SUMMARY
Acidic soils comprise a large portion of the Earth’s crust. In this environment, aluminum becomes soluble to plants affecting directly plant development. Among crops, rice is the most Al-resistant but the base of this tolerance is far from being elucidated. In this work, we showed a large-scale profile of Al-responsive genes in rice. Besides, we extended the study in relation to ASR5, a protein previously found by our group as having an important function in Al resistance.
Key words: Aluminum, ChIP-Seq, RNA-Seq, rice, ASR.
Abstract
Aluminum (Al) toxicity in plants is one of the primary constraints in crop production. Al3+, the most toxic form of Al, is released into soil under acidic conditions and causes extensive damage to plants, especially in the roots. In rice, Al tolerance requires the ASR5 gene, but the molecular function of ASR5 has remained unknown. Here, we perform genome-wide analyses to identify ASR5-dependent Al-responsive genes in rice. Based on ASR5_RNAi silencing in plants, a global transcriptome analysis identified a total of 961 genes that were responsive to Al treatment in wild-type rice roots. Of these genes, 909 did not respond to Al in the ASR5_RNAi plants, indicating a central role for ASR5 in Al-responsive gene expression. Under normal conditions, without Al treatment, the ASR5_RNAi plants expressed 1.756 genes differentially compared to the wild-type plants, and 446 of these genes responded to Al treatment in the wild-type plants. Chromatin immunoprecipitation followed by deep sequencing identified 104 putative target genes that were directly regulated by ASR5 binding to their promoters, including the STAR1 gene, which encodes an ABC transporter required for Al tolerance. Motif analysis of the binding peak sequences revealed the binding motif for ASR5, which was confirmed via in vitro DNA-binding assays using the STAR1 promoter. These results demonstrate that ASR5 acts as a key transcription factor that is essential for Al-responsive gene expression and Al tolerance in rice.
INTRODUCTION
Abiotic stress is a major cause of crop failure worldwide, leading to reduced crop productivity, which threatens agricultural sustainability (Mahajan and Tuteja, 2005). Aluminum (Al), an abundant metal in the Earth’s crust, is a component of clay soils. However, under acidic conditions, the trivalent form, Al3+, is solubilized in soil solutions and is highly toxic to plants (Famoso et al., 2010). Because approximately 30%–50% of the world’s arable land shows acidic conditions, Al toxicity is the primary limiting factor in crop productivity (Von Uexkull and Mutert, 1995). The major effect of Al toxicity is inhibition of root elongation, leading to poor ion and water uptake (Barcelo and Poschenrieder, 2002).
Over the course of evolutionary history, many plants have developed mechanisms that permit them to tolerate Al toxicity. These mechanisms are classified as either external or internal tolerance mechanisms (Kochian et al., 2004). External mechanisms, such as root exudation of organic acids (OAs) that bind to Al and prevent its entrance into cells, have been well characterized in several species, such as wheat, sorghum, and maize (Kochian et al., 2004). Internal mechanisms, such as compartmentalization of Al in the vacuole, have also been demonstrated in some species (Ma et al., 1997; Zheng et al., 1998; Wenzl et al., 2002).
Different species exhibit different levels of Al tolerance, and rice (Oryza sativa) is one of the most Al-resistant crops under field conditions (Foy, 1988). In general, rice is approximately two to five times more Al-tolerant than wheat, sorghum, or maize (Famoso et al., 2010). Al tolerance is mediated by Al-responsive gene expression (Tsutsui et al., 2012). For example, the Al-induced genes STAR1 and STAR2 encode an ATP-binding protein and a transmembrane domain protein, respectively. The STAR1–STAR2 complex transports UDP-glucose, a substrate used to modify the cell wall and mask Al-binding sites (Huang et al., 2009). Nrat1, a natural resistance-associated macrophage protein (Nramp), encodes an Al transporter (Xia et al., 2010), whereas FRDL4, a multidrug and toxic compound extrusion (MATE) protein, encodes an Al-induced citrate transporter that is involved in citrate secretion (Yokosho et al., 2011). These studies indicate that rice may display both external and internal Al detoxification mechanisms as well as additional unknown mechanisms to manage Al toxicity. Al-responsive genes have also been identified in other species such as Arabidopsis (Kumari et al., 2008; Goodwin and Sutter, 2009), Aspen (Grisel et al., 2010), Medicago truncatula (Chandran et al., 2008), common bean (Eticha et al., 2010), maize (Maron et al., 2008; Mattiello et al., 2010), and soybean (Guo et al., 2007; Houde and Diallo, 2008; Duressa et al., 2010; You et al., 2011). Nevertheless, little is known about the mechanisms of Al signal transduction that mediate Al-responsive gene expression. In rice, the transcription factor ART1 has been identified as an essential component of Al-responsive gene expression (Yamaji et al., 2009). However, the level and localization of ART1 are not affected by Al treatment, and it was therefore suggested that additional Al-regulated factors might be required for ART1 activation and Al-responsive gene expression (Yamaji et al., 2009).
The Abscisic Acid, Stress and Ripening (ASR) proteins constitute a low-molecular-weight, highly hydrophilic plant-specific protein family. Members of this family have been shown to be involved in processes such as fruit development (Çakir et al., 2003; Chen et al., 2011), abiotic stress (Vaidyanathan et al., 1999; Sugiharto et al., 2002; Yang et al., 2004; Kim et al., 2009) and biotic stress (Liu et al., 2010). Some ASR proteins present chaperone activity (Konrad and Bar-Zvi, 2008) and also may participate in transcriptional regulation (Çakir et al., 2003; Yang et al., 2008; Kim et al., 2009; Hsu et al., 2011).
We have previously shown that the ASR5 (LOC_Os11g06720) protein is localized in the chloroplast (Arenhart et al., 2012), cytoplasm, and nucleus (Arenhart et al., 2013). ASR5 transcript levels increase in response to Al in the roots and shoots, and ASR5-silenced plants are extremely sensitive to Al (Arenhart et al., 2013). To understand the mechanisms by which rice tolerates toxic Al concentrations, it is important to determine how ASR5 acts in the Al response pathway. In this study, we performed genome-wide transcriptome and protein–DNA interaction analyses of ASR5-mediated Al responses using ASR5_RNAi in plants. We show that ASR5 regulates a large number of Al-responsive genes, including STAR1.
RESULTS
Promoter Analysis Reveals a Correlation between ASR5 Expression and Its Role in the Al-Tolerance Mechanisms
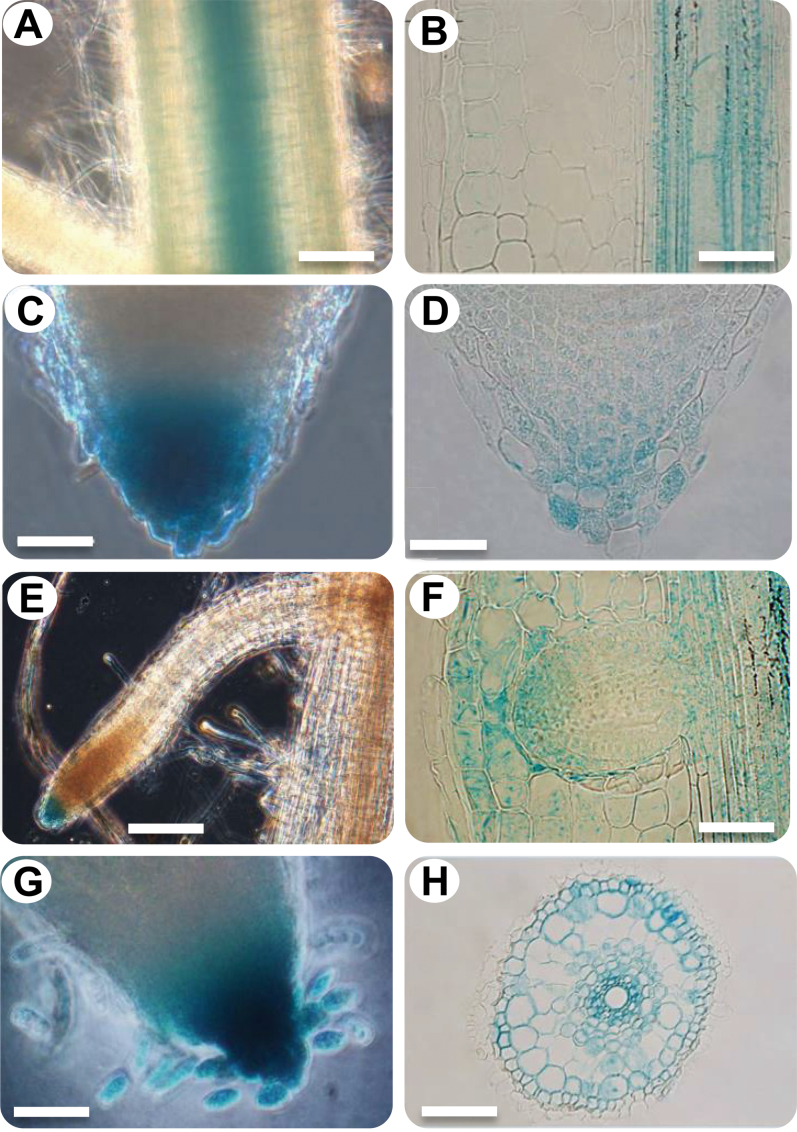
Previous analysis has shown that the ASR5 transcript level increases in response to Al (Arenhart et al., 2013). To characterize the tissue-specific expression conferred by the ASR5 promoter, a construct containing the 2060 base pairs upstream of the rice ASR5 start codon fused with GUS was transformed into rice. Regenerated plants from non-transformed calli were used as negative controls. GUS activity was detected in the vascular tissue of the root (Figure 1A and 1B), root cap (Figure 1C and 1D), lateral root cap (Figure 1E and 1F), and cells detaching from the root cap (root border cells) (Figure 1G). Transverse sections revealed that GUS was also expressed in the cells of the exodermal layer and the cortex and in the parenchymatous cells of the xylem and the companion cells of the phloem in the vascular tissue (Figure 1H). Lateral root emergence mechanically damages the cortical cells adjacent to the apical zone, and GUS expression was stronger in the cortical cells in the apical zone than in other cortical cells (Figure 1F). Under Al stress, there was a slight increase in GUS expression in the roots (Supplemental Figure 1).
