Abstract
G-quadruplex-forming oligonucleotides containing modified nucleotide chemistries have demonstrated promising pharmaceutical potential. In this work, we systematically investigate the effects of sugar-modified guanosines on the structure and stability of a (4+0) parallel and a (3+1) hybrid G-quadruplex using over 60 modified sequences containing a single-position substitution of 2′-O-4′-C-methylene-guanosine (LNAG), 2′-deoxy-2′-fluoro-riboguanosine (FG) or 2′-deoxy-2′-fluoro-arabinoguanosine (FANAG). Our results are summarized in two parts: (I) Generally, LNAG substitutions into ‘anti’ position guanines within a guanine-tetrad lead to a more stable G-quadruplex, while substitutions into ‘syn’ positions disrupt the native G-quadruplex conformation. However, some interesting exceptions to this trend are observed. We discover that a LNAG modification upstream of a short propeller loop hinders G-quadruplex formation. (II) A single substitution of either FG or FANAG into a ‘syn’ position is powerful enough to perturb the (3+1) G-quadruplex. Substitution of either FG or FANAG into any ‘anti’ position is well tolerated in the two G-quadruplex scaffolds. FANAG substitutions to ‘anti’ positions are better tolerated than their FG counterparts. In both scaffolds, FANAG substitutions to the central tetrad layer are observed to be the most stabilizing. The observations reported herein on the effects of LNAG, FG and FANAG modifications on G-quadruplex structure and stability will enable the future design of pharmaceutically relevant oligonucleotides.
INTRODUCTION
G-quadruplexes are four-stranded nucleic acid structures composed of stacked layers of guanine tetrads, stabilized by Hoogsteen hydrogen bonds and coordinating cations (1,2). Guanine-rich G-quadruplex-forming sequences are present in some critical regions of the human genome, and the formation of these structures has been shown to play important roles in various biological processes (3–10).
From a therapeutic perspective, many engineered G-quadruplex–forming sequences show high affinity towards biologically important protein targets. For example, G-quadruplex–forming oligonucleotides have been discovered with anti-coagulant, anti-cancer and anti-HIV activity (11–16). However, native DNA chemistry is prone to enzymatic digestion. The incorporation of alternative nucleic acid chemistries can enhance the lifetime and other pharmacological properties of G-quadruplex-based drugs.
Modification of the base (17–20) or phosphate–sugar backbone (20–26) can have beneficial effects on the stability, kinetics, resistance to enzymatic digestion and cellular uptake of biologically active G-quadruplexes. For example, past studies have investigated the effects of introducing modified base and sugar-backbone chemistries into the thrombin-binding aptamer (TBA) (19), known for its anti-coagulant properties. The use of modified chemistries in the TBA has lead to higher stability (22,25,27–31), increased binding affinity (25,30) and enhanced biological activity including studies in vivo (28,30,32–34). In a similar manner, modified nucleic acid chemistries have been used to enhance the pharmacological properties of anti-HIV aptamers (25,35–40).
One alternative DNA chemistry that has received notable attention is Locked Nucleic Acid (LNA) (41), a ribonucleotide analogue with a 2′-O-4′-C-methylene linkage (Figure 1A). Introduction of LNA can improve oligonucleotide stability towards enzymatic digestion as well as the thermal stability of duplexes and triplexes (41,42). Previous studies have shown that LNA modifications can greatly enhance the RNA cleaving rate of a DNAzyme (43). Additionally, LNA is generally soluble in water and non-toxic (44,45). In the context of G-quadruplexes, it has been reported that the introduction of LNA-modified guanosine (LNAG) stabilizes the tetrameric G-quadruplexes formed by the d[TLNAG3T], d[T(GLNAG)2T] and d[TLNAG4T] sequences (46,47). LNAG has been previously observed to favour an ‘anti’ glycosidic conformation of the base (48), and studies have taken advantage of this preference to engineer the G-quadruplex folding topology (49,50). Substitutions of LNAG into positions that adopt a ‘syn’ conformation tend to push structural equilibrium towards a parallel G-quadruplex where all guanines adopt an ‘anti’ conformation (48,49,51). Incorporation of LNAG has also been used to enhance the inhibitory properties of biologically active G-quadruplex molecules (28,40).
Figure 1.
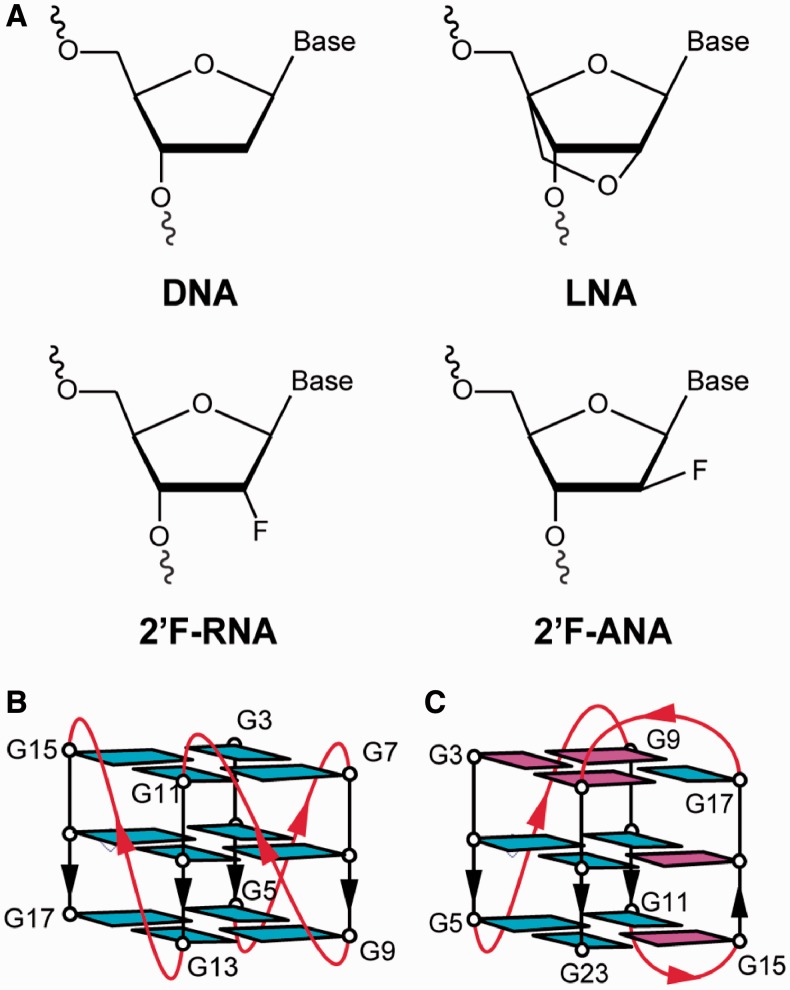
Modified nucleotides and G-quadruplex scaffolds used in this study: (A) Sugar chemistries of DNA, LNA, 2′F-RNA and 2′F-ANA. Schematic structures of the (B) (4+0) parallel G-quadruplex formed by d[T2(G3T)4] and the (C) (3+1) hybrid G-quadruplex formed by d[T2(G3T2A)3G3A]. Guanines are coloured based on their ‘syn’ (magenta) or ‘anti’ (cyan) conformation.
The sugar-modified nucleotides 2′-deoxy-2′-fluoro-riboguanosine (FG) and 2′-deoxy-2′-fluoro-arabinoguanosine (FANAG) represent another useful family of chemical tools, containing a proton to fluorine modification at the C2′ position of the sugar (Figure 1A). These chemistries have shown promise for increasing the stability and anti-sense potency of duplexes formed by short interference RNAs (52,53). 2′F-RNA and 2′F-ANA modified nucleotides have been observed to increase the resistance of modified oligonucleotides to degradation by nuclease (25,54,55). In the context of G-quadruplex, modification with 2′F-ANA nucleotides has allowed for enhanced G-quadruplex aptamer stability and nuclease resistance (25). Recent works from our laboratory and others have also shown that the FG and FANAG chemistries can be used to manipulate the folding topology of G-quadruplexes (26,56).
Considering the demonstrated potential of sugar-modified nucleotides for enhancing the drug-like properties of G-quadruplexes, we set out on a systematic study to characterize the effects of LNAG, FG and FANAG incorporation into two G-quadruplex DNA scaffolds: (i) The first scaffold is an intramolecular ‘(4+0)’ parallel-stranded (PS) G-quadruplex formed by the ‘PS-series’ sequence d[T2(G3T)4] (57). This structure contains all strands oriented in the same direction connected by single-nucleotide (1-nt) propeller loops, with all guanine bases adopting an ‘anti’ glycosidic conformation (Figure 1B). (ii) The second scaffold is an intramolecular ‘(3+1)’ hybrid G-quadruplex formed by the human telomeric (HT) sequence d[T2(G3T2A)3G3A] termed the ‘HT-series’ (58). This structure contains three strands oriented in one direction and the fourth in the opposite direction (Figure 1C). The strands are connected by edgewise and propeller loops that are three nucleotides (3-nt) in length. Furthermore, guanine bases within the G-tetrad core adopt a mixture of ‘syn’ and ‘anti’ glycosidic conformations. These two G-quadruplex scaffolds were chosen for study as they have been well characterized and represent distinct types of folding topologies, which exhibit a variety of different structural features.
