Abstract
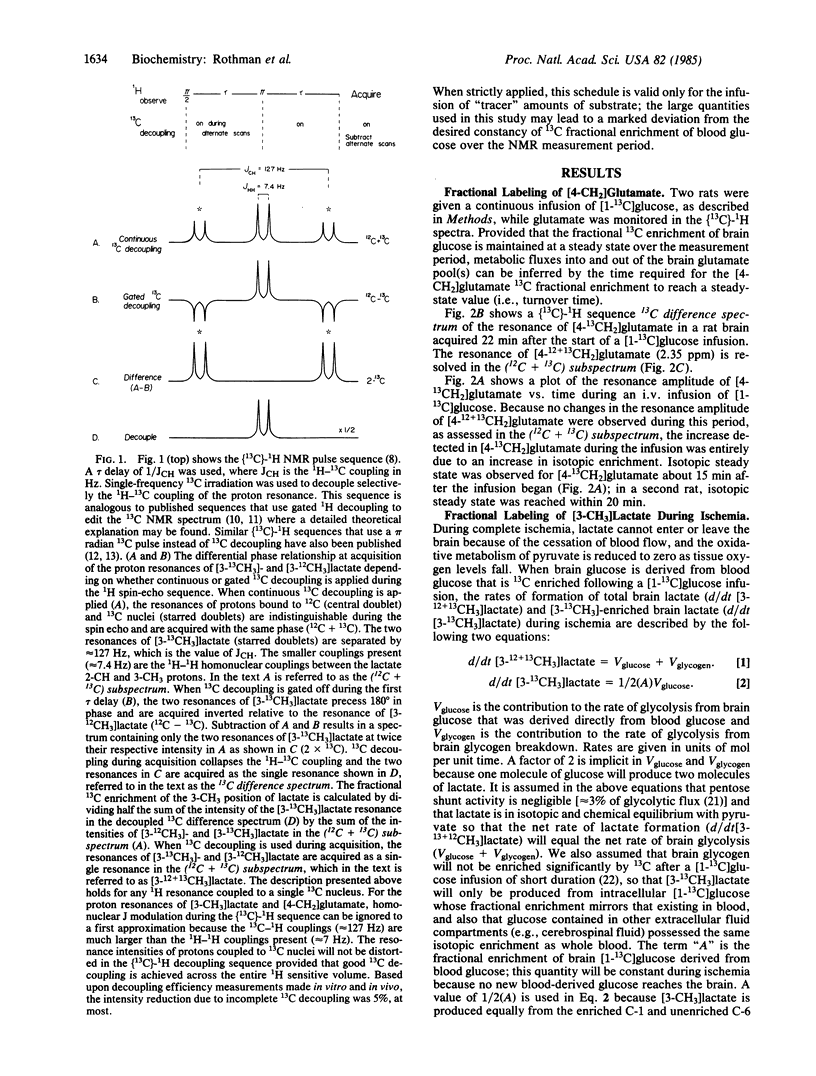
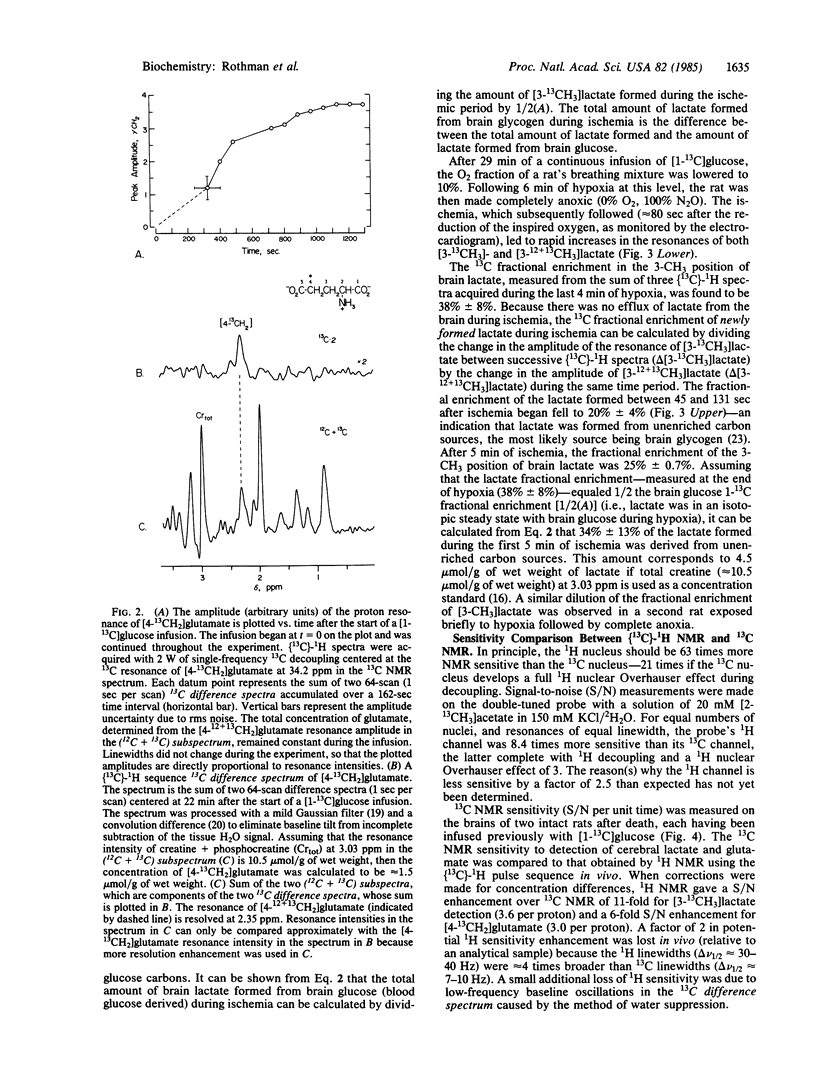
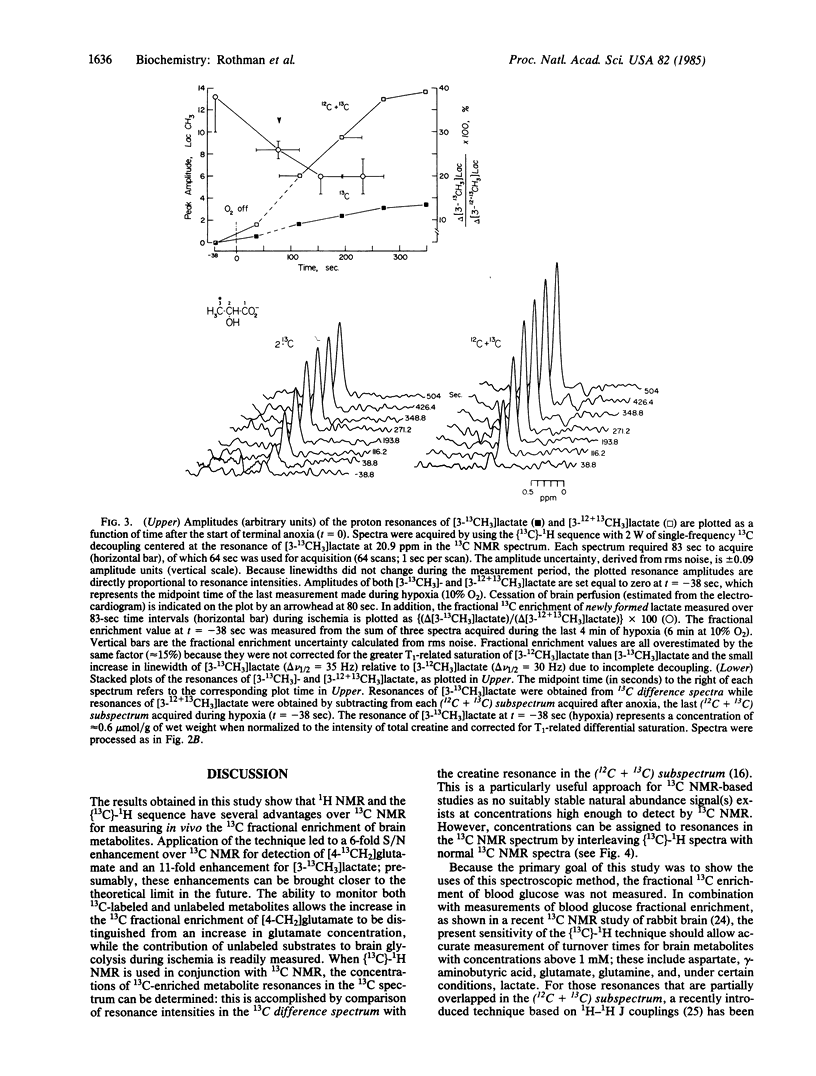
We have used (13C)-1H NMR spectroscopy at 360.13 MHz to resolve the 13C coupled proton resonance of glutamate and lactate in the rat brain in vivo. The time required for the 13C fractional enrichment of the 4-CH2 position of brain glutamate to reach isotopic steady state was determined during a continuous infusion of D-[1-13C]glucose. Under conditions of ischemia, measurements made of the 3-CH3 of lactate in (13C)-1H NMR spectra revealed the relative contribution of brain glucose and glycogen to lactate formation. (13C)-1H NMR was 11 times more sensitive than 13C NMR for the detection of 13C in the 3-CH3 position of lactate and 6 times more sensitive for the detection of 13C in the 4-CH2 of glutamate under similar in vivo conditions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alger J. R., Sillerud L. O., Behar K. L., Gillies R. J., Shulman R. G., Gordon R. E., Shae D., Hanley P. E. In vivo carbon-13 nuclear magnetic resonance studies of mammals. Science. 1981 Nov 6;214(4521):660–662. doi: 10.1126/science.7292005. [DOI] [PubMed] [Google Scholar]