Figure 1.
Expression Pattern of ASR5 prom:GUS in Rice Roots.
(A) View of the root elongation zone.
(B) Longitudinal section of the root elongation zone.
(C) Macroscopic view of the root cap.
(D) Longitudinal section of the root cap.
(E) Root cap of the lateral root and (F) mechanical damage in the cortical cells.
(G) Root cap with unstructured cells (root border cells).
(H) Transverse section of the root elongation zone showing a GUS-positive reaction in the exodermal cells (arrow), cortex, pericycle, parenchymatic cells of the xylem, and companion cells of the phloem. The bars in A = 150 μm, B = 50 μm, C = 50 μm, D = 50 μm, E = 100 μm, F = 100 μm, and G = 100 μm.
In the leaves, expression of ASR5prom:GUS was detected in vascular tissues (Supplemental Figure 2A) and in response to mechanical damage (Supplemental Figure 2B). In floral tissues, GUS was expressed in the vascular tissues of the anther (Supplemental Figure 2C), stigma (Supplemental Figure 2D), palea and lemma (Supplemental Figure 2E), and it was also detected in the trichomes of the palea and lemma (Supplemental Figure 2F).
Global Transcriptome Analysis Reveals a Central Role for ASR5 in Al-Responsive Gene Expression in Rice
In a previous study, we showed that ASR5-silenced plants (ASR5_RNAi) present high sensitivity to Al (Arenhart et al., 2013) (Supplemental Figure 3A). Furthermore, the function of ASR5 was found to be specific to Al, as cadmium treatment did not trigger different responses in wild-type non-transformed (NT) versus ASR5_RNAi plants (Arenhart et al., 2013). To understand the molecular function of ASR5, we performed transcriptome analysis of the ASR5_RNAi plants. NT and ASR5_RNAi transgenic plants were cultivated under control conditions for 12 d and then treated with a control solution (Cnt) or AlCl3 (Al, 450 μM) for 8h. The plant roots were harvested to generate four RNA libraries for RNA-Seq analysis. Illumina RNA-Seq generated a total of 9497649 reads mapped to the Rice Genome Annotation, version 7.0 for the NT_Cnt sample; 11257398 for the NT_Al sample; 2013886 for the ASR5_RNAi_Cnt sample; and 1831659 for the ASR5_RNAi_Al sample. Digital gene expression (DGE) determined from the counted reads was normalized via a statistical analysis performed using edgeR (Robinson et al., 2010) for the four libraries and was employed to perform three comparisons, as follows: (1) NT_Cnt versus NT_Al, (2) ASR5_RNAi_Cnt versus ASR5_RNAi_Al, and (3) NT_Cnt versus ASR5_RNAi_Cnt. Using this statistical method, we normalized the data, despite the variation in the number of uniquely mapped reads (Supplemental Figure 3B–3D). The ASR5 gene was up-regulated in response to Al in the NT plants and was silenced in the ASR5_RNAi plants (Supplemental Table 1). The raw data from these experiments are provided in the Supplemental Information 1.
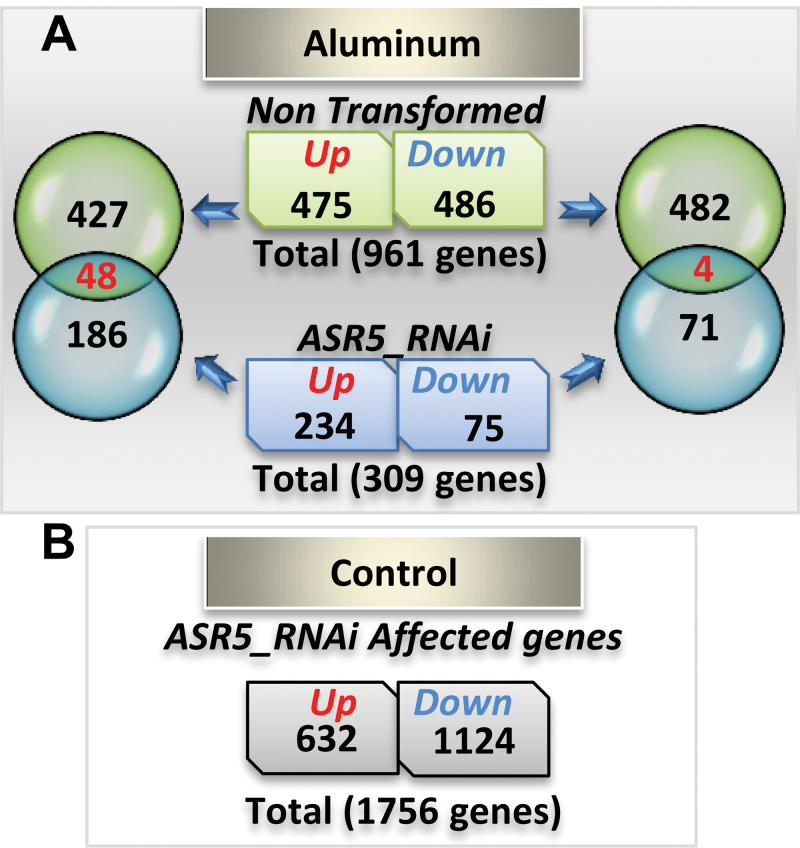
A total of 961 genes were differentially expressed in rice roots in response to Al treatment in the NT plants (475 up-regulated and 486 down-regulated). These included 72 genes (51 up- and 21 down-regulated) previously shown to be affected by a low concentration of Al (20 μM) in wild-type rice (Tsutsui et al., 2012) (Supplemental Information 1). In contrast, in the ASR5_RNAi plants, only 309 genes showed Al-induced differential expression (234 up-regulated and 75 down-regulated) (Figure 2A). Only 48 genes were up-regulated and 4 genes were down-regulated by Al treatment in both the NT and ASR5_RNAi plants (Figure 2A). Thus, approximately 95% of the rice genes regulated by Al in the NT plants did not respond similarly to Al treatment in the ASR5_RNAi plants (Figure 2A). A set of 23 known Al-responsive genes showed similar Al response patterns in the NT plants, as previously reported (Table 1) (Zhang et al., 2007; Huang et al., 2009; Yamaji et al., 2009; Dong et al., 2010; Krill et al., 2010; Xia et al., 2010; Zhang et al., 2010; Yokosho et al., 2011). However, 22 (95%) of these 23 genes did not respond to the Al treatment, or they responded in an opposite manner in the ASR5_RNAi plants. Therefore, the RNA-Seq data demonstrate an essential role of ASR5 in Al-responsive gene expression.
Figure 2.
Al-Responsive Genes in Non-Transformed (NT) and ASR5_RNAi Plants.
(A) Venn diagram showing the overlap of the Al-responsive up- and down-regulated genes between the NT and ASR5_RNAi plants.
(B) Number of genes affected by ASR5 silencing in the ASR5_RNAi plants.
Under normal growth conditions, without Al treatment, the ASR5_RNAi plants expressed 1.756 genes differentially (632 up-regulated and 1.124 down-regulated) compared to the NT plants, 446 of which responded to Al treatment in the wild-type plants, suggesting that ASR5 plays additional roles, independently of Al stress (Figure 2B and Supplemental Figure 3D). A hierarchical clustering and heat-map analysis showed that the expression patterns observed in the NT and ASR5_RNAi plants in the presence of Al clustered separately and revealed that hundreds of genes were affected by ASR5 silencing and showed clear opposite patterns when Al-responsive genes were compared in the NT and ASR5_RNAi plants (Supplemental Figure 3E).
Because basipetal transport of exogenous [3H] indole-3-acetic acid applied to the meristematic zone is significantly inhibited by Al application in the root apex DTZ (Distal Transition Zone) in the Al-sensitive maize cv. Lixis (Kollmeier et al., 2000), we searched our RNA-Seq data for auxin-related genes in the NT and RNAi plants that were responsive to Al (Supplemental Figure 4). We found one auxin response factor and an auxin-repressed protein gene showing increased transcript levels in response to Al only in NT plants (LOC_Os02g06910 and LOC_Os03g22270). Another auxin-repressed protein (LOC_Os11g44810) was increased in NT but down-regulated in RNAi plants. Furthermore, three auxin-induced proteins (LOC_Os05g01570, LOC_Os08g44750, and LOC_Os01g58910) and one auxin efflux carrier (LOC_Os02g50960) did not respond to Al but were down-regulated in the ASR5_RNAi plants.
A gene ontology (GO) search for biological processes within the RNA-Seq data set using the website software programs AgriGO (Du et al., 2010) and ReviGO (Supek et al., 2011) revealed several enriched terms. A total of 83 GO terms were found for genes that were up-regulated by Al in the NT plants. Some of the enriched GOs included programmed cell death (PCD)-like apoptotic processes, response to stress, signaling, and ion transport (Supplemental Figure 5A). Among the down-regulated genes, 131 GO terms were enriched, covering a wide range within the classes including primary metabolism, sugar pathways, cellulose biosynthesis (including glucan and carbohydrate biosynthetic processes and glucose and hexose metabolic processes), and cellular macromolecule complex assembly (including chromatin assembly, chromosome organization, and DNA packing) (Supplemental Figure 5B). The GO terms for the genes that were up-regulated in the ASR5_RNAi plants in response to Al treatment were enriched in classes including carbohydrate metabolic processes, molecule catabolic processes, signal transduction, and amine biosynthetic processes. All of the GO terms are included in Supplemental Information 2. Under the same experimental conditions, no significant terms were found for the 75 genes that were down-regulated by Al in the ASR5_RNAi plants when using the same threshold.
