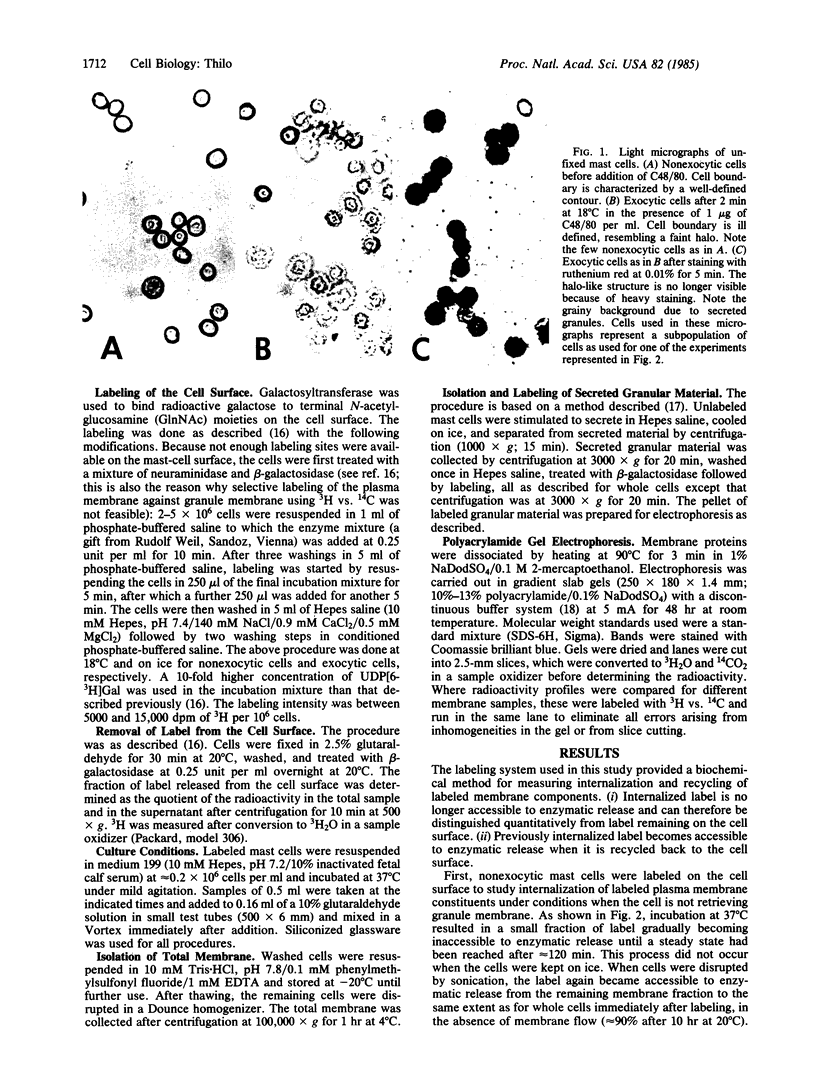
Abstract
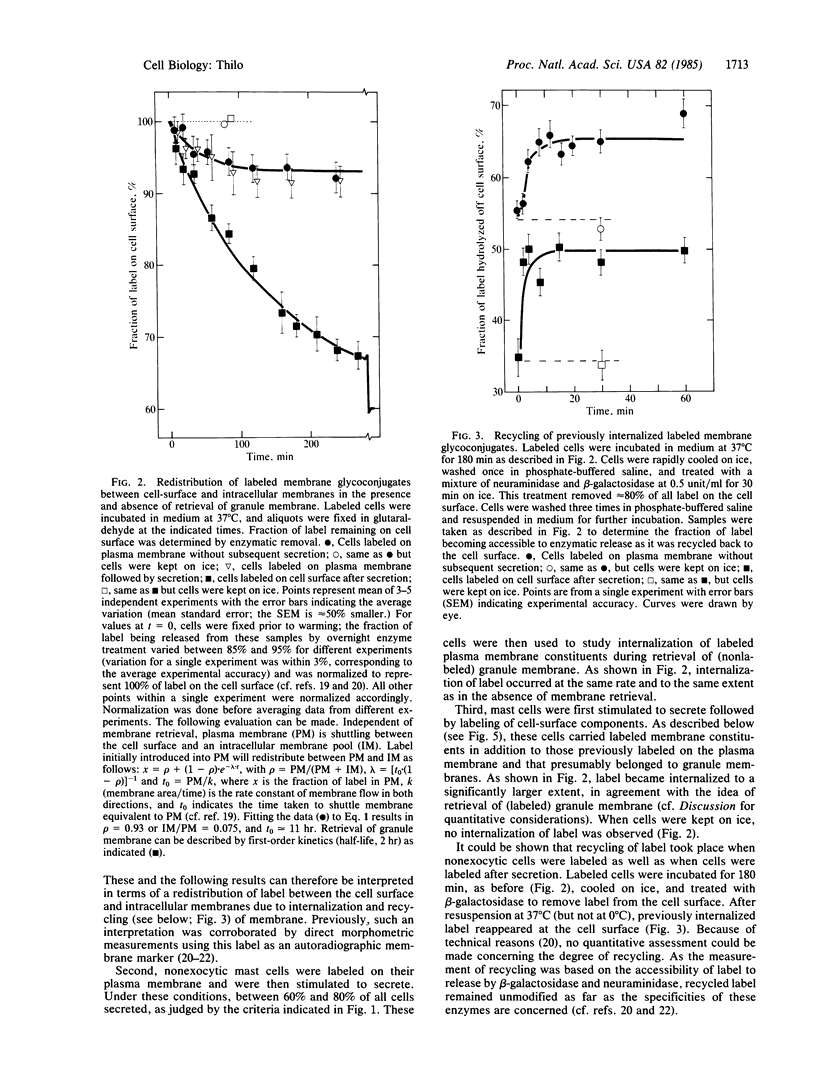
[3H]Galactose, covalently bound to cell surface glycoconjugates of rat peritoneal mast cells, was used to study internalization of labeled plasma membrane and granule membrane constituents before or after secretion stimulated by compound 48/80. Internalized label was distinguished quantitatively from label on the cell surface by its inaccessibility to enzymatic removal. Three different situations were compared. (i) With label only on the plasma membrane, and in the absence of secretion, incubation at 37 degrees C (but not at 0 degree C) resulted in a gradual decrease of label on the cell surface until, after approximately equal to 2 hr, a steady state was reached with 93% of all cell-bound label remaining on the cell surface. Recycling of internalized label was demonstrated. (ii) When cells were labeled on the plasma membrane and then stimulated to secrete, subsequent retrieval of (unlabeled) granule membrane did not affect the rate or extent of simultaneous internalization of labeled plasma membrane. (iii) When both plasma membrane and exposed granule membrane were labeled after secretion, subsequent incubation at 37 degrees C (but not at 0 degrees C) resulted in approximately equal to 33% of all cell-bound label becoming internalized during 4 hr, indicating additional internalization of label due to retrieval of labeled granule membrane. In all three cases, loss of label into the medium occurred with a half-life of 8-11 hr, showing that no extensive shedding of granule membrane occurred after secretion. The results suggest either that no mixing of labeled membrane constituents occurred between the plasma membrane and granule membrane or that during retrieval of granule membrane, sorting of membrane was taking place at the cell surface.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown W. J., Shannon W. A., Jr, Snell W. J. Specific and azurophilic granules from rabbit polymorphonuclear leukocytes. II. Cell surface localization of granule membrane and content proteins before and after degranulation. J Cell Biol. 1983 Apr;96(4):1040–1046. doi: 10.1083/jcb.96.4.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgert H. G., Thilo L. Internalization and recycling of plasma membrane glycoconjugates during pinocytosis in the macrophage cell line, P388D1. Kinetic evidence for compartmentation of internalized membranes. Exp Cell Res. 1983 Mar;144(1):127–142. doi: 10.1016/0014-4827(83)90447-0. [DOI] [PubMed] [Google Scholar]