In order to investigate how to effectively incorporate LNAG, FANAG and FG nucleotides into G-quadruplex structures, we characterize the conformation and thermal stability of G-quadruplexes formed by modified sequences using a combination of nuclear magnetic resonance (NMR), ultraviolet (UV) absorption and circular dichroism (CD) spectroscopic methods. Our systematic study reveals important considerations for use of these sugar-modified chemical tools within G-quadruplex nucleic acids. Such knowledge will enable the rational design of engineered G-quadruplexes containing these chemistries for pharmaceutical and nanotechnology applications.
MATERIALS AND METHODS
Sample preparation
Oligonucleotides were chemically synthesized using an Applied Biosystems 394 DNA/RNA synthesizer (Foster City, CA, USA). LNAG phosphoramidite was purchased from ScienceWerke (Singapore). Sugar-modified FG and FANAG phosphoramidites were purchased from Glen Research (Sterling, VA, USA). Oligonucleotides were purified using a Poly-Pak cartridge (Glen Research) following standard protocol. After purification, samples were dialysed successively against water, KCl solution (25 mM) and water again.
After lyophilization, DNA was dissolved and stored in potassium phosphate (KPi) buffer (pH 7) containing 10% D2O and 20 μM 4,4-dimethyl-4-silapentane-1-sulfonic acid (DSS). The concentrations of potassium in DNA stocks were as follows: 5 mM KPi for LNAG-modified PS-series, 20 mM KPi for LNAG-modified HT-series, 1 mM KPi for FG-modified and FANAG-modified PS-series, and 5 mM KPi supplemented with 5 mM KCl for FG-modified and FANAG-modified HT-series.
Nuclear magnetic resonance
NMR experiments were performed on a 600 or 700 MHz Bruker NMR spectrometer (Billerica, MA, USA) at 25°C using jump-and-return-type pulse sequences for water suppression (61,62). Chemical shifts were calibrated by DSS in NMR buffer. Samples were annealed by heating at 100°C for 5 min and cooled down slowly overnight before recording NMR spectra and subsequent CD studies. Samples for 1D NMR experiments were examined in DNA stock conditions described above with DNA strand concentrations as follows: LNAG PS-series 0.15 mM; LNAG HT-series 0.1–0.2 mM; FG and FANAG PS-series 0.05 mM; FG and FANAG HT-series 0.05–0.4 mM.
Circular Dichroism
CD experiments were performed on a JASCO-815 spectropolarimeter (Tokyo, Japan) using a 1-cm path length quartz cuvette. CD spectra were taken at 20°C in a range of 220–320 nm and accumulated over three scans for the LNAG PS-series and 10 scans for all other sequences. Spectra were baseline corrected and zero-corrected at 320 nm. CD spectra were normalized by DNA strand concentration determined by concurrent UV absorbance measurements.
Samples for the LNAG PS-series were prepared by diluting the DNA stock with 1 mM KPi to obtain DNA concentration of 3 µM and KPi concentration of 1.1 mM. Samples for the LNAG HT-series contained a DNA concentration of 10 µM in the stock salt conditions stated above. Samples for FG and FANAG modified PS-series and HT-series contained DNA strand concentration of 4–6 μM with stock salt conditions described in above section. DNA concentration was expressed in strand molarity with the extinction coefficient of modified sequences approximated to that of the unmodified sequence.
Thermal stability measurements
CD melting was performed for the LNAG-modified PS-series and loop-elongated samples by monitoring CD intensity at 260 nm against temperature over a range of 44–93°C. CD melting was performed by heating the samples at 93°C for 20 min and then cooling to 44°C at a rate of 0.2°C/min. Samples were then heated up again to 93°C at the same rate. For several samples, this range was extended to a lower temperature to monitor complete melting transitions. The melting temperature (Tm) values were obtained by baseline normalization of CD melting curves to create folded fraction curves (59,60). The Tm value is determined to be the temperature at which half of the sample is in the folded state. The Tm values presented are an average over cooling and heating curves.
UV melting experiments were performed for all other samples on a JASCO V-650 UV-Vis spectrophotometer (Tokyo, Japan). UV melting experiments were conducted by monitoring the UV absorbance at 295 nm (59) using a 1-cm path length quartz cuvette over a temperature range of 15–84°C for the HT-series and 43–89°C for the PS-series. This range was extended for several sequences to monitor the complete transition. UV melting was done by first heating the samples to their maximum temperature for 30 min, then cooling down to the minimum temperature at a rate of 0.1°C/min. Samples were then heated up to the maximum temperature at the same rate. The UV absorbance of samples was recorded at 295 nm and corrected by subtracting the absorbance at 320 nm as well as baseline corrected with a reference cell. Fraction folded curves were calculated in the manner described above. Samples were prepared as described in ‘Circular Dichroism’ section.
The effects of sugar-modifed guanosine substitutions were studied in low-salt conditions to monitor the full denaturation of these samples in thermal stability experiments.
RESULTS AND DISCUSSION
G-quadruplex formation of sugar-modified sequences (Table 1 and 2) was evaluated by monitoring the imino proton region (10.5–12.5 ppm) of 1H NMR spectra (63). Peaks in this region can be used to determine the number of G-quadruplex conformations present. High similarity between NMR spectra is a strong indicator that sequences adopt the same folding topology. Validating the folding topology of modified sequences is important, given that small changes in sequence can have large effects on the conformations adopted by G-quadruplex–forming sequences (64,65). CD spectroscopy was also used to probe the G-quadruplex folding topology based on well-characterized patterns in CD spectra (66). The thermal stability of sugar-modified sequences was evaluated through a series of UV and CD melting experiments. The Tm values of sequences are presented as a useful quantitative measure for comparing the thermal stability of modified G-quadruplexes. We also present thermodynamic parameters ΔH, ΔS and ΔG for modified sequences in Supplementary Data.
Table 1.
LNA-modified sequences used in this study and their thermal stability
| Namea | Sequence (5′→3′)b | Tm(°C)c | ΔTm(°C) |
|---|---|---|---|
| (4+0) native | TTGGGTGGGTGGGTGGGT | 77.1 ± 0.5 | – |
| PS-L3 | TTLGGTGGGTGGGTGGGT | 82.0 ± 0.4 | 4.9 |
| PS-L4 | TTGLGTGGGTGGGTGGGT | 85.3 ± 0.3 | 8.1 |
| PS-L5 | TTGGLTGGGTGGGTGGGT | 36.0 ± 0.4 | −41.2 |
| PS-L7 | TTGGGTLGGTGGGTGGGT | 84.3 ± 0.0 | 7.2 |
| PS-L8 | TTGGGTGLGTGGGTGGGT | 80.2 ± 0.2 | 3.1 |
| PS-L9 | TTGGGTGGLTGGGTGGGT | 32.2 ± 0.1 | −45.0 |
| PS-L11 | TTGGGTGGGTLGGTGGGT | 84.9 ± 0.1 | 7.8 |
| PS-L12 | TTGGGTGGGTGLGTGGGT | 79.8 ± 0.1 | 2.7 |
| PS-L13 | TTGGGTGGGTGGLTGGGT | 31.7 ± 0.5 | −45.5 |
| PS-L15 | TTGGGTGGGTGGGTLGGT | 83.2 ± 0.1 | 6.1 |
| PS-L16 | TTGGGTGGGTGGGTGLGT | 81.0 ± 0.1 | 3.8 |
| PS-L17 | TTGGGTGGGTGGGTGGLT | 80.4 ± 0.1 | 3.3 |
| (3+1) native | TTGGGTTAGGGTTAGGGTTAGGGA | 57.4 ± 0.2 | – |
| HT-L3 | TTLGGTTAGGGTTAGGGTTAGGGA | 55.4 ± 0.3 | −2.0 |
| HT-L4 | TTGLGTTAGGGTTAGGGTTAGGGA | 61.6 ± 0.0 | 4.2 |
| HT-L5 | TTGGLTTAGGGTTAGGGTTAGGGA | 56.8 ± 0.4 | −0.6 |
| HT-L9 | TTGGGTTALGGTTAGGGTTAGGGA | – | – |
| HT-L10 | TTGGGTTAGLGTTAGGGTTAGGGA | 59.3 ± 0.5 | 1.9 |
| HT-L11 | TTGGGTTAGGLTTAGGGTTAGGGA | 58.3 ± 0.3 | 0.9 |
| HT-L15 | TTGGGTTAGGGTTALGGTTAGGGA | – | – |
| HT-L16 | TTGGGTTAGGGTTAGLGTTAGGGA | – | – |
| HT-L17 | TTGGGTTAGGGTTAGGLTTAGGGA | 55.1 ± 0.4 | −2.3 |
| HT-L21 | TTGGGTTAGGGTTAGGGTTALGGA | – | – |
| HT-L22 | TTGGGTTAGGGTTAGGGTTAGLGA | – | – |
| HT-L23d | TTGGGTTAGGGTTAGGGTTAGGLA | 60.5 ± 0.1 | 3.1 |
aThe ‘HT-series’ denotes sequences modified from the (3+1) G-quadruplex-forming sequence, while the ‘PS-series’ denotes sequences modified from the (4+0) G-quadruplex-forming sequence.
bResidues with LNA-modified guanosine are denoted as (L).
cThermal stability data were obtained via UV melting (HT-series) and CD melting (PS-series) experiments. Salt conditions were 20 mM KPi for the HT-series and 1.1 mM KPi for the PS-series. Thermal stability data for the HT-series is presented for sequences that demonstrate a single major conformation in NMR spectra. The uncertainties (±values) indicate the hysteresis between heating and cooling curves. Information regarding thermodynamic parameters ΔH, ΔS and ΔG are presented in Supplementary Table S1.
dSequence contains a small secondary melting transition at the low temperature range.
