Abstract
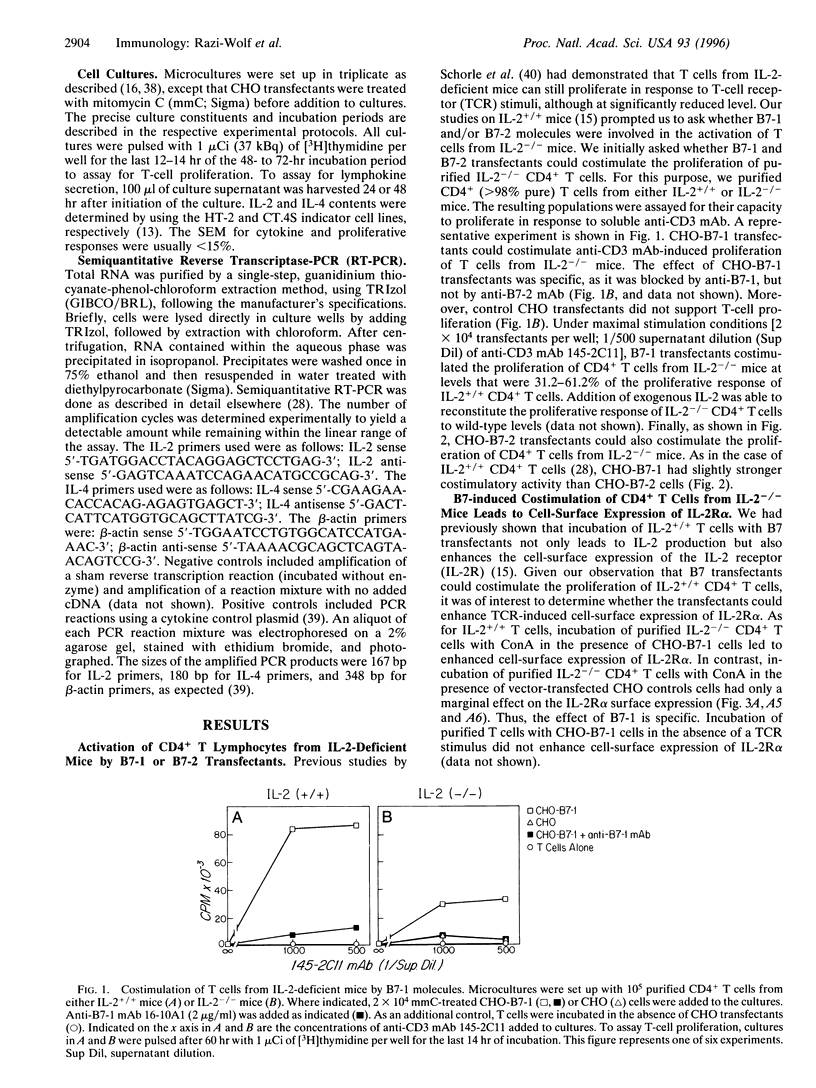
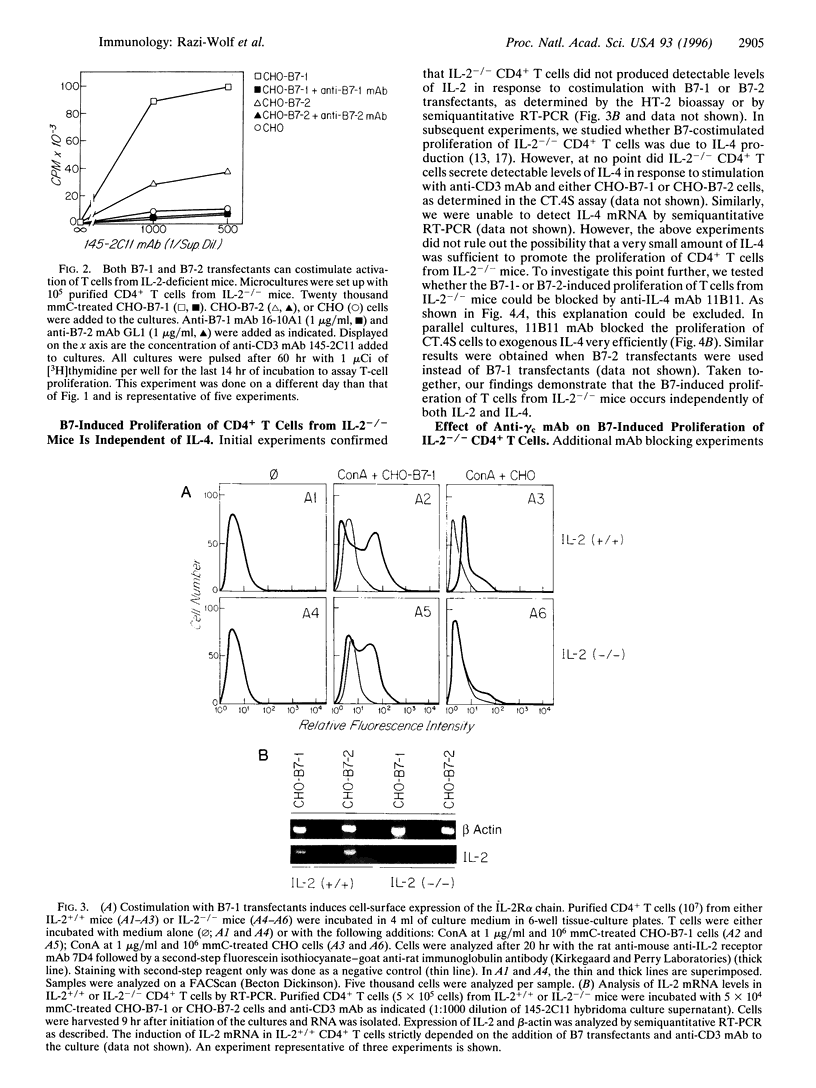
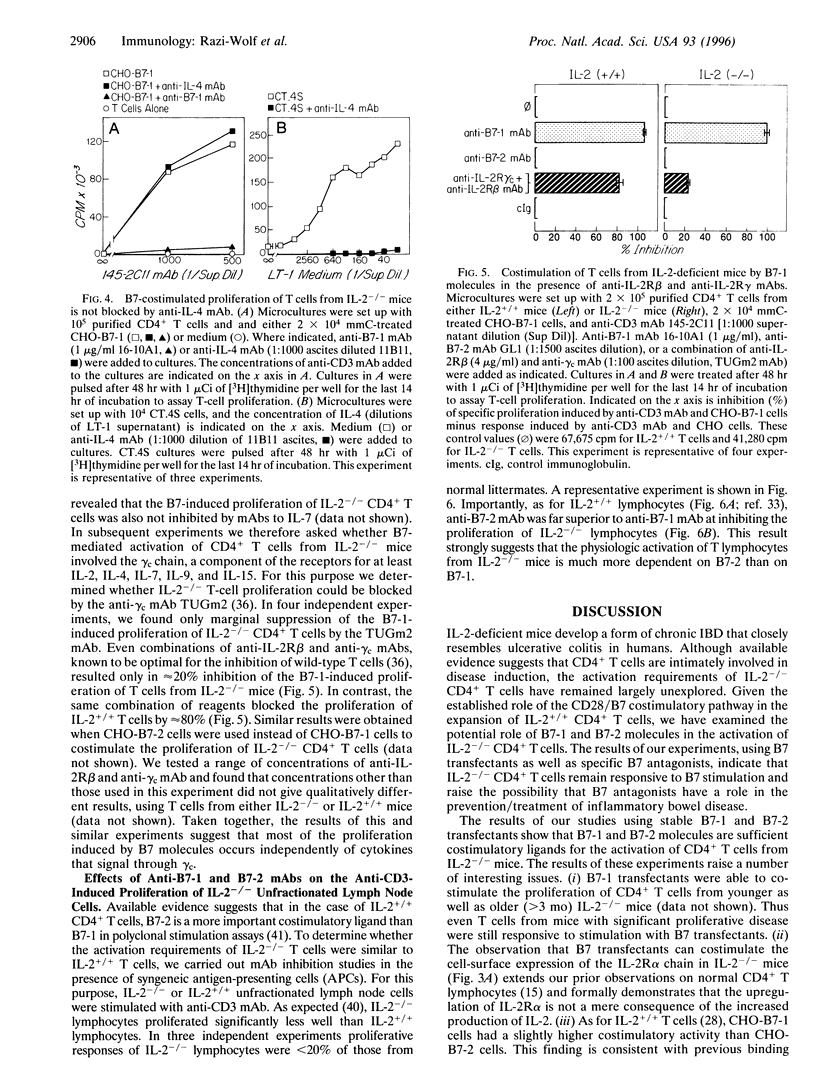
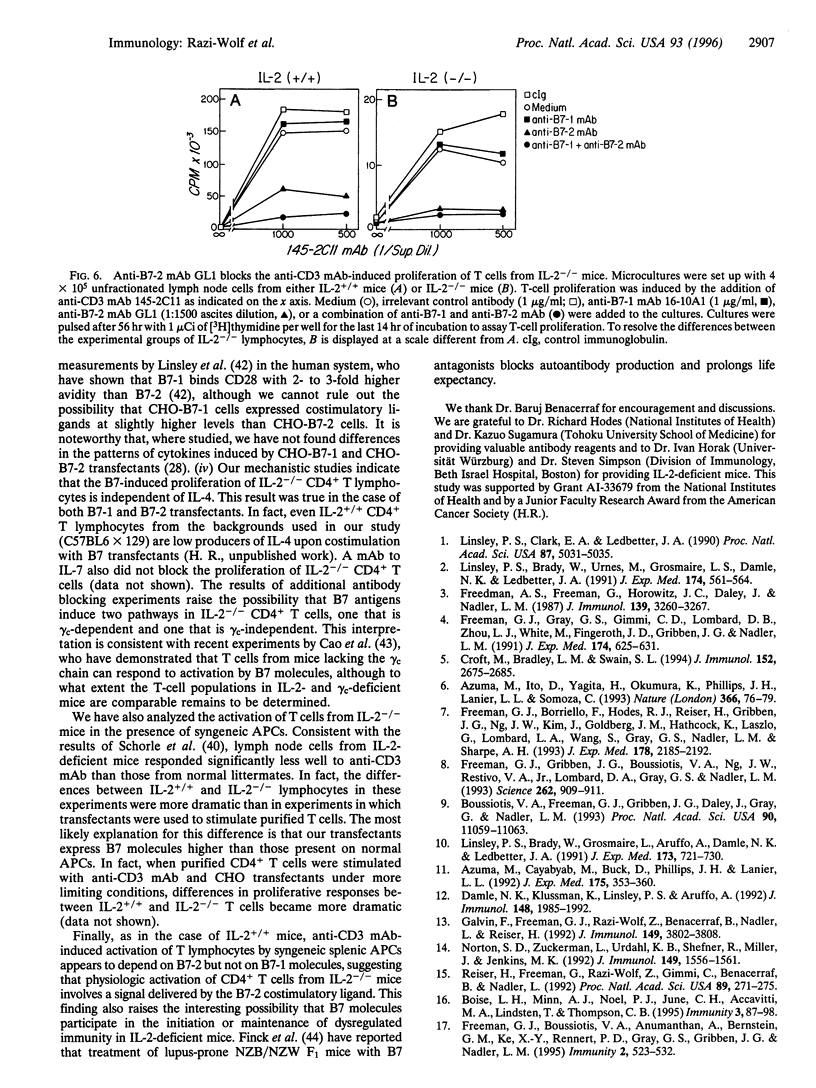
Interleukin 2 (IL-2)-deficient (IL-2-/-) mice develop hemolytic anemia and chronic inflammatory bowel disease. Importantly, the induction of disease in IL-2-deficient mice is critically dependent on CD4+ T cells. We have studied the requirements of T cells from IL-2-deficient mice for costimulation with B7 antigens. Stable B7-1 or B7-2 chinese hamster ovary (CHO) cell transfectants could synergize with anti-CD3 monoclonal antibody (mAb) to induce the proliferation of CD4+ T cells from IL-2-/- mutant mice. Further mechanistic studies established that B7-induced activation resulted in surface expression of the alpha chain of the IL-2 receptor. B7-induced proliferation occurred independently of IL-4 and was largely independent of the common gamma chain of the IL-2, IL-4, IL-7, IL-9, and IL-15 receptors. Finally, anti-B7-2 but not anti-B7-1 mAb was able to inhibit the activation of IL-2-/- T cells induced by anti-CD3 mAb in the presence of syngeneic antigen-presenting cells. The results of our experiments indicate that IL-2-/- CD4+ T cells remain responsive to B7 stimulation and raise the possibility that B7 antagonists have a role in the prevention/treatment of inflammatory bowel disease.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azuma M., Cayabyab M., Buck D., Phillips J. H., Lanier L. L. CD28 interaction with B7 costimulates primary allogeneic proliferative responses and cytotoxicity mediated by small, resting T lymphocytes. J Exp Med. 1992 Feb 1;175(2):353–360. doi: 10.1084/jem.175.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma M., Ito D., Yagita H., Okumura K., Phillips J. H., Lanier L. L., Somoza C. B70 antigen is a second ligand for CTLA-4 and CD28. Nature. 1993 Nov 4;366(6450):76–79. doi: 10.1038/366076a0. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A., Dorf M. E., Springer T. A. A shared alloantigenic determinant on Ia antigens encoded by the I-A and I-E subregions: evidence for I region gene duplication. J Immunol. 1981 Dec;127(6):2488–2495. [PubMed] [Google Scholar]