- Burwen S. J., Satir B. H. Plasma membrane folds on the mast cell surface and their relationship to secretory activity. J Cell Biol. 1977 Sep;74(3):690–697. doi: 10.1083/jcb.74.3.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bytzer P., Nielsen E. H., Clausen J. Surface morphology of rat peritoneal mast cells during in vitro regeneration after histamine secretion. Cell Tissue Res. 1981;216(3):647–654. doi: 10.1007/BF00238659. [DOI] [PubMed] [Google Scholar]
- De Camilli P., Peluchetti D., Meldolesi J. Dynamic changes of the luminal plasmalemma in stimulated parotid acinar cells. A freeze-fracture study. J Cell Biol. 1976 Jul;70(1):59–74. doi: 10.1083/jcb.70.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eady R. A. The mast cells: distribution and morphology. Clin Exp Dermatol. 1976 Dec;1(4):313–321. doi: 10.1111/j.1365-2230.1976.tb01436.x. [DOI] [PubMed] [Google Scholar]
- Johnson A. R., Moran N. C. Release of histamine from rat mast cells: a comparison of the effects of 48-80 and two antigen systems. Fed Proc. 1969 Sep-Oct;28(5):1716–1720. [PubMed] [Google Scholar]
- Krüger P. G., Lagunoff D. Mast cell restoration. A study of the rat peritoneal mast cells after depletion with polymyxin B. Int Arch Allergy Appl Immunol. 1981;65(3):278–290. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lagunoff D. Vital staining of mast cells with ruthenium red. J Histochem Cytochem. 1972 Nov;20(11):938–944. doi: 10.1177/20.11.938. [DOI] [PubMed] [Google Scholar]
- Lawson D., Raff M. C., Gomperts B., Fewtrell C., Gilula N. B. Molecular events during membrane fusion. A study of exocytosis in rat peritoneal mast cells. J Cell Biol. 1977 Feb;72(2):242–259. doi: 10.1083/jcb.72.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludowyke R. I., West G. B. Proteins in the plasma membranes of rat peritoneal mast cells. Agents Actions. 1983 Apr;13(2-3):141–143. doi: 10.1007/BF01967318. [DOI] [PubMed] [Google Scholar]
- Marom Z., Casale T. B. Mast cells and their mediators. Ann Allergy. 1983 Jun;50(6):367–370. [PubMed] [Google Scholar]
- Morré D. J., Kartenbeck J., Franke W. W. Membrane flow and intercoversions among endomembranes. Biochim Biophys Acta. 1979 Apr 23;559(1):71–52. doi: 10.1016/0304-4157(79)90008-x. [DOI] [PubMed] [Google Scholar]