We also performed GO searches for the 632 genes that were up-regulated and the 1.124 genes that were down-regulated by ASR silencing. Due the large number of genes, we performed these analyses using only the GO terms that presented a p-value, Log10 of lower than 0.0009. We retrieved 33 and 99 GO terms for the up- and down-regulated genes, respectively. The genes that were up-regulated due to ASR silencing were enriched in PCD (apoptosis) compared to the reference genome (Supplemental Figure 5C). The down-regulated genes belong to categories including cell-wall macromolecule catabolism, response to stress, oxidative stress, chemical stimulus, and antioxidant activity.
ASR5 Silencing Affects STAR1 Expression
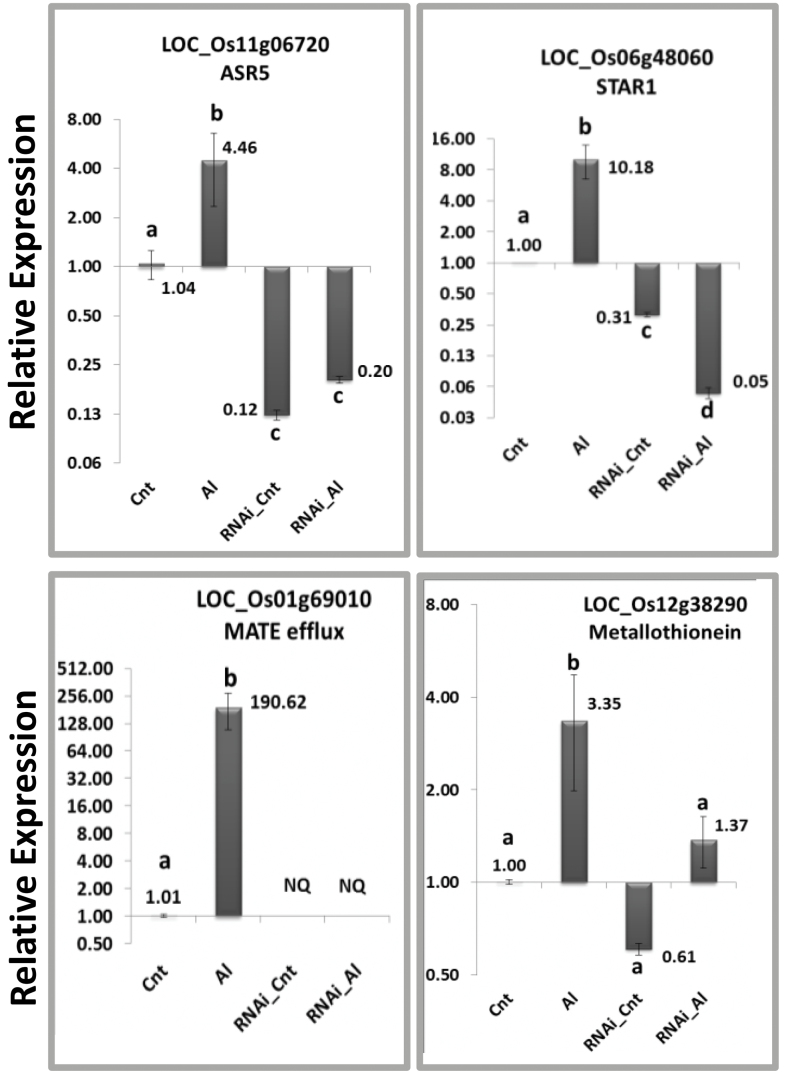
To identify downstream genes responsible for ASR5-mediated Al tolerance, we searched the RNA-Seq data set for rice Al-specific up-regulated genes with orthologs and homologs reported in the literature. We first searched for gene families whose transcript levels were increased in response to Al treatment in the NT plants, but not in the ASR5_RNAi plants. The genes that fit these criteria and were repressed in the ASR5_RNAi plants were also included. Using this approach, we found four ABC transporter genes showing Al-induced expression only in the NT plants (LOC_Os01g50100, LOC_Os03g54790, LOC_Os04g49900, and STAR1/LOC_Os06g48060). STAR1 was the only ABC gene whose transcript level was increased in the Al-treated NT plants but decreased in the ASR5_RNAi plants compared to the untreated NT plants (Supplemental Figure 6). To confirm the RNA-Seq results, real-time RT–qPCR experiments were performed to validate the expression patterns of 19 genes (including genes responsive to Al described in the literature, such as ASR5, STAR1, MATE, and a metallothionein) in NT and ASR5_RNAi plants that were treated with AlCl3 (450 μM) for 8h (Figure 3 and Supplemental Figure 7).
Figure 3.
Quantitative Real-Time RT–PCR of Four Selected Genes from the RNA-Seq Analysis.
Total RNA was extracted from the roots and used to synthesize cDNA. The relative expression was plotted using the expression levels of the FDH and Actin 2 genes as a reference. The roots of the Nipponbare cultivar were collected after 8h of treatment with AlCl3 (450 μM). The bars with different letters are significantly different (ANOVA, P < 0.05). Cnt, non-transformed plants under control conditions; Al, non-transformed plants under aluminum treatment; RNAi_Cnt, ASR5_RNAi plants under control conditions; RNAi_Al, ASR5_RNAi plants under aluminum treatment; NQ, not quantified.
Identification of In Vivo ASR5-Binding Sites
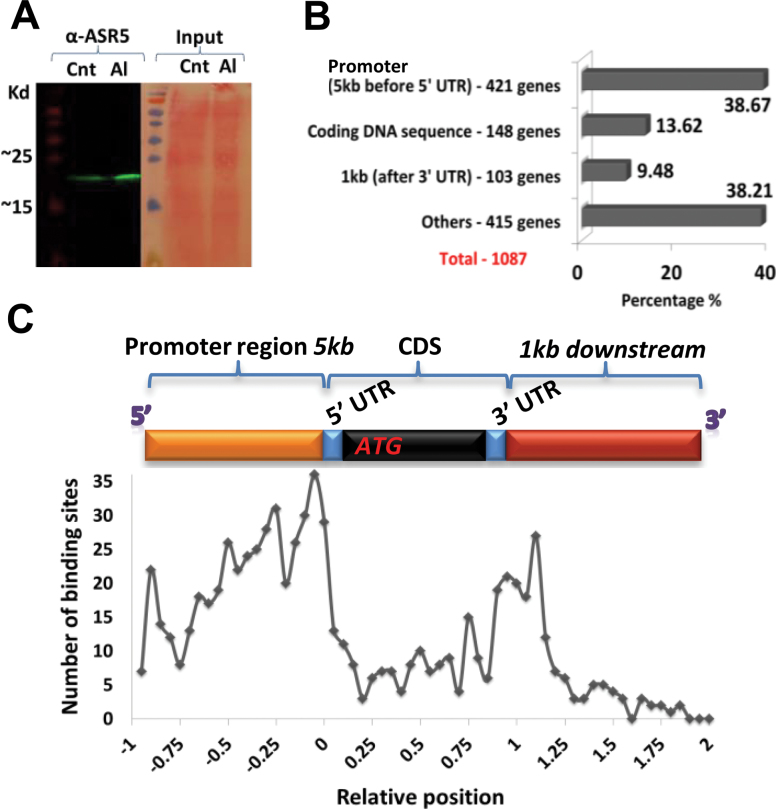
Because ASR5_RNAi plants are hypersensitive to Al (Arenhart et al., 2013) and an ASR protein was shown to act as a transcription factor in grape (Çakir et al., 2003), we searched for in vivo DNA-binding sites for ASR5. To accomplish this task, multiple chromatin-immunoprecipitation (ChIP) experiments (biological triplicates) were performed using an anti-ASR5 antibody in the roots of NT rice plants (12 d old) that were treated with AlCl3 (450 μM) for 8h at pH 4.5. The obtained DNA samples were then combined to perform ChIP-Seq analysis. The control ChIP experiments were performed using pre-immune serum. The anti-ASR5 antibody specifically detected increased ASR5 protein levels in response to Al treatment (Figure 4A). Sequencing of the anti-ASR5 ChIP DNA libraries generated 6.0 million reads, and the control ChIP experiments generated 1.74 million reads that mapped to the Rice Genome Annotation Version 7.0 (35bp/read with unique and up to two mismatches) using the MACS software program (Zhang et al., 2008). A total of 649 binding peaks led to the identification of 1.087 loci in the ChIP-Seq binding peak analyses, which were proposed as potential ASR5 target genes, including 421 loci (38.7%) that contained binding peaks in the promoter region (5000-bp upstream region), 148 loci (13.62%) in the transcribed region (5′UTR, CDS, or 3′UTR), and 518 loci (47.69%) outside of genes (Figure 4B and Supplemental Information 3). Consistently with a transcription factor function, most of the binding peaks were distributed near transcription start sites (Figure 4C). When a binding site was located in the gene body region (5kb upstream of the 5′UTR and 1kb downstream of the 3′UTR), the gene was designated as a potential ASR5 target gene. If the binding site was not located inside a gene body region, the nearest upstream gene and the nearest downstream gene to this binding site were included as potential ASR5 target genes.
Figure 4.
ChIP-Seq Analysis of ASR5 Target Genes in Al-Treated Rice Plants.
(A) Western blot showing increased ASR5 protein levels in rice plants in response to Al. ASR5 was detected with anti-ASR5. (Cnt) indicates the control untreated plants, whereas (Al) indicates the plants that were treated with 450 μM AlCl3 for 8h.
(B) Number and percentage of loci found in each binding region.
(C) Distribution of the binding sites. The x-axis displays the relative distance; the promoter region group is indicated in the top yellow bar; the coding region group is indicated by top blue and black bars; and the downstream region group is indicated by the top red bar. The y-axis displays the number of binding sites located in the different groups.