- Blazar B. R., Taylor P. A., Linsley P. S., Vallera D. A. In vivo blockade of CD28/CTLA4: B7/BB1 interaction with CTLA4-Ig reduces lethal murine graft-versus-host disease across the major histocompatibility complex barrier in mice. Blood. 1994 Jun 15;83(12):3815–3825. [PubMed] [Google Scholar]
- Boise L. H., Minn A. J., Noel P. J., June C. H., Accavitti M. A., Lindsten T., Thompson C. B. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity. 1995 Jul;3(1):87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- Boussiotis V. A., Freeman G. J., Gribben J. G., Daley J., Gray G., Nadler L. M. Activated human B lymphocytes express three CTLA-4 counterreceptors that costimulate T-cell activation. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11059–11063. doi: 10.1073/pnas.90.23.11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X., Shores E. W., Hu-Li J., Anver M. R., Kelsall B. L., Russell S. M., Drago J., Noguchi M., Grinberg A., Bloom E. T. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity. 1995 Mar;2(3):223–238. doi: 10.1016/1074-7613(95)90047-0. [DOI] [PubMed] [Google Scholar]
- Croft M., Bradley L. M., Swain S. L. Naive versus memory CD4 T cell response to antigen. Memory cells are less dependent on accessory cell costimulation and can respond to many antigen-presenting cell types including resting B cells. J Immunol. 1994 Mar 15;152(6):2675–2685. [PubMed] [Google Scholar]
- Damle N. K., Klussman K., Linsley P. S., Aruffo A. Differential costimulatory effects of adhesion molecules B7, ICAM-1, LFA-3, and VCAM-1 on resting and antigen-primed CD4+ T lymphocytes. J Immunol. 1992 Apr 1;148(7):1985–1992. [PubMed] [Google Scholar]
- Finck B. K., Linsley P. S., Wofsy D. Treatment of murine lupus with CTLA4Ig. Science. 1994 Aug 26;265(5176):1225–1227. doi: 10.1126/science.7520604. [DOI] [PubMed] [Google Scholar]