- Nielsen E. H., Bytzer P., Clausen J., Chakravarty N. Electron microscopic study of the regeneration in vitro of rat peritoneal mast cells after histamine secretion. Cell Tissue Res. 1981;216(3):635–645. doi: 10.1007/BF00238658. [DOI] [PubMed] [Google Scholar]
- Nielsen E. H., Clausen J., Bytzer P. Membrane retrieval in non-exocytic and exocytic rat peritoneal mast cells. Exp Cell Res. 1981 Oct;135(2):291–298. doi: 10.1016/0014-4827(81)90165-8. [DOI] [PubMed] [Google Scholar]
- Németh A., Röhlich P. Rapid separation of rat peritoneal mast cells with Percoll. Eur J Cell Biol. 1980 Feb;20(3):272–275. [PubMed] [Google Scholar]
- Palade G. E. Problems in intracellular membrane traffic. Ciba Found Symp. 1982;(92):1–14. doi: 10.1002/9780470720745.ch1. [DOI] [PubMed] [Google Scholar]
- Phillips J. H., Burridge K., Wilson S. P., Kirshner N. Visualization of the exocytosis/endocytosis secretory cycle in cultured adrenal chromaffin cells. J Cell Biol. 1983 Dec;97(6):1906–1917. doi: 10.1083/jcb.97.6.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röhlich P., Anderson P., Uvnäs B. Electron microscope observations on compounds 48-80-induced degranulation in rat mast cells. Evidence for sequential exocytosis of storage granules. J Cell Biol. 1971 Nov;51(21):465–483. doi: 10.1083/jcb.51.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz H., Thilo L. Membrane traffic in Dictyostelium discoideum: plasma membrane glycoconjugates internalized and recycled during fluid phase pinocytosis enter the Golgi complex. Eur J Cell Biol. 1983 Sep;31(2):212–219. [PubMed] [Google Scholar]
- Steinman R. M., Brodie S. E., Cohn Z. A. Membrane flow during pinocytosis. A stereologic analysis. J Cell Biol. 1976 Mar;68(3):665–687. doi: 10.1083/jcb.68.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thilo L. Labeling of plasma membrane glycoconjugates by terminal glycosylation (galactosyltransferase and glycosidase). Methods Enzymol. 1983;98:415–421. doi: 10.1016/0076-6879(83)98169-7. [DOI] [PubMed] [Google Scholar]
- Thilo L., Vogel G. Kinetics of membrane internalization and recycling during pinocytosis in Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1015–1019. doi: 10.1073/pnas.77.2.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Chastellier C., Ryter A., Thilo L. Membrane shuttle between plasma membrane, phagosomes, and pinosomes in Dictyostelium discoideum amoeboid cells. Eur J Cell Biol. 1983 May;30(2):233–243. [PubMed] [Google Scholar]