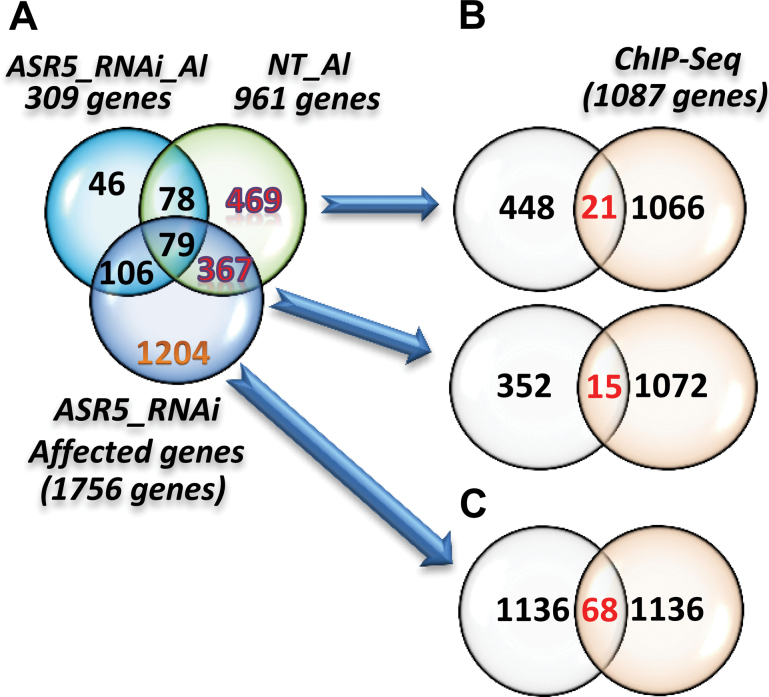
The list of ASR5 target genes was compared to the genes that responded to Al in the NT plants but not in the ASR5_RNAi plants in the RNA-Seq experiments (Figure 2A). This comparison identified 36 genes that were Al-responsive in an ASR5-dependent manner and that were bound by ASR5 in vivo (Figure 5A). Among these genes, 21 were unaffected, and 15 were affected in the ASR5_RNAi plants without Al treatment, including STAR1 (Supplemental Information 4). In addition, 68 ASR5 target genes were affected by ASR5_RNAi but were not responsive to Al treatment (Figure 5B and Supplemental Information 4). Together, these results identified 104 genes that were bound by ASR5 in vivo and were affected by Al treatment and/or silencing of ASR5. We consider these genes to be functional targets of ASR5.
Figure 5.
Overlap between the Genes Affected by ASR5_RNAi or Al Treatment and the Genes that Bound to ASR5 (ChIP-Seq Loci).
(A) Venn diagram showing the overlap of the Al-responsive genes between the non-transformed (NT) and ASR5_RNAi plants and the genes affected by ASR silencing.
(B) Venn diagram showing the overlap between the 469 Al-responsive genes found in the NT plants and ChIP-Seq loci. Venn diagram showing the overlap between the 367 Al-responsive genes found in the NT plants and those affected in the ASR5_RNAi plants due to ASR silencing with ChIP-Seq loci.
(C) Venn diagram showing the overlap between the 1.204 genes that were affected by ASR5 silencing but were unresponsive to Al.
Identification of the ASR5 DNA-Binding Motif and Binding Sites in the STAR1 Promoter Region
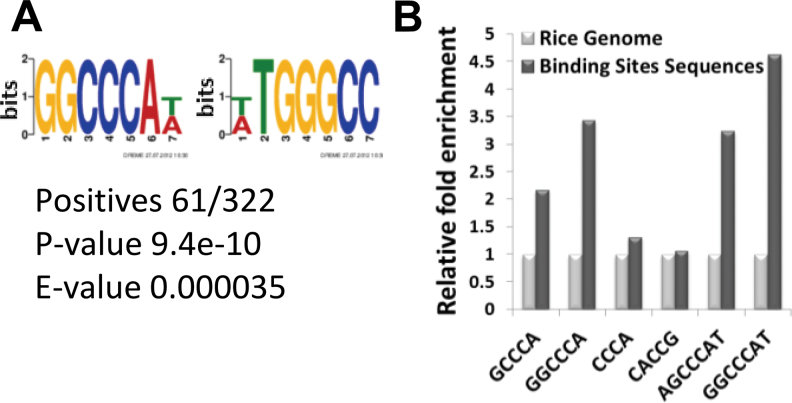
To identify potential ASR5-binding motifs, we used the DREME (Discriminative DNA Motif Discovery) tool (Bailey, 2011) to search for statistically overrepresented motifs in ASR5-binding regions base on the ChIP-Seq data. First, 649 binding sequences were used, which included all of the binding sites from the ChIP-Seq data set. The DREME analysis identified 10 potential enriched motif sequences (Supplemental Figure 8). The analysis was then repeated using only the binding peaks found in the promoter region (5kb, 322 sequences). This strategy allowed the identification of a consensus sequence GGCCCA(T/A) (Figure 6A). The sequences GGCCCA, AGCCCAT, and GGCCCAT were enriched in the ChIP-Seq data set compared to the rice genome (Figure 6B). However, the CACCG motif, which serves as the binding site for the ABI4 transcription factor in Arabidopsis (Shkolnik and Bar-zvi, 2008), was not enriched in the ASR5-bound sequences (Figure 6B).
Figure 6.
Discriminative Motif Discovery in the ASR5 ChIP-Seq Data Set.
(A) The most significant motif identified using only the promoter regions of the binding peaks from the ChIP-Seq data identified by DREME.
(B) Enrichment of possible motifs identified by DREME compared to the reference rice genome.
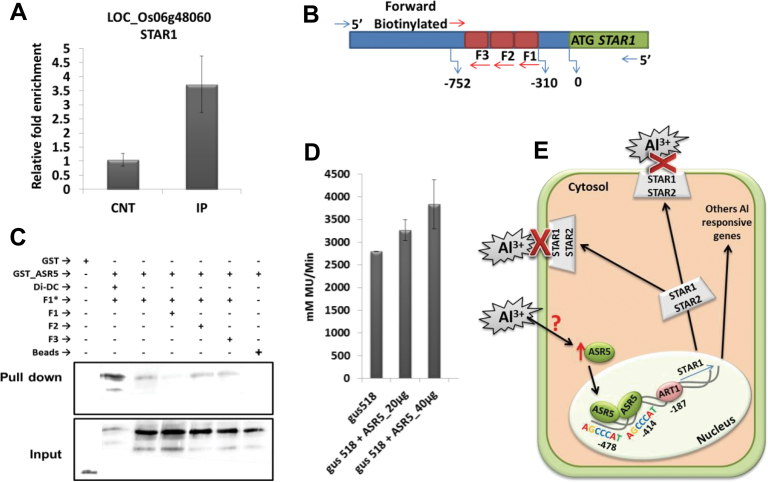
ChIP–qPCR experiments confirmed the in vivo binding of ASR5 to the STAR1 promoter region (Figure 7A). This region contains two ASR5-binding motifs (AGCCCAT) separated by 58bp. We further analyzed the binding of ASR5 to the STAR1 promoter through an in vitro DNA pull-down assay (Wu, 2006). A biotinylated DNA fragment containing the 442 base pairs of the STAR1 promoter region centered on the ASR5 ChIP-Seq peak was incubated with the GST–ASR5 protein or GST itself. The DNA-bound proteins were pulled down using Streptavidin-agarose beads. The results showed that GST–ASR5 bound to the biotinylated DNA fragment, whereas GST did not. The ASR5–DNA binding was competed away by the inclusion of a 25× concentrated non-biotinylated STAR1 fragment but not by Di-DC or DNA fragments harboring deletions of the putative ASR5-binding motif (AGCCCAT) (Figure 7B and 7C). Moreover, transient gene expression assays demonstrated the regulation of STAR1 by ASR5 (Figure 7D). These results indicated that rice ASR5 acts as a transcription factor that activates STAR1 expression through direct interaction with cis elements in the STAR1 promoter. Under Al stress, the ASR5 protein binds to the STAR1 promoter and other target genes to modulate their expression (Figure 7E).
Figure 7.
ASR5 Binds to the STAR1 Promoter In Vitro.
(A) ChIP qPCR results showing enrichment of the STAR1 promoter region using the α-ASR5 antibody.
(B) Scheme showing the amplification sites for the STAR1 promoter.
(C) SAPA pull-down system showing that ASR5–GST binds to an F1 biotinylated DNA fragment (F1*). Fragments F1, F2, F3, and Di-Dc were used as competitors. GST alone was used as a negative control.
(D) Transient gene expression assays demonstrating the regulation of STAR1 by ASR5 using GUS/luciferase assays.
(E) A proposed model for the ASR5–STAR1 promoter interaction. ART1 does not respond to Al in rice but maintains a housekeeping expression level of STAR1 under control conditions. In response to Al, ASR5 binds to the STAR1 promoter to enhance its expression. Adapted from Delhaize et al. (2012).
A previous study showed that Al-responsive expression of STAR1 requires the transcription factor ART1 (Yamaji et al., 2009). To test whether ASR5 can form homodimers and/or heterodimers with ART1 (a regulator of STAR1), a yeast two-hybrid assay was performed. The assay showed that ASR5 was unable to form homodimers or heterodimers with ART1 in the presence or absence of Al and Zinc (Supplemental Figure 9).
DISCUSSION
In previous studies, we have demonstrated an essential role for ASR5 in Al tolerance in rice. However, the molecular mechanism of ASR5 function has remained unknown. Here, through genome-wide gene expression and protein–DNA interaction analyses, we show that ASR5 is a DNA-binding protein that directly regulates a number of Al-responsive genes, including several genes previously shown to be required for full Al tolerance.