- Freedman A. S., Freeman G., Horowitz J. C., Daley J., Nadler L. M. B7, a B-cell-restricted antigen that identifies preactivated B cells. J Immunol. 1987 Nov 15;139(10):3260–3267. [PubMed] [Google Scholar]
- Freeman G. J., Borriello F., Hodes R. J., Reiser H., Gribben J. G., Ng J. W., Kim J., Goldberg J. M., Hathcock K., Laszlo G. Murine B7-2, an alternative CTLA4 counter-receptor that costimulates T cell proliferation and interleukin 2 production. J Exp Med. 1993 Dec 1;178(6):2185–2192. doi: 10.1084/jem.178.6.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman G. J., Boussiotis V. A., Anumanthan A., Bernstein G. M., Ke X. Y., Rennert P. D., Gray G. S., Gribben J. G., Nadler L. M. B7-1 and B7-2 do not deliver identical costimulatory signals, since B7-2 but not B7-1 preferentially costimulates the initial production of IL-4. Immunity. 1995 May;2(5):523–532. doi: 10.1016/1074-7613(95)90032-2. [DOI] [PubMed] [Google Scholar]
- Freeman G. J., Gray G. S., Gimmi C. D., Lombard D. B., Zhou L. J., White M., Fingeroth J. D., Gribben J. G., Nadler L. M. Structure, expression, and T cell costimulatory activity of the murine homologue of the human B lymphocyte activation antigen B7. J Exp Med. 1991 Sep 1;174(3):625–631. doi: 10.1084/jem.174.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman G. J., Gribben J. G., Boussiotis V. A., Ng J. W., Restivo V. A., Jr, Lombard L. A., Gray G. S., Nadler L. M. Cloning of B7-2: a CTLA-4 counter-receptor that costimulates human T cell proliferation. Science. 1993 Nov 5;262(5135):909–911. doi: 10.1126/science.7694363. [DOI] [PubMed] [Google Scholar]
- Galvin F., Freeman G. J., Razi-Wolf Z., Hall W., Jr, Benacerraf B., Nadler L., Reiser H. Murine B7 antigen provides a sufficient costimulatory signal for antigen-specific and MHC-restricted T cell activation. J Immunol. 1992 Dec 15;149(12):3802–3808. [PubMed] [Google Scholar]
- Guinan E. C., Gribben J. G., Boussiotis V. A., Freeman G. J., Nadler L. M. Pivotal role of the B7:CD28 pathway in transplantation tolerance and tumor immunity. Blood. 1994 Nov 15;84(10):3261–3282. [PubMed] [Google Scholar]
