;703(1):42–48. doi: 10.1016/0167-4838(82)90008-5. [DOI] [PubMed] [Google Scholar]
- Levy G., Yacobi A. Letter: Effect of plasma protein binding on elimination of warfarin. J Pharm Sci. 1974 May;63(5):805–806. doi: 10.1002/jps.2600630539. [DOI] [PubMed] [Google Scholar]
- Ockner R. K., Weisiger R. A., Gollan J. L. Hepatic uptake of albumin-bound substances: albumin receptor concept. Am J Physiol. 1983 Jul;245(1):G13–G18. doi: 10.1152/ajpgi.1983.245.1.G13. [DOI] [PubMed] [Google Scholar]
- Pang K. S., Rowland M. Hepatic clearance of drugs. I. Theoretical considerations of a "well-stirred" model and a "parallel tube" model. Influence of hepatic blood flow, plasma and blood cell binding, and the hepatocellular enzymatic activity on hepatic drug clearance. J Pharmacokinet Biopharm. 1977 Dec;5(6):625–653. doi: 10.1007/BF01059688. [DOI] [PubMed] [Google Scholar]
- Reed R. G. Kinetics of bilirubin binding to bovine serum albumin and the effects of palmitate. J Biol Chem. 1977 Nov 10;252(21):7483–7487. [PubMed] [Google Scholar]
- Scheider W. Dissociation rate of serum albumin-fatty acid complex from stop-flow dielectric study of ligand exchange. Biophys J. 1978 Oct;24(1):260–262. doi: 10.1016/S0006-3495(78)85371-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheider W. The rate of access to the organic ligand-binding region of serum albumin is entropy controlled. Proc Natl Acad Sci U S A. 1979 May;76(5):2283–2287. doi: 10.1073/pnas.76.5.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenson A., Holmer E., Andersson L. O. A new method for the measurement of dissociation rates for complexes between small ligands and proteins as applied to the palmitate and bilirubin complexes with serum albumin. Biochim Biophys Acta. 1974 Mar 14;342(1):54–59. doi: 10.1016/0005-2795(74)90105-6. [DOI] [PubMed] [Google Scholar]
- Tanford C. Chemical potential of bound ligand, an important parameter for free energy transduction. Proc Natl Acad Sci U S A. 1981 Jan;78(1):270–273. doi: 10.1073/pnas.78.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisiger R. A., Gollan J. L., Ockner R. K. The role of albumin in hepatic uptake processes. Prog Liver Dis. 1982;7:71–85. [PubMed] [Google Scholar]
- Weisiger R. A., Zacks C. M., Smith N. D., Boyer J. L. Effect of albumin binding on extraction of sulfobromophthalein by perfused elasmobranch liver: evidence for dissociation-limited uptake. Hepatology. 1984 May-Jun;4(3):492–501. doi: 10.1002/hep.1840040323. [DOI] [PubMed] [Google Scholar]
- Weisiger R., Gollan J., Ockner R. Receptor for albumin on the liver cell surface may mediate uptake of fatty acids and other albumin-bound substances. Science. 1981 Mar 6;211(4486):1048–1051. doi: 10.1126/science.6258226. [DOI] [PubMed] [Google Scholar]