ASR5 Is Expressed in Tissues that Are Important for Al Tolerance in Plants
The expression of GUS driven by the ASR5 promoter in the root apex (more specifically, in the root border cells) (Figure 1A–1G) provides new insights regarding Al resistance in rice. The root border cells (RBCs) are a population of mucilage-secreting cells that surround the root cap (Hawes et al., 2003). The expression of ASR5 in border cells is consistent with previous observations that physical removal of RBCs from the root tips results in higher Al accumulation in the root tips and more severe inhibition of root elongation, which indicates that these cells protect the root tip from Al toxicity (Miyasaka and Hawes, 2001). Therefore, the tissue-specific expression pattern of ASR5 and its increased level in the roots under Al treatment (Supplemental Figure 1) are consistent with the role of ASR5 in Al tolerance.
GUS expression was also observed in the trichomes of leaves (Supplemental Figure 2A) and of the palea and lemma (Supplemental Figure 2F). In tobacco, trichomes have been shown to secrete metals complexed with calcium crystals when the plants are grown in a medium with toxic levels of zinc (Sarret et al., 2006). Additionally, ASR5_RNAi plants display a reduced number of trichomes in the leaves, palea, and lemma (Arenhart et al., 2013). Together, these observations suggest that ASR5 acts on trichome development and may contribute to the management of toxic Al levels in leaf and inflorescence tissues in rice.
ASR5 Is Required for Al-Responsive Gene Expression in Rice
Our RNA-Seq experiments demonstrated that approximately 95% of the 961 identified Al-responsive genes depend on ASR5 for normal Al-responsive expression. The robustness of the applied statistical approach is supported by the fact that the ASR5 gene was up-regulated in response to Al in the NT plants and was silenced in the ASR5_RNAi plants (Supplemental Table 1 and Supplemental Figure 3). In addition, our RNA-Seq data were consistent with previous reports indicating 72 genes that respond to a low concentration of Al in wild-type rice (Tsutsui et al., 2012) (Supplemental Information 1) and a set of 23 other known Al-responsive genes (Supplemental Table 1). The observation that 95% of the genes that responded to Al in NT plants did not show a similar Al response in the ASR_RNAi plants demonstrates a central role for ASR5 in Al-responsive gene expression.
Among these ASR5-dependent Al-responsive genes, STAR1 (Huang et al., 2009), Nrat1 (Xia et al., 2010), and FRDL4 (Yokosho et al., 2011) were previously shown to play important roles in the rice response to Al. STAR1 belongs to the ABC family of genes and, in rice, the STAR1 protein, together with STAR2, mediates the transport of UDP-glucose to the cell wall, participating in masking Al-binding sites (Huang et al., 2009). An Arabidopsis homolog of STAR1, ALS3, has been documented to be essential for Al resistance (Larsen et al., 2005; Kumari et al., 2008). Nrat1 transports trivalent Al ions but not other divalent ions, such as manganese, iron, and cadmium, or the Al–citrate complex (Xia et al., 2010). FRDL4, another Al-induced protein, participates in external Al detoxification (organic acid release) via citrate transport (Yokosho et al., 2011).
Several gene classes described previously as being Al-responsive were also detected in our RNA-Seq experiments. For example, nine genes encoding metallothionein and metal transporters (LOC_Os05g39540, LOC_Os03g38970, LOC_Os02g50730, LOC_Os12g38290, LOC_Os12g38270, LOC_Os12g38010, LOC_Os12g38040, LOC_Os12g38051, and LOC_Os12g38300) were up-regulated by Al in the NT plants (Supplemental Information 1). LOC_Os12g38051 and LOC_Os12g38300 were repressed in the ASR5_RNAi plants. These metallothioneins may have evolved for the detoxification of heavy metals by chelating metals and buffering their cytosolic concentration (Cobbett and Goldsbrough, 2002). Six glycosyl hydrolases (LOC_Os01g71820, LOC_Os10g28080, LOC_Os10g28120, LOC_Os01g47070, LOC_Os04g40490, and LOC_Os01g71350) were up-regulated by Al only in the NT plants (Supplemental Information 1); all of these genes except for LOC_Os01g71820 were repressed in the ASR5_RNAi plants. Glycosyl hydrolases have also been shown to be up-regulated by Al in Arabidopsis and Populus (Kumari et al., 2008; Grisel et al., 2010). Al-induced root growth inhibition is the earliest symptom of Al toxicity and occurs as a result of the rapid inhibition of cell elongation (Kochian et al., 2005). Plants employ a set of different mechanisms to loosen the cell wall during cell extension. Glycosyl hydrolases may play a crucial role in this loosening process by negating the Al-induced cell-wall-stiffening effect. ASR5 also regulates many genes with potential functions in the auxin response (Supplemental Figure 4), including an auxin response factor homolog (LOC_Os02g06910), a putative auxin efflux carrier (LOC_Os02g50960), two auxin-repressed proteins (LOC_Os03g22270 and LOC_Os11g44810), and three auxin-induced proteins (LOC_Os05g01570, LOC_Os08g44750, and LOC_Os01g58910), suggesting a role of ASR5 in regulating plant growth by modulating the auxin pathway.
A GO search for genes that were differentially expressed in NT plants in response to Al (Supplemental Figure 5A and 5B) found that many classes of stress-responsive genes were up-regulated, while primary metabolism and cell cycle genes were down-regulated in response to Al. The down-regulation of primary metabolism may be required to manage stressful conditions (Chandran et al., 2008). These findings are consistent with a previous report in Medicago truncatula (Chandran et al., 2008).
Although the ASR5_RNAi plants exhibited a reduced overall transcriptional response to Al, a number of genes responded to Al in the ASR5_RNAi plants that did not respond in the NT control plants (Figure 2A). These genes may have been affected by Al damage in the absence of ASR5-mediated adaptive protection. Consistently with this notion, the star1 mutant, which exhibits a defect in Al detoxification, showed a much broader Al-induced gene expression response than wild-type plants (Tsutsui et al., 2012).
Many genes were also affected by ASR5 silencing but not by Al treatment (Figure 2B). The genes that were up-regulated due to ASR5 silencing were enriched for genes involved in PCD compared to the reference genome (Supplemental Figure 5C). Under normal growth conditions in wild-type rice plants, PCD may be a mechanism for managing Al toxicity, as PCD can occur as a result of an oxidative burst due to various stresses. ROS production due to Al toxicity induces cell death in wheat (Delisle et al., 2001) and barley (Simonovicova et al., 2004), and this process has been proposed to remove cells that accumulate Al and therefore serve as a mechanism of Al resistance (Chandran et al., 2008). However, the ASR5_RNAi plants displayed this pattern under normal conditions (without Al treatment), suggesting that the basal level of ASR5 expression plays a role in protecting cells from apoptosis under normal conditions (or when plants were stressed by an unknown factor under our normal growth conditions). The genes that were down-regulated due to ASR5 silencing belonged to categories including the response to stress, oxidative stress, chemical stimulus, and antioxidant activity, among others, and showed a positive correlation with the genes that were up-regulated by Al in the NT plants (Supplemental Figure 5D and Supplemental Information 2), consistently with Al activation of ASR5.
These results indicate that ASR5 is required for the Al-responsive expression of large numbers of genes, and the lack of Al induction of these genes may contribute to the high Al sensitivity displayed in the ASR5_RNAi plants. In the absence of ASR5-mediated protection, such as when ASR5 is silenced, plants experience greater intracellular damage induced by Al. Under non-Al-stress conditions, ASR5 appears to also play a role in protecting plant cells from PCD, although this phenotype is only detectable at the gene expression level.
ASR5 Directly Activates Al-Responsive Genes, including STAR1
Our RNA-Seq and ChIP-Seq experiments identified STAR1 as a direct target gene of ASR5. STAR1 transcript levels were increased in the Al-treated NT plants but decreased in the ASR5_RNAi plants compared to the untreated NT plants (Supplemental Table 1, Figure 3, and Supplemental Figure 6). STAR1 was one of the ASR5 target genes identified in the ChIP-Seq analysis (Figure 7A), and ASR5 binding to the STAR1 promoter region was confirmed via in vitro DNA-binding assays (Figure 7C). Furthermore, transient reporter gene assays showed that ASR5 activates STAR1 expression in protoplasts (Figure 7D).
A previous study (Kalifa et al., 2004) suggested that tomato ASR1 binds to a specific DNA sequence (C2-3 (C/G) A). Using the DREME tool, we identified four putative ASR5-binding sequences from the ChIP_seq data (A(C/A), (G/A)GCCCA, (G/A)GCCCAT), GGCCCA(A/C), and (GGCCCA(T/A) (Supplemental Figure 8 and Figure 6A), which showed high similarity to the tomato ASR1-binding site identified in a SELEX-binding experiment (Kalifa et al., 2004). The STAR1 promoter region used for ChIP–qPCR contains two ASR5-binding motifs (AGCCCAT) separated by 58bp. We propose that the consensus sequence (AGCCCAT) represents a novel motif for the binding of the ASR5 protein.
ASR1 from tomato forms homodimers via zinc-dependent DNA-binding activity (Goldgur et al., 2007), though ASR1 monomers can also bind DNA (Maskin et al., 2007). In the same study (Maskin et al., 2007), ASR1 was only found as homodimers in specific organs. Although ASR5 homodimerization could not be demonstrated in our yeast two-hybrid assays (Supplemental Figure 9), this possibility cannot be discarded because ASR proteins are able to form dimers in tomato (Rom et al., 2006; Goldgur et al., 2007; Konrad and Bar-Zvi, 2008) in the nucleus and even in the cytoplasm (Ricardi et al., 2012). Our results suggest that rice ASR5 can show activity as a monomer, or ASR5 might require another protein for its function, as grape ASR forms heterodimers with drought response element-binding (DREB) proteins (Saumonneau et al., 2008).
Al-responsive gene expression also requires the transcription factor ART1 (Yamaji et al., 2009). However, the ART1 gene is not affected by Al treatment, and Yamaji et al. suggested that other Al-regulated factors may be required for ART1 to activate its target genes in response to Al stress. Our results suggest that ASR5 is the Al-activated factor that binds to the STAR1 promoter to enhance its expression. The requirement of both ASR5 and ART1 for Al-induced STAR1 expression suggests that ASR5 and ART1 may interact with each other directly and function cooperatively. ASR5-binding sites (AGCCCAT) are found in STAR1 promoter 218 and 282 nucleotides upstream of the ART1-binding motif (Tsutsui et al., 2011). However, we did not detect any ASR5–ART1 interaction in our yeast two-hybrid assay, even in the presence of Al and Zinc (Supplemental Figure 9). Further research will be required to understand the mechanism underlying the transcriptional activities of these two proteins.