- Hathcock K. S., Laszlo G., Dickler H. B., Bradshaw J., Linsley P., Hodes R. J. Identification of an alternative CTLA-4 ligand costimulatory for T cell activation. Science. 1993 Nov 5;262(5135):905–907. doi: 10.1126/science.7694361. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kondo M., Takeshita T., Ishii N., Nakamura M., Watanabe S., Arai K., Sugamura K. Sharing of the interleukin-2 (IL-2) receptor gamma chain between receptors for IL-2 and IL-4. Science. 1993 Dec 17;262(5141):1874–1877. doi: 10.1126/science.8266076. [DOI] [PubMed] [Google Scholar]
- Lenschow D. J., Zeng Y., Thistlethwaite J. R., Montag A., Brady W., Gibson M. G., Linsley P. S., Bluestone J. A. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4lg. Science. 1992 Aug 7;257(5071):789–792. doi: 10.1126/science.1323143. [DOI] [PubMed] [Google Scholar]
- Leo O., Foo M., Sachs D. H., Samelson L. E., Bluestone J. A. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H., Bolling S. F., Linsley P. S., Wei R. Q., Gordon D., Thompson C. B., Turka L. A. Long-term acceptance of major histocompatibility complex mismatched cardiac allografts induced by CTLA4Ig plus donor-specific transfusion. J Exp Med. 1993 Nov 1;178(5):1801–1806. doi: 10.1084/jem.178.5.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley P. S., Brady W., Grosmaire L., Aruffo A., Damle N. K., Ledbetter J. A. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J Exp Med. 1991 Mar 1;173(3):721–730. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley P. S., Brady W., Urnes M., Grosmaire L. S., Damle N. K., Ledbetter J. A. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991 Sep 1;174(3):561–569. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley P. S., Clark E. A., Ledbetter J. A. T-cell antigen CD28 mediates adhesion with B cells by interacting with activation antigen B7/BB-1. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5031–5035. doi: 10.1073/pnas.87.13.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley P. S., Greene J. L., Brady W., Bajorath J., Ledbetter J. A., Peach R. Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity. 1994 Dec;1(9):793–801. doi: 10.1016/s1074-7613(94)80021-9. [DOI] [PubMed] [Google Scholar]