- Behar K. L., den Hollander J. A., Stromski M. E., Ogino T., Shulman R. G., Petroff O. A., Prichard J. W. High-resolution 1H nuclear magnetic resonance study of cerebral hypoxia in vivo. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4945–4948. doi: 10.1073/pnas.80.16.4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brindle K. M., Boyd J., Campbell I. D., Porteous R., Soffe N. Observation of carbon labelling in cell metabolites using proton spin echo NMR. Biochem Biophys Res Commun. 1982 Dec 15;109(3):864–871. doi: 10.1016/0006-291x(82)92020-4. [DOI] [PubMed] [Google Scholar]
- Campbell I. D., Dobson C. M., Jeminet G., Williams R. J. Pulsed NMR methods for the observation and assignment of exchangeable hydrogens: application to bacitracin. FEBS Lett. 1974 Dec 1;49(1):115–119. doi: 10.1016/0014-5793(74)80645-9. [DOI] [PubMed] [Google Scholar]
- Hostetler K. Y., Landau B. R. Estimation of the pentose cycle contribution to glucose metabolism in tissue in vivo. Biochemistry. 1967 Oct;6(10):2961–2964. doi: 10.1021/bi00862a001. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., HASSELBERGER F. X., SCHULZ D. W. EFFECT OF ISCHEMIA ON KNOWN SUBSTRATES AND COFACTORS OF THE GLYCOLYTIC PATHWAY IN BRAIN. J Biol Chem. 1964 Jan;239:18–30. [PubMed] [Google Scholar]