No Al receptors have been found in rice, and our results have shown that ASR5 likely does not serve as the receptor for Al, as IMAC (immobilized-metal affinity chromatography) experiments indicated no Al binding (data not shown). However, similarly to ASR1 from tomato (Goldgur et al., 2007), ASR5 was able to bind zinc ions in IMAC assays (data not shown). Interestingly, ART1, a putative C2H2 zinc finger protein, was unable to bind zinc ions in IMAC assays (data not shown).
This study demonstrates that ASR5 is a key transcription factor that mediates Al-responsive gene expression to provide Al tolerance in rice. Under Al stress, the ASR5 protein binds to promoter DNA sequences to mediate the Al-responsive expression of a large number of genes, many of which play important roles in Al detoxification and stress adaptation. Taken together, our results provide new insights into the molecular mechanisms of Al resistance in rice. Furthermore, the large number of both ASR5-dependent and ASR5-independent Al-responsive genes identified defines a structured transcriptome that mediates Al tolerance in rice. Our findings not only shed light on the molecular mechanisms of Al resistance in rice, but also provide important information for future research on improving Al tolerance in other crops.
METHODS
Aluminum Treatment and Sample Preparation for Transcriptome Sequencing
Rice seeds were germinated on filter paper for 4 d in the dark at 28°C. The seedlings were grown in hydroponic Baier’s solution (Baier et al., 1995) for 12 d in a growth chamber at 28°C under 12h of light. The hydroponic solution was replaced every 4 d. After 12 d, root samples from NT and ASR5-silenced (ASR5_RNAi) rice plants (ssp. Japonica cv. Nipponbare) grown under control conditions or subjected to Al treatment (8h with 450 μM AlCl3 at pH 4.5) were collected and immediately frozen in liquid nitrogen. The total RNA was then extracted with TRIzol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The total RNA (>10 μg) was then sent to Fasteris Life Sciences SA (Plan-les-Ouates, Switzerland) for sample preparation (conversion to cDNA) and shotgun sequencing using Illumina HiSeq 2000 (Illumina, San Diego, CA, USA). Polyadenylated transcript sequencing (mRNA-seq) was performed using the following successive steps: poly-A purification, cDNA synthesis using the poly-T primer shotgun to generate inserts of 300–500 nucleotides, 3p and 5p adapter ligations, pre-amplification, colony generation, and sequencing. The cDNA sequencing reaction was performed using a single-end and a 100 nucleotide read length.
Transcriptome Sequencing Data Analysis
Mapping Method
The reads were aligned with the Bowtie v. 0.12.7 software program (Langmead et al., 2009) using the default parameters. The first seed alignment was >28 nucleotides in size, allowing zero mismatches and unique mapped reads. The rice genome sequence RGAP v7 (http://rice.plantbiology.msu.edu/) was used as a reference. The SAM files from Bowtie were then processed using Python scripts to assign the counted reads that mapped to each gene region.
Statistical Methods
The scaling normalization method was employed to normalize the data according to Robinson and Oshlack (2010). Quantification of differential gene expression was performed with the R package EdgeR (Robinson et al., 2010). Briefly, EdgeR uses a negative binomial model to estimate overdispersion from a gene count. The dispersion parameter for each gene is estimated based on the tagwise dispersion. Finally, differential expression is assessed for each gene using an adapted exact test for overdispersed data.
Western Blot Analysis
Twelve-day-old roots from rice plants grown under control or Al-treated (8h, 450 μM AlCl3) conditions were macerated and homogenized in 0.5 M Tris–HCl (pH 8.3), 2% Triton X-100, 20mM MgCl2, 2% β-mercaptoethanol, 1mM PMSF, 2.5% PEG, and 1mM EDTA, followed by incubation at 4°C for 1h. Aliquots of each sample were then loaded and separated via SDS–PAGE in 15% gels. The ASR5 protein was detected with a rabbit polyclonal ASR5 antibody (1:500 dilution). Goat anti-rabbit IgG (1:1000) conjugated with alkaline phosphatase was used as the secondary antibody. The resultant bands were detected with a premixed BCIP/NBT substrate solution (Sigma, St. Louis, MO, USA) and recorded on X-ray film.
ChIP-Seq Data Analysis
Mapping Method
The ChIP-Seq libraries were sequenced using an Illumina Genome Analyzer IIx. Thirty-six sequencing cycles were performed, generating a total of more than 40 million sequence reads per sample. The sequencing reads were then mapped to the Released RGAP v7 Rice Genome Annotation using the SOAPaligner2.21 software program (Li et al., 2009), allowing up to two mismatched nucleotides and no gaps. Only unique mapped reads were filtered out for further analyses.
Binding Site Detection Method
The MACS software program (Zhang et al., 2008) was employed to search for binding peaks with the default parameters. All binding sites were detected under the following conditions: (1) binding site detection using only our sample data (α-ASR5); (2) binding site detection adding our control (Pre-Serum) data set to the MACS software; and (3) comparison of these two conditions to filter the binding sites that overlapped in the two conditions. Additionally, binding sites that presented less than 50% overlap with the control binding sites were included, and the resulting binding sites were considered to represent real aluminum-binding sites in rice.
ChIP qPCR
The chromatin-immunoprecipitation (ChIP) experiments were performed as previously described (He et al., 2005) using plants grown under the same conditions as in the ChIP-Seq experiment in triplicate biological repeats. The ChIP products were analyzed via quantitative real-time PCR (the primer sequences are listed in Supplemental Information 4), and enrichment was calculated as the ratio between the control sample and the α-ASR5 sample. Ubiquitin (LOC_Os04g57220) was used as a reference gene.
ASR5-Binding Motif Identification
To perform motif searches, 200-bp sequences (100bp upstream and 100bp downstream) surrounding the peak of each binding site were extracted and used to search for consensus transcription factor-binding motifs with the bioinformatics tool DREME (Bailey, 2011). The enrichment of motifs found by DREME was calculated based on the frequencies of these consensus binding motifs per 1000bp of sequence in the entire rice genome.
Yeast Two-Hybrid Assay
To investigate the interaction between the ASR5 and ART1 proteins, the coding sequences of both genes were amplified via PCR using specific primers. Sequential PCR was performed to amplify the ART1 gene (ART1 first step: forward CACCATGGATCGCGACCAGATG and reverse TCACTTGTCACCA TTCTCCTCCT; ART1 second step: forward AGTGATTCCCCCT GCTTGAT and reverse TCATATGCAACTCGCTACGC; ASR5 forward CACCATGGCGGAGGAGAAGCAC and ASR5 reverse TCA GCCGAAGAGGTGGTG). Yeast strain AH109 was co-transformed with pXDGATcy86 (GAL-4-binding domain) and pGADT7 (GAL-4 activation domain) plasmids containing these genes. The lithium acetate yeast transformation method was applied with some modifications to introduce the constructs into the cells (Gietz and Woods, 2002). Different concentrations of zinc and aluminum were used to verify whether interactions could occur in the presence of metal ions (Zn = 0.01, 0.05, 0.1mM; Al = 0.05, 0.1, 0.25, 0.5mM).
Pull-Down Assay
Pull-down assays were performed as previously described (Wu, 2006). Biotin-labeled and non-labeled forward and reverse primers were used to amplify fragments via PCR employing rice genomic DNA as a template. The amplified products were bound to Streptavidin-agarose beads and used to precipitate the ASR5 protein. A Western blot assay was subsequently performed. The primers used in these assays are described in Supplemental Information 4.
Transient Gene Expression Assays
Protoplast isolation and PEG transformation were performed using the tape method (Wu et al., 2009). Plasmid DNAs were extracted using the QIAGEN Plasmid Maxi Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Approximately 1×104 isolated mesophyll protoplasts were transfected with 10 μg of each plasmid (35S::Renilla Luciferase and 35S::STAR1prom_GUS, designated Gus518) plus 20 or 40 μg of 35S::ASR5 and incubated for 48h. Protoplasts were harvested via centrifugation and lysed in 100 μl of CCLR buffer (25mM K-phosphate pH 7.5, 1mM EDTA, 7mM 2-mercaptoethanol, 1% triton X-100, 1-% Glycerol). Renilla activity was measured using Coelenterazine (Sigma), while GUS activity was measured using MUG (4-methylumbelliferyl-β-D-glucuronide) and MU (4-methylumbelliferone), and 35S:GUS was used as a positive control.
Real-Time RT–qPCR
The plant materials and Al conditions were the same as described in a previous section (‘Aluminum Treatment and Sample Preparation for Transcriptome Sequencing’). RNA was extracted with TRIzol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. cDNA was synthesized using M-MLV RT reverse transcriptase (Promega, Madison, WI, USA). For the real-time RT–qPCR assays, the stock solution was diluted 10×. The protocol applied for real-time RT–qPCR can be summarized as follows: an initial step of 5min at 94ºC was followed by 40 cycles of 10 s at 94ºC, 15 s at 60ºC, and 15 s at 72ºC. The samples were incubated for 2min at 40ºC to promote re-annealing, and they were then warmed from 55ºC to 99ºC to generate relative denaturing curve data for the amplification products. Relative changes in gene expression levels were calculated using the 2–ΔΔCt method (Livak and Schmittgen, 2001). All of the reactions were performed in four technical replicates. Quantitative PCR was conducted using the specific primer pairs listed in Supplemental Information 4. Real-time RT–qPCR was performed in a StepOne Applied Biosystems real-time cyclerTM. FDH (LOC_Os02g57040) and Actin2 (LOC_Os08g29650) were used as reference genes.