Table 2.
2′F-RNA- and 2′F-ANA-modified sequences used in this study and their thermal stability
| Namea | Sequence (5′→3′)b | Tm(°C)c | ΔTm(°C) |
|---|---|---|---|
| (4+0) native | TTGGGTGGGTGGGTGGGT | 76.5 ± 0.4 | |
| PS-F-3 | TTFGGTGGGTGGGTGGGT | 76.8 ± 0.1 | 0.3 |
| PS-F-4 | TTGFGTGGGTGGGTGGGT | 77.0 ± 0.0 | 0.5 |
| PS-F-5 | TTGGFTGGGTGGGTGGGT | 75.4 ± 0.1 | −1.1 |
| PS-F-8 | TTGGGTGFGTGGGTGGGT | 76.9 ± 0.1 | 0.3 |
| PS-F-11 | TTGGGTGGGTFGGTGGGT | 77.2 ± 0.2 | 0.7 |
| PS-F-12 | TTGGGTGGGTGFGTGGGT | 75.4 ± 0.1 | −1.1 |
| PS-F-13 | TTGGGTGGGTGGFTGGGT | 76.6 ± 0.3 | 0.1 |
| PS-F-17 | TTGGGTGGGTGGGTGGFT | 75.2 ± 0.0 | −1.4 |
| PS-FANA-3 | TTFGGTGGGTGGGTGGGT | 77.1 ± 0.2 | 0.6 |
| PS-FANA-4 | TTGFGTGGGTGGGTGGGT | 79.7 ± 0.0 | 3.2 |
| PS-FANA-5 | TTGGFTGGGTGGGTGGGT | 77.2 ± 0.3 | 0.6 |
| PS-FANA-8 | TTGGGTGFGTGGGTGGGT | 79.7 ± 0.0 | 3.2 |
| PS-FANA-11 | TTGGGTGGGTFGGTGGGT | 77.2 ± 0.2 | 0.6 |
| PS-FANA-12 | TTGGGTGGGTGFGTGGGT | 79.3 ± 0.0 | 2.7 |
| PS-FANA-13 | TTGGGTGGGTGGFTGGGT | 76.6 ± 0.3 | 0.1 |
| PS-FANA-17 | TTGGGTGGGTGGGTGGFT | 76.3 ± 0.2 | −0.2 |
| (3+1) native | TTGGGTTAGGGTTAGGGTTAGGGA | 51.4 ± 0.2 | |
| HT-F-3 | TTFGGTTAGGGTTAGGGTTAGGGA | ||
| HT-F-4 | TTGFGTTAGGGTTAGGGTTAGGGA | 51.6 ± 0.4 | 0.2 |
| HT-F-5 | TTGGFTTAGGGTTAGGGTTAGGGA | 51.2 ± 0.3 | −0.2 |
| HT-F-9 | TTGGGTTAFGGTTAGGGTTAGGGA | ||
| HT-F-10 | TTGGGTTAGFGTTAGGGTTAGGGA | 48.9 ± 1.2 | −2.5 |
| HT-F-11 | TTGGGTTAGGFTTAGGGTTAGGGA | 49.8 ± 0.8 | −1.6 |
| HT-F-15 | TTGGGTTAGGGTTAFGGTTAGGGA | ||
| HT-F-16 | TTGGGTTAGGGTTAGFGTTAGGGA | ||
| HT-F-17 | TTGGGTTAGGGTTAGGFTTAGGGA | 48.7 ± 0.5 | −2.7 |
| HT-F-21 | TTGGGTTAGGGTTAGGGTTAFGGA | ||
| HT-F-22 | TTGGGTTAGGGTTAGGGTTAGFGA | 49.8 ± 0.8 | −1.6 |
| HT-F-23 | TTGGGTTAGGGTTAGGGTTAGGFA | 49.5 ± 0.1 | −1.8 |
| HT-FANA-3 | TTFGGTTAGGGTTAGGGTTAGGGA | ||
| HT-FANA-4 | TTGFGTTAGGGTTAGGGTTAGGGA | 54.5 ± 0.3 | 3.1 |
| HT-FANA-5 | TTGGFTTAGGGTTAGGGTTAGGGA | 51.9 ± 0.6 | 0.5 |
| HT-FANA-9 | TTGGGTTAFGGTTAGGGTTAGGGA | ||
| HT-FANA-10 | TTGGGTTAGFGTTAGGGTTAGGGA | 54.5 ± 0.7 | 3.2 |
| HT-FANA-11 | TTGGGTTAGGFTTAGGGTTAGGGA | 52.9 ± 0.5 | 1.5 |
| HT-FANA-15 | TTGGGTTAGGGTTAFGGTTAGGGA | ||
| HT-FANA-16 | TTGGGTTAGGGTTAGFGTTAGGGA | ||
| HT-FANA-17 | TTGGGTTAGGGTTAGGFTTAGGGA | 53.8 ± 0.0 | 2.4 |
| HT-FANA-21 | TTGGGTTAGGGTTAGGGTTAFGGA | ||
| HT-FANA-22 | TTGGGTTAGGGTTAGGGTTAGFGA | 53.5 ± 0.7 | 2.2 |
| HT-FANA-23 | TTGGGTTAGGGTTAGGGTTAGGFA | 51.9 ± 0.5 | 0.6 |
aThe ‘HT-series’ denotes sequences modified from the (3+1) G-quadruplex-forming sequence, while the ‘PS-series’ denotes sequences modified from a (4+0) G-quadruplex-forming sequence.
bResidues with modified nucleotides are denoted as such: 2′F-RNA-guanosine (F) and 2′F-ANA-guanosine (F).
cThermal stability data were obtained via UV melting experiments. Salt conditions were (5 mM KCl and 5 mM KPi) for the HT-series and (1 mM KPi) for the PS-series. Data for the HT-series are presented only for sequences that demonstrate a single major conformation in NMR spectra. The uncertainties (±values) indicate the hysteresis between heating and cooling curves. Information regarding thermodynamic parameters ΔH, ΔS and ΔG are presented in Supplementary Table S2.
Part I: LNA-guanosine
Substitution of LNAG into a (4+0) parallel G-quadruplex: LNA modifications are detrimental when substituted before short propeller loops
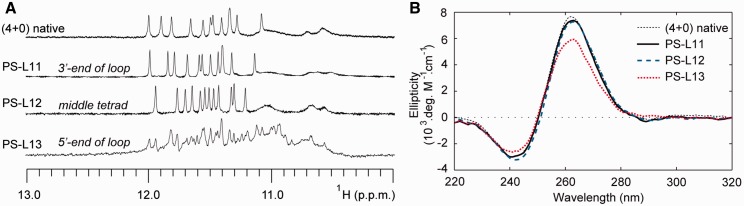
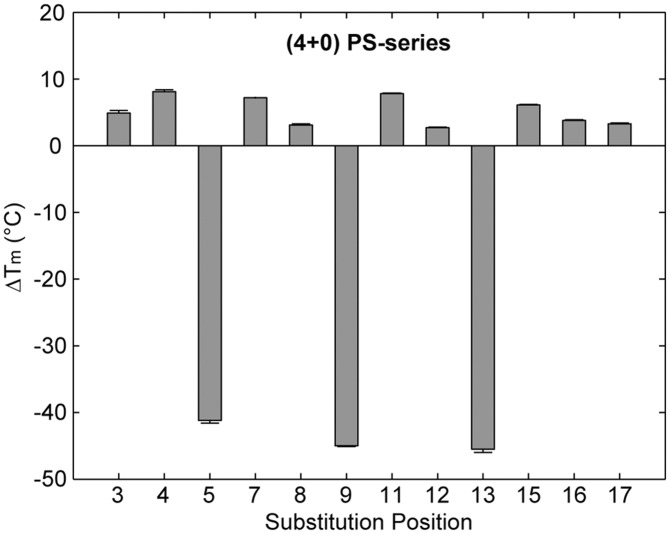
Within the PS-series, 9 of the 12 single-position LNAG-modified sequences were observed by NMR to form a single major G-quadruplex conformation and gave imino proton spectra which were similar to the native sequence (Figure 2A and Supplementary Figure S1). CD spectra of these sequences were characteristic of a (4+0) parallel G-quadruplex conformation, with a maximum at ∼260 nm and a minimum at ∼240 nm (Figure 2B and Supplementary Figure S3). Conversely, substitution of LNAG into positions G5, G9 and G13 lead to multiple conformations in NMR spectra and a reduction in the intensity of CD spectra at 260 nm (Figure 2). Sequences that formed multiple conformations also demonstrated a large decrease in thermal stability with a drop in melting temperature of >40°C (Figure 3 and Supplementary Figures S5–S6). Alternatively, sequences that formed a single conformation displayed an increase in Tm with changes in melting temperature (ΔTm) between +2.7°C and +8.1°C.