- Linsley P. S., Wallace P. M., Johnson J., Gibson M. G., Greene J. L., Ledbetter J. A., Singh C., Tepper M. A. Immunosuppression in vivo by a soluble form of the CTLA-4 T cell activation molecule. Science. 1992 Aug 7;257(5071):792–795. doi: 10.1126/science.1496399. [DOI] [PubMed] [Google Scholar]
- Ma A., Datta M., Margosian E., Chen J., Horak I. T cells, but not B cells, are required for bowel inflammation in interleukin 2-deficient mice. J Exp Med. 1995 Nov 1;182(5):1567–1572. doi: 10.1084/jem.182.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek T. R., Robb R. J., Shevach E. M. Identification and initial characterization of a rat monoclonal antibody reactive with the murine interleukin 2 receptor-ligand complex. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5694–5698. doi: 10.1073/pnas.80.18.5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton S. D., Zuckerman L., Urdahl K. B., Shefner R., Miller J., Jenkins M. K. The CD28 ligand, B7, enhances IL-2 production by providing a costimulatory signal to T cells. J Immunol. 1992 Sep 1;149(5):1556–1561. [PubMed] [Google Scholar]
- Ohara J., Paul W. E. Production of a monoclonal antibody to and molecular characterization of B-cell stimulatory factor-1. Nature. 1985 May 23;315(6017):333–336. doi: 10.1038/315333a0. [DOI] [PubMed] [Google Scholar]
- Platzer C., Richter G., Uberla K., Müller W., Blöcker H., Diamantstein T., Blankenstein T. Analysis of cytokine mRNA levels in interleukin-4-transgenic mice by quantitative polymerase chain reaction. Eur J Immunol. 1992 May;22(5):1179–1184. doi: 10.1002/eji.1830220511. [DOI] [PubMed] [Google Scholar]
- Powrie F. T cells in inflammatory bowel disease: protective and pathogenic roles. Immunity. 1995 Aug;3(2):171–174. doi: 10.1016/1074-7613(95)90086-1. [DOI] [PubMed] [Google Scholar]