GUS Expression
GUS histochemical assays were performed in different organs of the transgenic rice plants as previously described (Jefferson et al., 1987). A region 2060 base pairs upstream of the start codon of the ASR5 gene was amplified using specific primers (listed in Supplemental Information 4). The amplified product was cloned in the pENTR-D TOPO vector and recombined via an LR reaction into the vector pHGWFS7 (Karimi et al., 2002). The resulting plasmid was used to transform rice calli (Upadhyaya et al., 2000). The regenerated plants were incubated in 1mM X-Gluc, 100mM phosphate buffer (pH 7.0), 2mM KH2Fe, and 0.5% Triton X-100. The samples were incubated for 16h at 37ºC. After the reaction had completed, the green tissues were incubated in 70% ethanol to remove any chlorophyll. The tissues were fixed in a solution of 4% formaldehyde and 1% glutaraldehyde in sodium phosphate buffer (pH 7.2); they were then dehydrated in increasingly concentrated ethanol solutions and treated with hydroxyethylmethacrylate. Sections with a thickness of 10 μm were generated with a rotative microtome, and the analysis was performed using a Leica DMR-HC microscope equipped with a Leica DFC500 camera. Three independent lines were used in this analysis.
SUPPLEMENTARY DATA
Supplementary Data are available at Molecular Plant Online.
FUNDING
This work was supported by the Coordenação de Aper- feiçoamento de Pessoal de Nível Superior (CAPES: www.capes.gov.br), the Fundação de apoio a Pesquisa do Rio Grande do Sul (FAPERGS), and the Brazilian National Council of Technological and Scientific Development (CNPq). This research was partially supported by a grant from the NIH (R01GM066258) to Z.-Y.W.
Supplementary Material
ACKNOWLEDGMENTS
We thank the Stanford Center for Genomics and Personalized Medicine (SCGPM) service center, which is led by M. Snyder and A. Sidow, for providing sequencing services, and Z. Wen for performing the sequencing reactions. Conceived and designed the experiments: M.M.P., Z.-Y.W., R.M. Performed the experiments: R.A.A., L.B.N., M.S., A.S. Wrote the paper: R.A.A. Performed some of the data analysis: R.A.A., Y.B., L.F.V.O., J.M., G.S.-M. Revised the paper: M.M.P., F.S.M., Z.-Y.W., R.M. No conflict of interest declared.
REFERENCES
- Arenhart R.A, Lima J.C de, Pedron M., Carvalho F.E.L., Silveira J.A., Rosa S.B., Caverzan A., Andrade C.M.B., Schünemann M., Margis R., et al. (2013). Involvement of ASR genes in aluminium tolerance mechanisms in rice. Plant, Cell & Environment. 36, 52–67 [DOI] [PubMed] [Google Scholar]
- Arenhart R.A., Margis R., Margis-Pinheiro M. (2012). A putative role in the response to aluminum photosynthesis disturbance: the rice ASR5 protein. Plant Signaling and Behavior. 7, 1263–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier A.C., Somers D.J., Gusiafson J.P. (1995). Aluminium tolerance in wheat: correlating hydroponic evaluations with field and soil performances. Plant Breeding. 114, 291–296 [Google Scholar]
- Bailey T.L. (2011). DREME : motif discovery in transcription factor ChIP-seq data. Bioinformatics. 27, 1653–1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcelo J., Poschenrieder C. (2002). Fast root growth responses, root exudates, and internal detoxification as clues to the mechanisms of aluminium toxicity and resistance: a review. Environmental and Experimental Botany. 48, 75–92 [Google Scholar]
- Çakir B., Agasse A., Gaillard C., Saumonneau A., Delrot S., Atanassova R. (2003). A grape ASR protein involved in sugar and abscisic acid signaling. Plant Cell. 15, 2165–2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandran D., Sharopova N., Ivashuta S., Gantt J.S., Vandenbosch K.A., Samac D. (2008). Transcriptome profiling identified novel genes associated with aluminum toxicity, resistance and tolerance in Medicago truncatula . Planta. 228, 151–166 [DOI] [PubMed] [Google Scholar]
- Chen J., Liu D., Jiang Y., Zhao M., Shan W., Kuang J., Lu W. (2011). Molecular characterization of a strawberry FaASR gene in relation to fruit ripening. PloS One. 6, e24649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbett C., Goldsbrough P. (2002). Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Ann. Rev. Plant Biol. 53, 159–182 [DOI] [PubMed] [Google Scholar]
- Delhaize E., Ma J.F., Ryan P.R. (2012). Transcriptional regulation of aluminium tolerance genes. Trends Plant Sci. 17, 341–348 [DOI] [PubMed] [Google Scholar]
- Delisle G., Champoux M., Houde M. (2001). Characterization of oxalate oxidase and cell death in Al-sensitive and tolerant wheat roots. Plant Cell. 42, 324–333 [DOI] [PubMed] [Google Scholar]
- Dong C.-J., Wang Y., Yu S.-S., Liu J.-Y. (2010). Characterization of a novel rice metallothionein gene promoter: its tissue specificity and heavy metal responsiveness. Journal of Integrative Plant Biology. 52, 914–924 [DOI] [PubMed] [Google Scholar]
- Du Z., Zhou X., Ling Y., Zhang Z., Su Z. (2010). agriGO : a GO analysis toolkit for the agricultural community. Nucleic Acids Res. 38, 64–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duressa D., Soliman K., Chen D. (2010). Identification of aluminum responsive genes in Al-tolerant soybean line PI 416937. International Journal of Plant Genomics. 2010, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eticha D., Zahn M., Bremer M., Yang Z., Horst W.J. (2010). Transcriptomic analysis reveals differential gene expression in response to aluminium in common bean (Phaseolus vulgaris) genotypes. Ann. Bot. (Lond.). 105, 1119–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famoso A.N., Clark R.T., Shaff J.E., Craft E., McCouch S.R., Kochian L.V. (2010). Development of a novel aluminum tolerance phenotyping platform used for comparisons of cereal aluminum tolerance and investigations into rice aluminum tolerance mechanisms. Plant Physiol. 153, 1678–1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foy C. (1988). Plant adaptation to acid, aluminum-toxic soils. Soil Sci. Plant Anal. 19, 959–987 [Google Scholar]
- Gietz R.D., Woods R.A. (2002). Transformation of yeast by the Liac/SS carrier DNA/PEG method. Methods Enzymol. 350, 87–96 [DOI] [PubMed] [Google Scholar]
- Goldgur Y., Rom S., Ghirlando R., Shkolnik D., Shadrin N., Konrad Z., Bar-Zvi D. (2007). Desiccation and zinc binding induce transition of tomato abscisic acid stress ripening 1, a water stress- and salt stress-regulated plant-specific protein, from unfolded to folded state. Plant Physiol. 143, 617–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin S.B., Sutter T.R. (2009). Microarray analysis of Arabidopsis genome response to aluminum stress. Biologia Plantarum. 53, 85–99 [Google Scholar]
- Grisel N., Zoller S., Künzli-Gontarczyk M., Lampart T., Münsterkötter M., Brunner I., Bovet L., Métraux J.-P., Sperisen C. (2010). Transcriptome responses to aluminum stress in roots of aspen (Populus tremula). BMC Plant Biology. 10, 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo P., Bai G., Carver B., Li R., Bernardo A., Baum M. (2007). Transcriptional analysis between two wheat near-isogenic lines contrasting in aluminum tolerance under aluminum stress. Molecular Genetics and Genomics: MGG. 277, 1–12 [DOI] [PubMed] [Google Scholar]
- Hawes M.C., Bengough G., Cassab G., Ponce G. (2003). Root caps and rhizosphere. Journal of Plant Growth Regulation. 21, 352–367 [Google Scholar]
- He J., Gendron J.M., Sun Y., Gampala S.S.L., Gendron N., Sun C.Q., Wang Z. (2005). BZR1 is a transcriptional repressor with dual roles in brassinosteroid homeostasis and growth responses. Science. 307, 1634–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houde M., Diallo A.O. (2008). Identification of genes and pathways associated with aluminum stress and tolerance using transcriptome profiling of wheat. BMC Genomics. 9, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu Y.-F., Yu S.-C., Yang C.-Y., Wang C.-S. (2011). Lily ASR protein-conferred cold and freezing resistance in Arabidopsis . Plant Physiology and Biochemistry. 49, 937–945 [DOI] [PubMed] [Google Scholar]
- Huang C., Yamaji N., Mitani N., Yano M., Nagamura Y., Ma J.F. (2009). A bacterial-type ABC transporter is involved in aluminum tolerance in rice. Plant Cell. 21, 655–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson R.A., Kavanagh T.A., Bevan M.W. (1987). GUS fusions: B-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6, 3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalifa Y., Gilad A., Konrad Z., Zaccai M., Scolnik P. A., Bar-Zvi D. (2004). The water- and salt-stress-regulated Asr1 (abscisic acid stress ripening) gene encodes a zinc-dependent DNA-binding protein. Biochem. J. 381, 373–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi M., Inzé D., Depicker A. (2002). Gateway vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci. 7, 193–195 [DOI] [PubMed] [Google Scholar]
- Kim S.-J., Lee S., Hong S.K., An K., An G., Kim S. (2009). Ectopic expression of a cold-responsive OsAsr1 cDNA gives enhanced cold tolerance in transgenic rice plants. Molecules and Cells. 27, 449–458 [DOI] [PubMed] [Google Scholar]
- Kochian L.V., Hoekenga O.A., Pineros M.A. (2004). How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Ann. Rev. Plant Biol. 55, 459–493 [DOI] [PubMed] [Google Scholar]
- Kochian L.V., Piñeros M.A., Hoekenga O.A. (2005). The physiology, genetics and molecular biology of plant aluminum resistance and toxicity. Plant and Soil. 274, 175–195 [Google Scholar]