Figure 2.
(A) NMR imino proton spectra and (B) CD spectra of select PS-series sequences containing single-position LNAG substitutions on the 3′-end of a loop, middle tetrad or 5′-end of a loop.
Figure 3.
ΔTm of the PS-series sequences containing single-position LNAG modifications with respect to that of the native sequence, as determined by CD melting experiments.
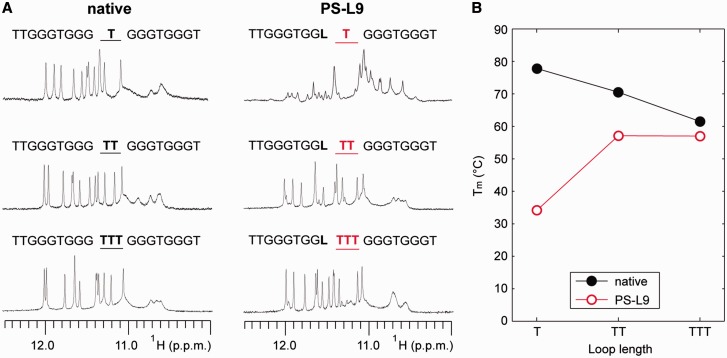
We note that the unfavourable substitutions at positions G5, G9 and G13 are located upstream and adjacent to 1-nt propeller loops within this intramolecular (4+0) G-quadruplex scaffold (Figure 1B). Our results suggest the incompatibility of LNA incorporation into residues preceding short propeller loops. Considering this observation, we proceeded to explore the role of the loop length in the destabilization induced by a LNA modification upstream of propeller loops. We expanded the central 1-nt loop at position T10 to two (TT) and three (TTT) nucleotides for both the native and the LNAG9-modified PS-L9 sequence. The effects of loop elongation were monitored using NMR and CD melting experiments (Figure 4). While NMR spectra indicated a single conformation for all three native sequences, the emergence of a single major conformation was only observed for LNA-modified sequences with the central loop longer than 1-nt. The Tm values of the loop-extended native and LNA-modified PS-L9 sequences converged as the loop length increased. The expansion of the T10 loop in the PS-L9 sequence by a single thymine led to a dramatic recovery of the thermal stability, with an increase in Tm of 23°C. The extension of this loop by a third thymine led to a relatively small increase in stability. At a loop length of 3-nt, the difference in Tm between the loop-extended native and LNA-modified PS-L9 sequences converged to a mere 4.5°C compared with a 43.6°C difference in sequences containing a 1-nt loop.
Figure 4.
Effect of loop length on the compatibility of LNA incorporation preceding a propeller loop. (A) NMR imino proton spectra of (4+0) G-quadruplex–forming sequences with the central loop of different length. The length of the central 1-nt T10 loop of the native DNA sequence and the modified PS-L9 sequence containing a LNA substitution at position 9 were varied, resulting in sequences containing a loop T, TT or TTT. (B) The Tm value of the native and PS-L9 sequences with different loop lengths.
In this study, we show that LNAG substitution upstream of short propeller loops strongly destabilizes G-quadruplex structure. LNAG substitution to positions G5, G9 and G13 in the (4+0) G-quadruplex is observed to be disruptive, despite the ‘anti’ glycosidic conformation of the substituted guanine base. Elongation of these propeller loops is shown to reduce the detrimental effects of LNAG substitutions. It is likely that the conformational constraints on the sugar-backbone geometry imposed by the short propeller loop are incompatible with the conformationally restricted LNA sugar-backbone. The loop-dependent nature of LNAG substitutions into ‘anti’-position guanines is in direct contrast to the FG and FANAG nucleotides discussed in Part II. The disruptive effect of LNAG-modification at the 5′-end of short loops constitutes an important consideration for future design of G-quadruplexes containing LNA modifications.
Substitution of LNAG into a (3+1) hybrid G-quadruplex: LNA modifications generally affect structure in a syn/anti dependent manner
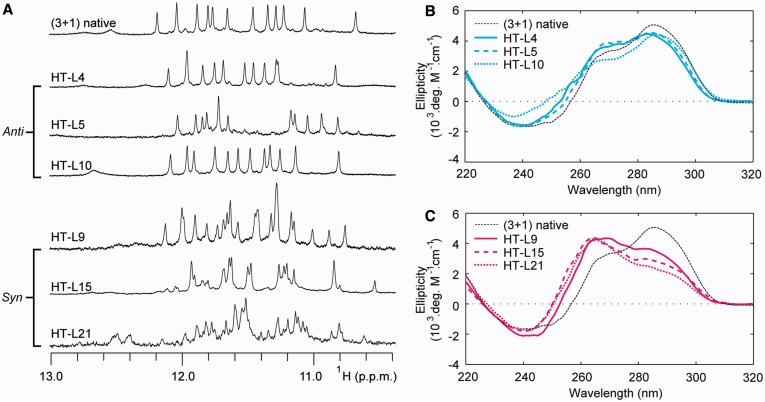
Similar to our study of the (4+0) G-quadruplex scaffold, we systematically substituted LNAG into guanines within the (3+1) G-quadruplex structure formed by the HT-series, which contains guanine residues in both ‘syn’ and ‘anti’ conformations (Figure 1C). Within the HT-series, 7 of the 12 modified sequences demonstrated a single major conformation as monitored by NMR spectra (Figure 5A and Supplementary Figure S2). CD spectra of these sequences displayed the profile of a (3+1) G-quadruplex, similar to that of the native sequence (Figure 5B and Supplementary Figure S4). Conversely, the other five LNAG substitutions induced multiple conformations as observed in NMR spectra (Figure 5A). The CD spectra of these five modified sequences exhibit a profile that deviates from that of the native (3+1) G-quadruplex, with an increase in intensity at 260 nm and a decrease at 295 nm (Figure 5C).
Figure 5.
The ‘syn’/‘anti’ preference of LNA substitutions in the HT-series. (A) NMR imino proton spectra and (B–C) CD spectra are shown for select sequences containing single-position LNA substitutions to (B) ‘anti’ positions and (C) ‘syn’ positions.
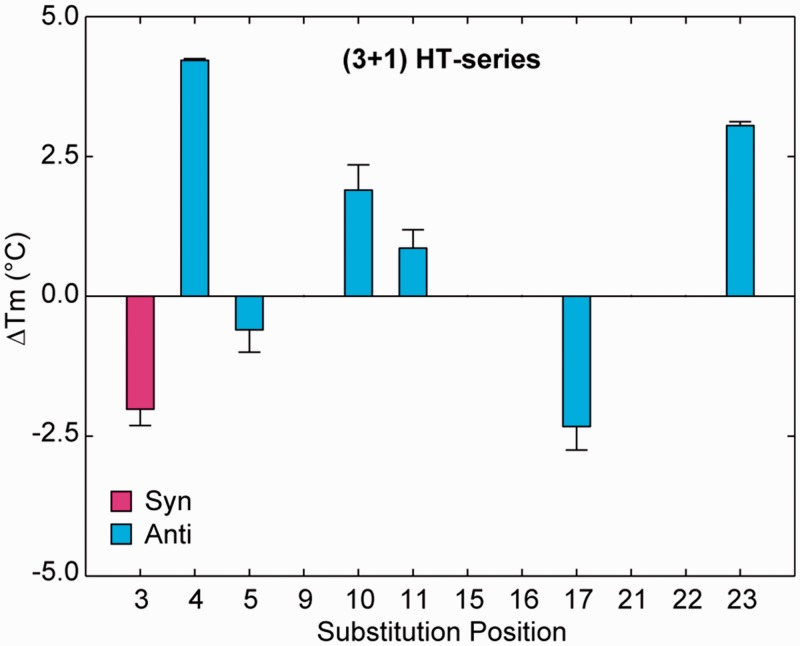
Among the seven modified sequences displaying a single (3+1) G-quadruplex conformation, six sequences involved a LNAG substitution to a guanine in an ‘anti’ glycosidic conformation, and surprisingly, one involved a LNAG substitution to ‘syn’ guanine G3. Substitution with LNAG produced mixed effects on the thermal stability of modified sequences (Figure 6 and Supplementary Figures S7 and S8). Substitution into ‘anti’ guanines led to ΔTm values in the range of −2.3°C to +4.2°C. Substitution to the ‘syn’-position G3 led to a destabilization, with a ΔTm of −2.0°C. Among the five LNAG substitutions that induced multiple conformations, four were made to ‘syn’-position guanines and one was made to ‘anti’-position G22. Interpretation of Tm values for sequences that adopt multiple species is complicated by complex melting curves and uncertainty about which structural transition is being analysed. Therefore we do not discuss the Tm values of these sequences in detail.
Figure 6.