- Neurohr K. J., Barrett E. J., Shulman R. G. In vivo carbon-13 nuclear magnetic resonance studies of heart metabolism. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1603–1607. doi: 10.1073/pnas.80.6.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogino T., Arata Y., Fujiwara S. Proton correlation nuclear magnetic resonance study of metabolic regulations and pyruvate transport in anaerobic Escherichia coli cells. Biochemistry. 1980 Aug 5;19(16):3684–3691. doi: 10.1021/bi00557a008. [DOI] [PubMed] [Google Scholar]
- Patlak C. S., Pettigrew K. D. A method to obtain infusion schedules for prescribed blood concentration time courses. J Appl Physiol. 1976 Mar;40(3):458–463. doi: 10.1152/jappl.1976.40.3.458. [DOI] [PubMed] [Google Scholar]
- Rothman D. L., Behar K. L., Hetherington H. P., Shulman R. G. Homonuclear 1H double-resonance difference spectroscopy of the rat brain in vivo. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6330–6334. doi: 10.1073/pnas.81.20.6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman R. G., Brown T. R., Ugurbil K., Ogawa S., Cohen S. M., den Hollander J. A. Cellular applications of 31P and 13C nuclear magnetic resonance. Science. 1979 Jul 13;205(4402):160–166. doi: 10.1126/science.36664. [DOI] [PubMed] [Google Scholar]
- Watanabe H., Passonneau J. V. Factors affecting the turnover of cerebral glycogen and limit dextrin in vivo. J Neurochem. 1973 Jun;20(6):1543–1554. doi: 10.1111/j.1471-4159.1973.tb00272.x. [DOI] [PubMed] [Google Scholar]