- Kollmeier M., Felle H.H., Horst W.J. (2000). Genotypical differences in aluminum resistance of maize are expressed in the distal part of the transition zone: is reduced basipetal auxin flow involved in inhibition of root elongation by aluminum? Plant Physiol. 122, 945–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad Z., Bar-Zvi D. (2008). Synergism between the chaperone-like activity of the stress regulated ASR1 protein and the osmolyte glycine-betaine. Planta. 227, 1213–1219 [DOI] [PubMed] [Google Scholar]
- Krill A.M., Kirst M., Kochian L.V., Buckler E.S., Hoekenga O.A. (2010). Association and linkage analysis of aluminum tolerance genes in maize. PloS One. 5, e9958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari M., Taylor G.J., Deyholos M.K. (2008). Transcriptomic responses to aluminum stress in roots of Arabidopsis thaliana. Mol. Genet. Genomics. 279, 339–357 [DOI] [PubMed] [Google Scholar]
- Langmead B., Trapnell C., Pop M., Salzberg S.L. (2009). Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen P.B., Geisler M.J.B., Jones C.A., Williams K.M., Cancel J.D. (2005). ALS3 encodes a phloem-localized ABC transporter-like protein that is required for aluminum tolerance in Arabidopsis . Plant J. 41, 353–363 [DOI] [PubMed] [Google Scholar]
- Li R., Yu C., Li Y., Lam T., Yiu S., Kristiansen K., Wang J. (2009). SOAP2: an improved ultrafast tool for short read alignment. Bioinformatics. 25, 1966–1967 [DOI] [PubMed] [Google Scholar]
- Liu H.-Y., Dai J.-R., Feng D.-R., Liu B., Wang H.-B., Wang J.-F. (2010). Characterization of a novel plantain Asr gene, MpAsr, that is regulated in response to infection of Fusarium oxysporum f. sp. cubense and abiotic stresses. Journal of Integrative Plant Biology. 52, 315–323 [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2 Ϫ ⌬⌬ C T method. Methods. 25, 402–408 [DOI] [PubMed] [Google Scholar]
- Ma J., Hiradate S., Nomoto K., Iwashita T., Matsumoto H. (1997). Internal detoxification mechanism of Al in hydrangea. Plant Physiol. 113, 1033–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan S., Tuteja N. (2005). Cold, salinity and drought stresses: an overview. Archives of Biochemistry and Biophysics. 444, 139–158 [DOI] [PubMed] [Google Scholar]
- Maron L.G., Kirst M., Mao C., Milner M.J., Menossi M., Kochian L.V. (2008). Transcriptional profiling of aluminum toxicity and tolerance responses in maize roots. New Phytologist. 179, 116–128 [DOI] [PubMed] [Google Scholar]
- Maskin L., Frankel N., Gudesblat G., Demergasso M.J., Pietrasanta L.I., Iusem N.D. (2007). Dimerization and DNA-binding of ASR1, a small hydrophilic protein abundant in plant tissues suffering from water loss. Biochem. Biophys. Res. Commun. 352, 831–835 [DOI] [PubMed] [Google Scholar]
- Mattiello L., Kirst M., Silva F.R., Jorge R.A., Menossi M. (2010). Transcriptional profile of maize roots under acid soil growth. BMC Plant Biol. 10, 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyasaka S.C., Hawes M.C. (2001). Possible role of root border cells in detection and avoidance of aluminum toxicity. Plant Physiol. 125, 1978–1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricardi M.M., Guaimas F.F., González R.M., Burrieza H.P., López-Fernández M.P., Jares-Erijman E. A., Estévez J.M., Iusem N.D. (2012). Nuclear import and dimerization of tomato ASR1, a water stress-inducible protein exclusive to plants. PloS One. 7, e41008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M.D., Oshlack A. (2010). A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biology. 11, R:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M.D., Mccarthy D.J., Smyth G.K. (2010). edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 26, 139–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rom S., Gilad A., Kalifa Y., Konrad Z., Karpasas M.M., Goldgur Y., Bar-Zvi D. (2006). Mapping the DNA- and zinc-binding domains of ASR1 (abscisic acid stress ripening), an abiotic-stress regulated plant specific protein. Biochimie. 88, 621–628 [DOI] [PubMed] [Google Scholar]
- Sarret G., Harada E., Choi Y., Isaure M., Geoffroy N., Fakra S., Marcus M.A., Birschwilks M., Clemens S., Manceau A. (2006). Trichomes of tobacco excrete zinc as zinc-substituted calcium carbonate and other zinc-containing compounds. Plant Physiol. 141, 1021–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saumonneau A., Agasse A., Bidoyen M.-T., Lallemand M., Cantereau A., Medici A., Laloi M., Atanassova R. (2008). Interaction of grape ASR proteins with a DREB transcription factor in the nucleus. FEBS Lett. 582, 3281–3287 [DOI] [PubMed] [Google Scholar]
- Shkolnik D., Bar-zvi D. (2008). Tomato ASR1 abrogates the response to abscisic acid and glucose in Arabidopsis by competing with ABI4 for DNA binding. Plant Biotechnology Journal. 6, 368–378 [DOI] [PubMed] [Google Scholar]
- Simonovicova M., Huttová J., Mistrík I., Siroká B., Tamás L. (2004). Root growth inhibition by aluminum is probably caused by cell death due to peroxidase-mediated hydrogen peroxide production. Protoplasma. 224, 91–98 [DOI] [PubMed] [Google Scholar]
- Sugiharto B., Ermawati N., Mori H., Aoki K., Yonekura-Sakakibara K., Yamaya T., Sugiyama T., Sakakibara H. (2002). Identification and characterization of a gene encoding drought-inducible protein localizing in the bundle sheath cell of sugarcane. Plant Cell Physiol. 43, 350–354 [DOI] [PubMed] [Google Scholar]
- Supek F., Bosnjak M., Skunca N., Smuc T. (2011). REVIGO summarizes and visualizes long lists of gene ontology terms. PloS One. 6, e21800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui T., Yamaji N., Feng Ma J. (2011). Identification of a cis-acting element of ART1, a C2H2-type zinc-finger transcription factor for aluminum tolerance in rice. Plant Physiol. 156, 925–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui T., Yamaji N., Huang C.F., Motoyama R., Nagamura Y., Ma J.F. (2012). Comparative genome-wide transcriptional analysis of Al-responsive genes reveals novel Al tolerance mechanisms in rice. PloS One. 7, e48197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyaya N., Surin B., Ramm K., Gaudron J., Schunmann P., Taylor W., Waterhouse P., Wang M. (2000). Agrobacterium-mediated transformation of australian rice Cultivars jarrah and amaroo using modified promoters and selectable markers. Aust. J. Plant Physiol. 27, 201–210 [Google Scholar]
- Vaidyanathan R., Kuruvilla S., Thomas G. (1999). Characterization and expression pattern of an abscisic acid and osmotic stress responsive gene from rice. Plant Science. 140, 21–30 [Google Scholar]
- Von Uexkull H.R., Mutert E. (1995). Global extent, development and economic impact of acid soils. Plant Soil. 171, 1–15 [Google Scholar]
- Wenzl P., Chaves A.L., Patiæo G.M., Mayer J.E., Rao I.M., Internacional C., Tropical D.A. (2002). Aluminum stress stimulates the accumulation of organic acids in root apices of Brachiaria species. Journal of Plant Nutrition and Soil Science. 165, 582–588 [Google Scholar]
- Wu F.-H., Shen S.-C., Lee L.-Y., Lee S.-H., Chan M.-T., Lin C.-S. (2009). Tape-Arabidopsis Sandwich—a simpler Arabidopsis protoplast isolation method. Plant Methods. 5, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K.K. (2006). Analysis of protein–DNA binding by streptavidin–agarose pulldown. Methods Mol. Biol. 338, 281–290 [DOI] [PubMed] [Google Scholar]
- Xia J., Yamaji N., Kasai T., Ma J.F. (2010). Plasma membrane-localized transporter for aluminum in rice. Proc. Natl Acad. Sci. U S A. 107, 18381–18385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaji N., Huang C.F., Nagao S., Yano M., Sato Y., Nagamura Y., Ma J.F. (2009). A zinc finger transcription factor ART1 regulates multiple genes implicated in aluminum tolerance in rice. Plant Cell. 21, 3339–3349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C.-Y., Wu C.-H., Jauh G.Y., Huang J.-C., Lin C.-C., Wang C.-S. (2008). The LLA23 protein translocates into nuclei shortly before desiccation in developing pollen grains and regulates gene expression in Arabidopsis . Protoplasma. 233, 241–254 [DOI] [PubMed] [Google Scholar]
- Yang L., Zheng B., Mao C, Qi X., Liu F., Wu P. (2004). Analysis of transcripts that are differentially expressed in three sectors of the rice root system under water deficit. Mol. Genet. Genom. 272, 433–442 [DOI] [PubMed] [Google Scholar]
- Yokosho K., Yamaji N., Ma J.F. (2011). An Al-inducible MATE gene is involved in external detoxification of Al in rice. Plant J. 68, 1061–1069 [DOI] [PubMed] [Google Scholar]
- You J., Zhang H., Liu N., Gao L., Kong L., Yang Z.Y. (2011). Transcriptomic responses to aluminum stress in soybean roots. Genome. 54, 1–11 [DOI] [PubMed] [Google Scholar]
- Zhang J., He Z., Tian H., Zhu G., Peng X. (2007). Identification of aluminium-responsive genes in rice cultivars with different aluminium sensitivities. J. Exp. Bot. 58, 2269–2278 [DOI] [PubMed] [Google Scholar]
- Zhang J., Yin Y., Wang Y., Peng X. (2010). Identification of rice Al-responsive genes by semi-quantitative polymerase chain reaction using sulfite reductase as a novel endogenous control. Journal of Integrative Plant Biology. 52, 505–514 [DOI] [PubMed] [Google Scholar]
- Zhang Y., et al. (2008). Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, R137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S.J., Ma J.F., Matsumoto H. (1998). High aluminum resistance in buckwheat. Plant Physiol. 117, 745–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.