ΔTm of HT-series sequences containing single-position LNAG modifications. Values are plotted for sequences containing single-position LNAG substitutions that demonstrated a single conformation. Error bars indicate the hysteresis between heating and cooling melting curves.
It is generally observed that substitution of LNAG into ‘syn’-position guanines within the HT-series disrupted the folding topology of the G-quadruplex and led to the coexistence of multiple species, while ‘anti’ positions substitutions are generally tolerated. This is in agreement with the findings of previous studies (46–48,50,51). Despite the general ‘syn’/‘anti’ dependence of the LNAG modifications, we note some important exceptions to this trend. As discussed in the above section, substitutions into ‘anti’ guanines directly upstream of 1-nt propeller loops were highly disruptive within the PS-series (Figures 2 and 3). Interestingly, LNAG modification to G5, G11 and G17 within the HT-series, located upstream of a 3-nt propeller or edgewise loop, were the least favourable ‘anti’-position substitutions tested in the (3+1) G-quadruplex with ΔTm values of −0.6°C, +0.9°C and −2.3°C, respectively (Figure 6). Additionally, a substitution of LNAG into an ‘anti’-conformation guanine before a 2-nt edgewise loop has been previously reported to destabilize the TBA (28). These data suggest that LNAG substitutions into ‘anti’-position guanines before many types of loops of short to medium length may have an adverse effect on G-quadruplex stability.
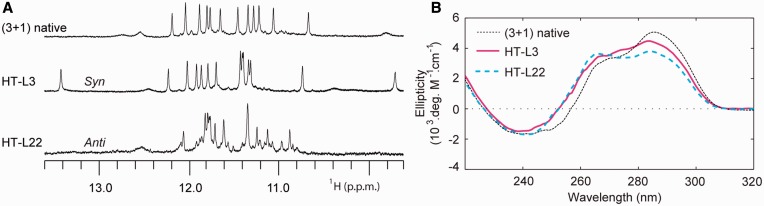
Other exceptions to the ‘syn’/‘anti’ dependence of LNAG modifications are also observed. Within the (3+1) G-quadruplex, LNAG substitution to ‘anti’-position G22 is observed to disrupt the native conformation (Figure 7). It is not yet clear why G22 does not tolerate LNAG modification in this (3+1) G-quadruplex conformation. Additionally, a single instance of ‘syn’-position substitution to residue G3 unexpectedly resulted in a presumably undisrupted (3+1) G-quadruplex conformation (Figure 7). The NMR spectrum of this sequence contains sharp peaks at 9.7 and 13.4 ppm not present in the native (3+1) G-quadruplex, suggesting enhanced external base pairing (Figure 7A). However, this ‘syn’-substitution comes at a cost of reduced thermal stability (Figure 6). The exceptions we observe to the generalized ‘syn’/‘anti’ dependence of LNAG substitutions illustrates the need for cautious substitution of this sugar-modified chemistry into G-quadruplex nucleic acids.
Figure 7.
(A) NMR imino proton spectra and (B) CD spectra are presented for the ‘syn’-position modified HT-L3 sequence and the ‘anti’-position modified HT-L22 sequence.
Part II: 2′F-RNA- and 2′F-ANA-guanosine
2′F-RNA and 2′F-ANA modifications destabilize G-quadruplex DNA in syn positions and are universally tolerated in anti positions
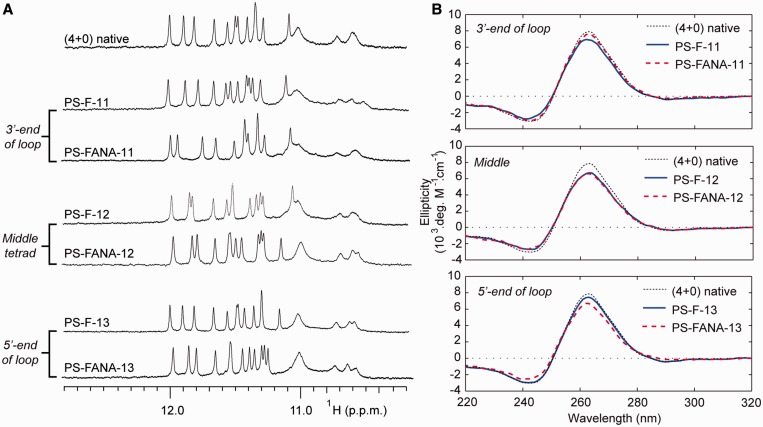
Within the (4+0) G-quadruplex, substitutions were made into eight ‘anti'- position guanines, G3-G5, G8, G11-G13 and G17, to explore a variety of structural environments including guanines adjacent to loops, guanines within the central tetrad layer and guanines toward the flanking ends of the sequence. All of the FG and FANAG modifications into the (4+0) G-quadruplex demonstrated highly similar NMR and CD spectra compared with the unmodified sequence with no sign of disruption to G-quadruplex conformation (Figure 8).
Figure 8.
Incorporation of FG and FANAG substitutions into the (4+0) G-quadruplex scaffold: (A) NMR and (B) CD spectra of select PS-series samples containing single FG and FANAG substitutions to guanines located in a variety of structural environments. FG and FANAG are well tolerated in all positions of the PS-series.
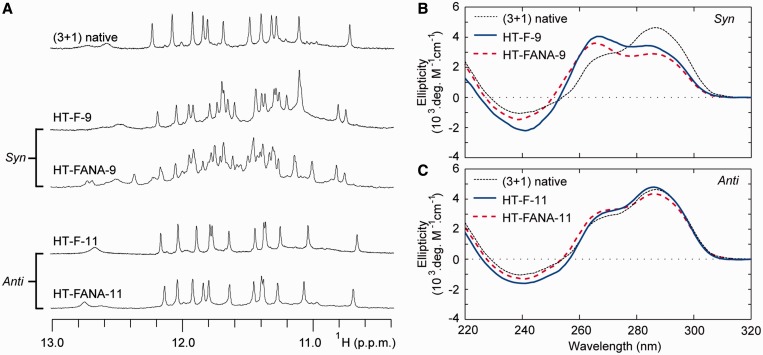
Substitutions of FG and FANAG were also made into the (3+1) G-quadruplex scaffold of the HT-series. The substitutions with guanines that adopt a ‘syn’ conformation in the native (3+1) G-quadruplex resulted in multiple sets of imino proton peaks in NMR spectra (Figure 9 and Supplementary Figures S9 and S10). Furthermore, these sequences generally demonstrated notable changes in CD spectra compared with the unmodified sequence, with a decrease in signal at 295 nm and an increase at 260 nm being observed for most sequences (Figure 9 and Supplementary Figures S13 and S14). Alternatively, FG and FANAG were well tolerated when substituted into ‘anti’-position guanines within the (3+1) G-quadruplex. Sequences containing modifications to ‘anti’-position guanines were observed to form a single major conformation in NMR spectra with chemical shift patterns highly similar to that of the unmodified sequence. CD spectra of these sequences were also similar to that of the unmodified sequence, suggesting that modified sequences maintain the same (3+1) G-quadruplex conformation upon substitution of FG or FANAG (Figure 9).
Figure 9.
The ‘syn'/‘anti' preference of FG and FANAG substitutions into the (3+1) hybrid G-quadruplex: (A) Illustrative NMR spectra of the ‘(3+1) native’ sequence and sequences containing FG or FANAG substitutions to ‘syn’ and ‘anti’ positions. CD spectra are shown for FG and FANAG substitutions to (B) ‘syn’ guanine 9 and (C) ‘anti’ guanine 11.
Collectively, these data suggest that modification of FG or FANAG to ‘syn’-position guanines consistently perturbs the folding topology of the (3+1) G-quadruplex and induces a mixture of conformers. The presence of multiple conformations in NMR spectra of ‘syn’-modified sequences is sometimes found to occur without notable change in CD spectra (Supplementary Figures S13 and S14). Such sequences may adopt a mixture of different (3+1) G-quadruplex folding topologies. Previous studies have shown that modifications of FANAG to multiple ‘syn’-positions within a G-quadruplex scaffold can drive conformational changes (25). Works from our laboratory and others have expanded on this to show that rationally placed FANAG modifications can alter structural equilibrium (26,56). In the current work, we demonstrate that a single substitution of either FG or FANAG into any of the ‘syn’-positions tested is powerful enough to perturb the native (3+1) G-quadruplex folding topology. These findings suggest a general destabilizing characteristic of FG or FANAG substitution into ‘syn’-position guanines.
In contrast, ‘anti’-position substitutions of FG and FANAG chemistries are tolerated in both the (3+1) and (4+0) G-quadruplex scaffolds without disrupting the folding topology. The tolerance of these chemistries in all ‘anti’ positions is interesting considering the wide range of structural environments studied in this work. Comparatively, LNAG is less versatile in its ability to be substituted into ‘anti’ conformation guanines. Our work here suggests that, as a general rule, the FG and FANAG chemistries can be universally incorporated into ‘anti’-position guanines in G-quadruplex DNA.