- Razi-Wolf Z., Freeman G. J., Galvin F., Benacerraf B., Nadler L., Reiser H. Expression and function of the murine B7 antigen, the major costimulatory molecule expressed by peritoneal exudate cells. Proc Natl Acad Sci U S A. 1992 May 1;89(9):4210–4214. doi: 10.1073/pnas.89.9.4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser H., Freeman G. J., Razi-Wolf Z., Gimmi C. D., Benacerraf B., Nadler L. M. Murine B7 antigen provides an efficient costimulatory signal for activation of murine T lymphocytes via the T-cell receptor/CD3 complex. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):271–275. doi: 10.1073/pnas.89.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser H. sgp-60, a signal-transducing glycoprotein concerned with T cell activation through the T cell receptor/CD3 complex. J Immunol. 1990 Oct 1;145(7):2077–2086. [PubMed] [Google Scholar]
- Sadlack B., Merz H., Schorle H., Schimpl A., Feller A. C., Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993 Oct 22;75(2):253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- Schorle H., Holtschke T., Hünig T., Schimpl A., Horak I. Development and function of T cells in mice rendered interleukin-2 deficient by gene targeting. Nature. 1991 Aug 15;352(6336):621–624. doi: 10.1038/352621a0. [DOI] [PubMed] [Google Scholar]
- Simpson S. J., Mizoguchi E., Allen D., Bhan A. K., Terhorst C. Evidence that CD4+, but not CD8+ T cells are responsible for murine interleukin-2-deficient colitis. Eur J Immunol. 1995 Sep;25(9):2618–2625. doi: 10.1002/eji.1830250932. [DOI] [PubMed] [Google Scholar]
- Turka L. A., Linsley P. S., Lin H., Brady W., Leiden J. M., Wei R. Q., Gibson M. L., Zheng X. G., Myrdal S., Gordon D. T-cell activation by the CD28 ligand B7 is required for cardiac allograft rejection in vivo. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):11102–11105. doi: 10.1073/pnas.89.22.11102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace P. M., Johnson J. S., MacMaster J. F., Kennedy K. A., Gladstone P., Linsley P. S. CTLA4Ig treatment ameliorates the lethality of murine graft-versus-host disease across major histocompatibility complex barriers. Transplantation. 1994 Sep 15;58(5):602–610. doi: 10.1097/00007890-199409150-00013. [DOI] [PubMed] [Google Scholar]