Substitutions at anti positions: 2′F-ANA-guanosine generally stabilizes G-quadruplex DNA while 2′F-RNA-guanosine induces mixed effects on stability
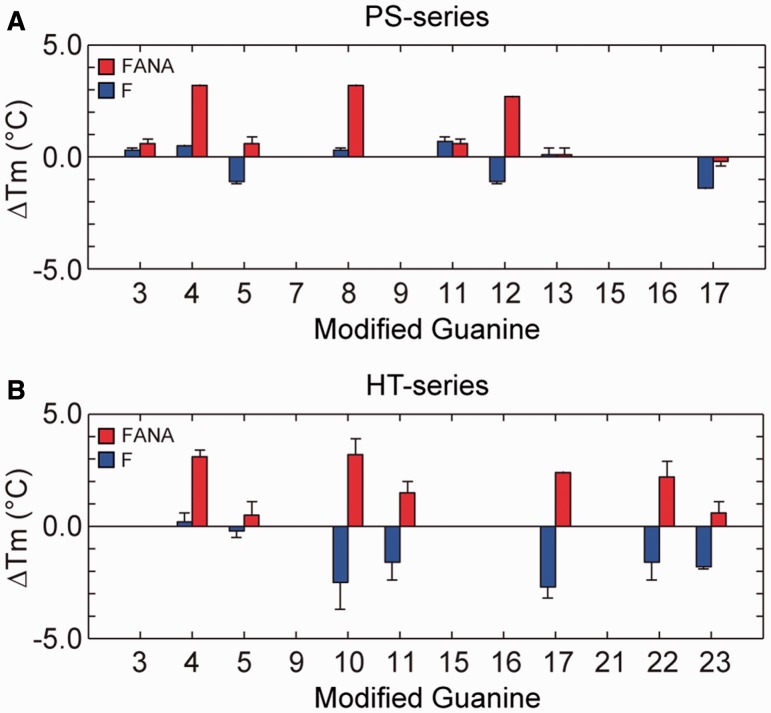
The effects of single-position FG and FANAG substitutions on the Tm values of G-quadruplexes was determined through thermal denaturing experiments monitored by UV absorption spectroscopy (Figure 10). Samples from the PS-series were analysed in 1 mM KPi, while those from the HT-series were analysed in solution containing 5 mM KCl and 5 mM KPi. Only sequences containing single-position substitutions into anti guanines were analysed as they adopt the same conformation as their parent sequences, a prerequisite to a meaningful quantitative comparison of thermal stability.
Figure 10.
ΔTm for single-position FG and FANAG substitutions to (A) select positions in the (4+0) G-quadruplex of the PS-series and (B) ‘anti’ positions in the (3+1) G-quadruplex of the HT-series. Error bars indicate the hysteresis between heating and cooling melting curves.
FANAG substitutions were generally stabilizing within the (4+0) G-quadruplex scaffold with ΔTm values in the range of −0.2 to +3.2°C and moderately stabilizing in the (3+1) G-quadruplex scaffold with ΔTm values in the range of +0.5 to +3.1°C (Figure 10). Substitutions of FANAG into central tetrad layers in both scaffolds were observed to be particularly stabilizing, with ΔTm values of +2.2 to +3.2°C observed in the (3+1) G-quadruplex HT-series and ΔTm values of +2.7 to +3.2°C observed for the (4+0) G-quadruplex PS-series. On the contrary, sequences containing FG substitutions were generally mildly destabilizing in the HT-series with ΔTm values between −2.7 and +0.2°C and were slightly more favourable in the PS-series with ΔTm values between −1.4 and +0.7°C. No clear tetrad-layer preference is observed for FG substitutions.
The results of our thermal stability studies indicate that FANAG modifications generally lead to a moderate increase in stability. FG substitutions are observed to be tolerated within the two scaffolds, generally decreasing the melting temperature of the (3+1) G-quadruplex while having minor and mixed effects on the stability of the (4+0) G-quadruplex. In all ‘anti’ positions tested over both scaffolds, FANAG is observed to be of equal or greater effectiveness in stabilizing a G-quadruplex structure compared with the FG counterpart. This observation is in agreement with recent work describing FANAG to be a more powerful substituent than FG for driving structural equilibrium (26,56). The different effects of FG and FANAG on the thermal stability of modified G-quadruplexes reported in this work may be attributed to a variety of differing structural features of 2′F-RNA and 2′F-ANA chemistries. Firstly, the sugar pucker in 2′F-ANA adopts a South/East orientation similar to DNA, while 2′F-RNA adopts a North orientation (56,67). These nucleotides also differ in their steric penalties and abilities to form intra-residue hydrogen bonds through substituent fluorine atoms (56,67,68). Interestingly, substitutions of FANAG to guanosines in the central G-tetrad of the three-layered (3+1) and (4+0) G-quadruplexes were found to be most stabilizing compared with other positions. Our current study demonstrates that FANAG are generally better tolerated than FG when substituted into the G-tetrad core of G-quadruplex DNA.
CONCLUSION
This work examines the effects of systematic single-substitutions of LNAG, FG and FANAG nucleotides into a (3+1) and a (4+0) G-quadruplex scaffold. (I) We discover that modification of LNAG directly upstream of short propeller loops is highly disruptive to G-quadruplex formation. LNAG substitutions are generally tolerated in a manner dependent on the ‘syn’ or ‘anti’ glycosidic conformation of the guanine base. Substitution of LNAG into most ‘anti’ positions leads to a more stable G-quadruplex, while substitution into most ‘syn’ positions generally disrupts the native (3+1) G-quadruplex conformation. However, we identify some noteworthy exceptions to this generalization in the course of our study. (II) A single modification of FG or FANAG to a ‘syn’ guanine in the (3+1) G-quadruplex perturbs this conformation. Alternatively, substitutions into all ‘anti’-positions are well tolerated and do not disrupt the conformation of the (3+1) or the (4+0) G-quadruplexes, suggesting that these nucleotides are universally well-suited for substitution into ‘anti’-position guanine within the G-tetrad core of G-quadruplexes. FANAG is observed to be more stabilizing than FG in both scaffolds, with FANAG substitutions into central G-tetrad guanines being particularly stabilizing. The insight gained from this work will be valuable to the future design of sugar-modified G-quadruplexes for pharmaceutical and engineering applications.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Singapore Ministry of Education and Nanyang Technological University (to A.T.P.); Nanyang Technological University Undergraduate Research Experience on Campus (URECA) program (to Z.L.). Funding for open access charge: Singapore Ministry of Education.
Conflict of interest statement. None declared.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Mee Chea Chin for her participation in the early stage of this work.
REFERENCES
- 1.Davis JT. G-quartets 40 years later: from 5′-GMP to molecular biology and supramolecular chemistry. Angew. Chem. Int. Ed. 2004;43:668–698. doi: 10.1002/anie.200300589. [DOI] [PubMed] [Google Scholar]
- 2.Burge S, Parkinson GN, Hazel P, Todd AK, Neidle S. Quadruplex DNA: sequence, topology and structure. Nucleic Acids Res. 2006;34:5402–5415. doi: 10.1093/nar/gkl655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel DJ, Phan AT, Kuryavyi V. Human telomere, oncogenic promoter and 5′-UTR G-quadruplexes: diverse higher order DNA and RNA targets for cancer therapeutics. Nucleic Acids Res. 2007;35:7429–7455. doi: 10.1093/nar/gkm711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maizels N, Gray LT. The G4 genome. PLoS Genet. 2013;9:e1003468. doi: 10.1371/journal.pgen.1003468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neidle S, Parkinson G. Telomere maintenance as a target for anticancer drug discovery. Nat. Rev. Drug Discov. 2002;1:383–393. doi: 10.1038/nrd793. [DOI] [PubMed] [Google Scholar]
- 6.Mergny JL, Riou JF, Mailliet P, Teulade-Fichou MP, Gilson E. Natural and pharmacological regulation of telomerase. Nucleic Acids Res. 2002;30:839–865. doi: 10.1093/nar/30.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ribeyre C, Lopes J, Boule JB, Piazza A, Guédin A, Zakian VA, Mergny JL, Nicolas A. The yeast Pif1 helicase prevents genomic instability caused by G-Quadruplex-forming CEB1 sequences in vivo. PLoS Genet. 2009;5:e1000475. doi: 10.1371/journal.pgen.1000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balasubramanian S, Hurley LH, Neidle S. Targeting G-quadruplexes in gene promoters: a novel anticancer strategy? Nat. Rev. Drug Discov. 2011;10:261–275. doi: 10.1038/nrd3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biffi G, Tannahill D, McCafferty J, Balasubramanian S. Quantitative visualization of DNA G-quadruplex structures in human cells. Nat. Chem. 2013;5:182–186. doi: 10.1038/nchem.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez R, Miller KM, Forment JV, Bradshaw CR, Nikan M, Britton S, Oelschlaegel T, Xhemalce B, Balasubramanian S, Jackson SP. Small-molecule-induced DNA damage identifies alternative DNA structures in human genes. Nat. Chem. Biol. 2012;8:301–310. doi: 10.1038/nchembio.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang KY, Mccurdy S, Shea RG, Swaminathan S, Bolton PH. A DNA aptamer which binds to and inhibits thrombin exhibits a new structural motif for DNA. Biochemistry. 1993;32:1899–1904. doi: 10.1021/bi00059a003. [DOI] [PubMed] [Google Scholar]
- 12.Wyatt JR, Vickers TA, Roberson JL, Buckheit RW, Klimkait T, Debaets E, Davis PW, Rayner B, Imbach JL, Ecker DJ. Combinatorially selected guanosine-quartet structure is a potent inhibitor of human-immunodeficiency-virus envelope-mediated cell-fusion. Proc. Natl Acad. Sci. USA. 1994;91:1356–1360. doi: 10.1073/pnas.91.4.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mergny JL, Helene C. G-quadruplex DNA: A target for drug design. Nat. Med. 1998;4:1366–1367. doi: 10.1038/3949. [DOI] [PubMed] [Google Scholar]
- 14.Qi HY, Lin CP, Fu X, Wood LM, Liu AA, Tsai YC, Chen YJ, Barbieri CM, Pilch DS, Liu LF. G-quadruplexes induce apoptosis in tumor cells. Cancer Res. 2006;66:11808–11816. doi: 10.1158/0008-5472.CAN-06-1225. [DOI] [PubMed] [Google Scholar]
- 15.Gatto B, Palumbo M, Sissi C. Nucleic acid aptamers based on the G-quadruplex structure: therapeutic and diagnostic potential. Curr. Med. Chem. 2009;16:1248–1265. doi: 10.2174/092986709787846640. [DOI] [PubMed] [Google Scholar]
- 16.Collie GW, Parkinson GN. The application of DNA and RNA G-quadruplexes to therapeutic medicines. Chem. Soc. Rev. 2011;40:5867–5892. doi: 10.1039/c1cs15067g. [DOI] [PubMed] [Google Scholar]
- 17.Gros J, Rosu F, Amrane S, De Cian A, Gabelica V, Lacroix L, Mergny JL. Guanines are a quartet's best friend: impact of base substitutions on the kinetics and stability of tetramolecular quadruplexes. Nucleic Acids Res. 2007;35: 3064–3075. doi: 10.1093/nar/gkm111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lech CJ, Lim JKC, Lim JMW, Amrane S, Heddi B, Phan AT. Effects of site-specific guanine C8-modifications on an intramolecular DNA G-quadruplex. Biophys. J. 2011;101:1987–1998. doi: 10.1016/j.bpj.2011.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Avino A, Fabrega C, Tintore M, Eritja R. Thrombin binding aptamer, more than a simple aptamer: chemically modified derivatives and biomedical applications. Curr. Pharm. Des. 2012;18:2036–2047. doi: 10.2174/138161212799958387. [DOI] [PubMed] [Google Scholar]
- 20.Sagi J. G-quadruplexes incorporating modified constituents: a review. J. Biomol. Struc. Dyn. 2013;32:477–511. doi: 10.1080/07391102.2013.775074. [DOI] [PubMed] [Google Scholar]
- 21.Haaima G, Hansen HF, Christensen L, Dahl O, Nielsen PE. Increased DNA binding and sequence discrimination of PNA oligomers containing 2,6-diaminopurine. Nucleic Acids Res. 1997;25:4639–4643. doi: 10.1093/nar/25.22.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sacca B, Lacroix L, Mergny JL. The effect of chemical modifications on the thermal stability of different G-quadruplex-forming oligonucleotides. Nucleic Acids Res. 2005;33:1182–1192. doi: 10.1093/nar/gki257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diaz-Mochon JJ, Bialy L, Watson J, Sanchez-Martin RM, Bradley M. Synthesis and cellular uptake of cell delivering PNA-peptide conjugates. Chem. Comm. 2005;26:3316–3318. doi: 10.1039/b503777h. [DOI] [PubMed] [Google Scholar]
- 24.Paul A, Sengupta P, Krishnan Y, Ladame S. Combining G-quadruplex targeting motifs on a single peptide nucleic acid scaffold: a hybrid (3+1) PNA-DNA bimolecular quadruplex. Chem. Eur. J. 2008;14:8682–8689. doi: 10.1002/chem.200800605. [DOI] [PubMed] [Google Scholar]
- 25.Peng CG, Damha MJ. G-quadruplex induced stabilization by 2′-deoxy-2′-fluoro-D-arabinonucleic acids (2′F-ANA) Nucleic Acids Res. 2007;35:4977–4988. doi: 10.1093/nar/gkm520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lech CJ, Li Z, Heddi B, Phan AT. 2′-F-ANA-guanosine and 2′-F-guanosine as powerful tools for structural manipulation of G-quadruplexes. Chem. Comm. 2012;48:11425–11427. doi: 10.1039/c2cc35097a. [DOI] [PubMed] [Google Scholar]
- 27.Tang CF, Shafer RH. Engineering the quadruplex fold: nucleoside conformation determines both folding topology and molecularity in guanine quadruplexes. J. Am. Chem. Soc. 2006;128:5966–5973. doi: 10.1021/ja0603958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonifacio L, Church FC, Jarstfer MB. Effect of locked-nucleic acid on a biologically active G-quadruplex. A structure-activity relationship of the thrombin aptamer. Int. J. Mol. Sci. 2008;9:422–433. doi: 10.3390/ijms9030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zaitseva M, Kaluzhny D, Shchyolkina A, Borisova O, Smirnov I, Pozmogova G. Conformation and thermostability of oligonucleotide d(GGTTGGTGTGGTTGG) containing thiophosphoryl internucleotide bonds at different positions. Biophys. Chem. 2010;146:1–6. doi: 10.1016/j.bpc.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 30.Pasternak A, Hernandez FJ, Rasmussen LM, Vester B, Wengel J. Improved thrombin binding aptamer by incorporation of a single unlocked nucleic acid monomer. Nucleic Acids Res. 2011;39:1155–1164. doi: 10.1093/nar/gkq823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Avino A, Mazzini S, Ferreira R, Gargallo R, Marquez VE, Eritja R. The effect on quadruplex stability of North-nucleoside derivatives in the loops of the thrombin-binding aptamer. Bioorg. Med. Chem. 2012;20:4186–4193. doi: 10.1016/j.bmc.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He GX, Krawczyk SH, Swaminathan S, Shea RG, Dougherty JP, Terhorst T, Law VS, Griffin LC, Coutre S, Bischofberger N. N-2- and C-8-substituted oligodeoxynucleotides with enhanced thrombin inhibitory activity in vitro and in vivo. J. Med. Chem. 1998;41:2234–2242. doi: 10.1021/jm970434d. [DOI] [PubMed] [Google Scholar]
- 33.He GX, Williams JP, Postich MJ, Swaminathan S, Shea RG, Terhorst T, Law VS, Mao CT, Sueoka C, Coutre S, et al. In vitro and in vivo activities of oligodeoxynucleotide-based thrombin inhibitors containing neutral formacetal linkages. J. Med. Chem. 1998;41:4224–4231. doi: 10.1021/jm970766i. [DOI] [PubMed] [Google Scholar]
- 34.Mendelboum Raviv S, Horvath A, Aradi J, Bagoly Z, Fazakas F, Batta Z, Muszbek L, Harsfalvi J. 4-thio-deoxyuridylate-modified thrombin aptamer and its inhibitory effect on fibrin clot formation, platelet aggregation and thrombus growth on subendothelial matrix. J. Thromb. Haemost. 2008;6:1764–1771. doi: 10.1111/j.1538-7836.2008.03106.x. [DOI] [PubMed] [Google Scholar]
- 35.Bishop JS, Guy-Caffey JK, Ojwang JO, Smith SR, Hogan ME, Cossum PA, Rando RF, Chaudhary N. Intramolecular G-quartet motifs confer nuclease resistance to a potent anti-HIV oligonucleotide. J. Biol. Chem. 1996;271:5698–5703. doi: 10.1074/jbc.271.10.5698. [DOI] [PubMed] [Google Scholar]
- 36.Koizumi M, Koga R, Hotoda H, Ohmine T, Furukawa H, Agatsuma T, Nishigaki T, Abe K, Kosaka T, Tsutsumi S, et al. Biologically active oligodeoxyribonucleotides. Part 11: the least phosphate-modification of quadruplex-forming hexadeoxyribonucleotide TGGGAG, bearing 3-and 5-end-modification, with anti-HIV-1 activity. Bioorg. Med. Chem. 1998;6:2469–2475. doi: 10.1016/s0968-0896(98)80021-7. [DOI] [PubMed] [Google Scholar]
- 37.Koizumi M, Akahori K, Ohmine T, Tsutsumi S, Sone J, Kosaka T, Kaneko M, Kimura S, Shimada K. Biologically active oligodeoxyribonucleotides. Part 12: N2-methylation of 2′-deoxyguanosines enhances stability of parallel G-quadruplex and anti-HIV-1 activity. Bioorg Med. Chem. Lett. 2000;10:2213–2216. doi: 10.1016/s0960-894x(00)00432-7. [DOI] [PubMed] [Google Scholar]
- 38.Kuwasaki T, Hatta M, Takeuchi H, Takaku H. Inhibition of human immunodeficiency virus 1 replication in vitro by a self-stabilized oligonucleotide with 2′-O-methyl-guanosine-uridine quadruplex motifs. J. Antimicrob. Chemother. 2003;51:813–819. doi: 10.1093/jac/dkg174. [DOI] [PubMed] [Google Scholar]
- 39.Urata H, Kumashiro T, Kawahata T, Otake T, Akagi M. Anti-HIV-1 activity and mode of action of mirror image oligodeoxynucleotide analogue of Zintevir. Biochem. Biophys. Res. Commun. 2004;313:55–61. doi: 10.1016/j.bbrc.2003.11.094. [DOI] [PubMed] [Google Scholar]
- 40.Pedersen EB, Nielsen JT, Nielsen C, Filichev VV. Enhanced anti-HIV-1 activity of G-quadruplexes comprising locked nucleic acids and intercalating nucleic acids. Nucleic Acids Res. 2011;39:2470–2481. doi: 10.1093/nar/gkq1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh SK, Nielsen P, Koshkin AA, Wengel J. LNA (locked nucleic acids): synthesis and high-affinity nucleic acid recognition. Chem. Comm. 1998;4:455–456. [Google Scholar]
- 42.Sorensen JJ, Nielsen JT, Petersen M. Solution structure of a dsDNA:LNA triplex. Nucleic Acids Res. 2004;32:6078–6085. doi: 10.1093/nar/gkh942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vester B, Lundberg LB, Sorensen MD, Babu BR, Douthwaite S, Wengel J. LNAzymes: incorporation of LNA-type monomers into DNAzymes markedly increases RNA cleavage. J. Am. Chem. Soc. 2002;124:13682–13683. doi: 10.1021/ja0276220. [DOI] [PubMed] [Google Scholar]
- 44.Wahlestedt C, Salmi P, Good L, Kela J, Johnsson T, Hokfelt T, Broberger C, Porreca F, Lai J, Ren KK, et al. Potent and nontoxic antisense oligonucleotides containing locked nucleic acids. Proc. Natl Acad. Sci. USA. 2000;97:5633–5638. doi: 10.1073/pnas.97.10.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petersen M, Wengel J. LNA: a versatile tool for therapeutics and genomics. Trends Biotechnol. 2003;21:74–81. doi: 10.1016/S0167-7799(02)00038-0. [DOI] [PubMed] [Google Scholar]
- 46.Randazzo A, Esposito V, Ohlenschlager O, Ramachandran R, Mayol L. NMR solution structure of a parallel LNA quadruplex. Nucleic Acids Res. 2004;32:3083–3092. doi: 10.1093/nar/gkh629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nielsen JT, Arar K, Petersen M. NMR solution structures of LNA (locked nucleic acid) modified quadruplexes. Nucleic Acids Res. 2006;34:2006–2014. doi: 10.1093/nar/gkl144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pradhan D, Hansen LH, Vester B, Petersen M. Selection of G-quadruplex folding topology with LNA-modified human telomeric sequences in K+ Solution. Chem. Eur. J. 2011;17:2405–2413. doi: 10.1002/chem.201001961. [DOI] [PubMed] [Google Scholar]
- 49.Randazzo A, Esposito V, Ohlenschlager O, Ramachandran R, Virgilio A, Mayol L. Structural studies on LNA quadruplexes. Nucleosides Nucleotides Nucleic Acids. 2005;24:795–800. doi: 10.1081/ncn-200060279. [DOI] [PubMed] [Google Scholar]
- 50.Nielsen JT, Arar K, Petersen M. Solution structure of a locked nucleic acid modified quadruplex: Introducing the V4 folding topology. Angew. Chem. Int. Ed. 2009;48:3099–3103. doi: 10.1002/anie.200806244. [DOI] [PubMed] [Google Scholar]
- 51.Dominick PK, Jarstfer MB. A conformationally constrained nucleotide analogue controls the folding topology of a DNA G-quadruplex. J. Am. Chem. Soc. 2004;126:5050–5051. doi: 10.1021/ja039192z. [DOI] [PubMed] [Google Scholar]
- 52.Deleavey GF, Watts JK, Alain T, Robert F, Kalota A, Aishwarya V, Pelletier J, Gewirtz AM, Sonenberg N, Damha MJ. Synergistic effects between analogs of DNA and RNA improve the potency of siRNA-mediated gene silencing. Nucleic Acids Res. 2010;38:4547–4557. doi: 10.1093/nar/gkq181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pallan PS, Greene EM, Jicman PA, Pandey RK, Manoharan M, Rozners E, Egli M. Unexpected origins of the enhanced pairing affinity of 2′-fluoro-modified RNA. Nucleic Acids Res. 2011;39:3482–3495. doi: 10.1093/nar/gkq1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kawasaki AM, Casper MD, Freier SM, Lesnik EA, Zounes MC, Cummins LL, Gonzalez C, Cook PD. Uniformly modified 2′-deoxy-2′-fluoro phosphorothioate oligonucleotides as nuclease-resistant antisense compounds with high affinity and specificity for RNA targets. J. Med. Chem. 1993;36:831–841. doi: 10.1021/jm00059a007. [DOI] [PubMed] [Google Scholar]
- 55.Kalota A, Karabon L, Swider CR, Viazovkina E, Elzagheid M, Damha MJ, Gewirtz AM. 2′-deoxy-2′-fluoro-beta-D-arabinonucleic acid (2′F-ANA) modified oligonucleotides (ON) effect highly efficient, and persistent, gene silencing. Nucleic Acids Res. 2006;34:451–461. doi: 10.1093/nar/gkj455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martin-Pintado N, Yahyaee-Anzahaee M, Deleavey GF, Portella G, Orozco M, Damha MJ, Gonzalez C. Dramatic effect of furanose C2′ substitution on structure and stability: directing the folding of the human telomeric quadruplex with a single fluorine atom. J. Am. Chem. Soc. 2013;135:5344–5347. doi: 10.1021/ja401954t. [DOI] [PubMed] [Google Scholar]
- 57.Do NQ, Phan AT. Monomer-dimer equilibrium for the 5′-5′ stacking of propeller-type parallel-stranded G-quadruplexes: NMR structural study. Chem. Eur. J. 2012;18:14752–14759. doi: 10.1002/chem.201103295. [DOI] [PubMed] [Google Scholar]
- 58.Luu KN, Phan AT, Kuryavyi V, Lacroix L, Patel DJ. Structure of the human telomere in K+ solution: an intramolecular (3+1) G-quadruplex scaffold. J. Am. Chem. Soc. 2006;128:9963–9970. doi: 10.1021/ja062791w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mergny JL, Phan AT, Lacroix L. Following G-quartet formation by UV-spectroscopy. FEBS Lett. 1998;435:74–78. doi: 10.1016/s0014-5793(98)01043-6. [DOI] [PubMed] [Google Scholar]
- 60.Rachwal PA, Fox KR. Quadruplex melting. Methods. 2007;43:291–301. doi: 10.1016/j.ymeth.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 61.Phan AT, Guéron M, Leroy JL. Investigation of unusual DNA motifs. Methods Enzymol. 2001;338:341–371. doi: 10.1016/s0076-6879(02)38228-4. [DOI] [PubMed] [Google Scholar]
- 62.Plateau P, Guéron M. Exchangeable Proton NMR without base-line distortion, using new strong-pulse sequences. J. Am. Chem. Soc. 1982;104:7310–7311. [Google Scholar]
- 63.Adrian M, Heddi B, Phan AT. NMR spectroscopy of G-quadruplexes. Methods. 2012;57:11–24. doi: 10.1016/j.ymeth.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 64.Crnugelj M, Sket P, Plavec J. Small change in a G-rich sequence, a dramatic change in topology: new dimeric G-quadruplex folding motif with unique loop orientations. J. Am. Chem. Soc. 2003;125:7866–7871. doi: 10.1021/ja0348694. [DOI] [PubMed] [Google Scholar]
- 65.Phan AT, Patel DJ. Two-repeat human telomeric d(TAGGGTTAGGGT) sequence forms interconverting parallel and antiparallel G-quadruplexes in solution: Distinct topologies, thermodynamic properties, and folding/unfolding kinetics. J. Am. Chem. Soc. 2003;125:15021–15027. doi: 10.1021/ja037616j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vorlickova M, Kejnovska I, Sagi J, Renciuk D, Bednarova K, Motlova J, Kypr J. Circular dichroism and guanine quadruplexes. Methods. 2012;57:64–75. doi: 10.1016/j.ymeth.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 67.Martin-Pintado N, Deleavey GF, Portella G, Campos-Olivas R, Orozco M, Damha MJ, Gonzalez C. Backbone FC-H · · · O hydrogen bonds in 2′F-substituted nucleic acids. Angew. Chem. Int. Ed. 2013;52:12065–12068. doi: 10.1002/anie.201305710. [DOI] [PubMed] [Google Scholar]
- 68.Martin-Pintado N, Yahyaee-Anzahaee N, Campos-Olivas R, Noronha AM, Wilds CJ, Damha MJ, Gonzalez C. The solution structure of double helical arabino nucleic acids (ANA and 2′F-ANA): effect of arabinoses in duplex-hairpin interconversion. Nucleic Acids Res. 2012;40:9329–9339. doi: 10.1093/nar/gks672. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.