Abstract
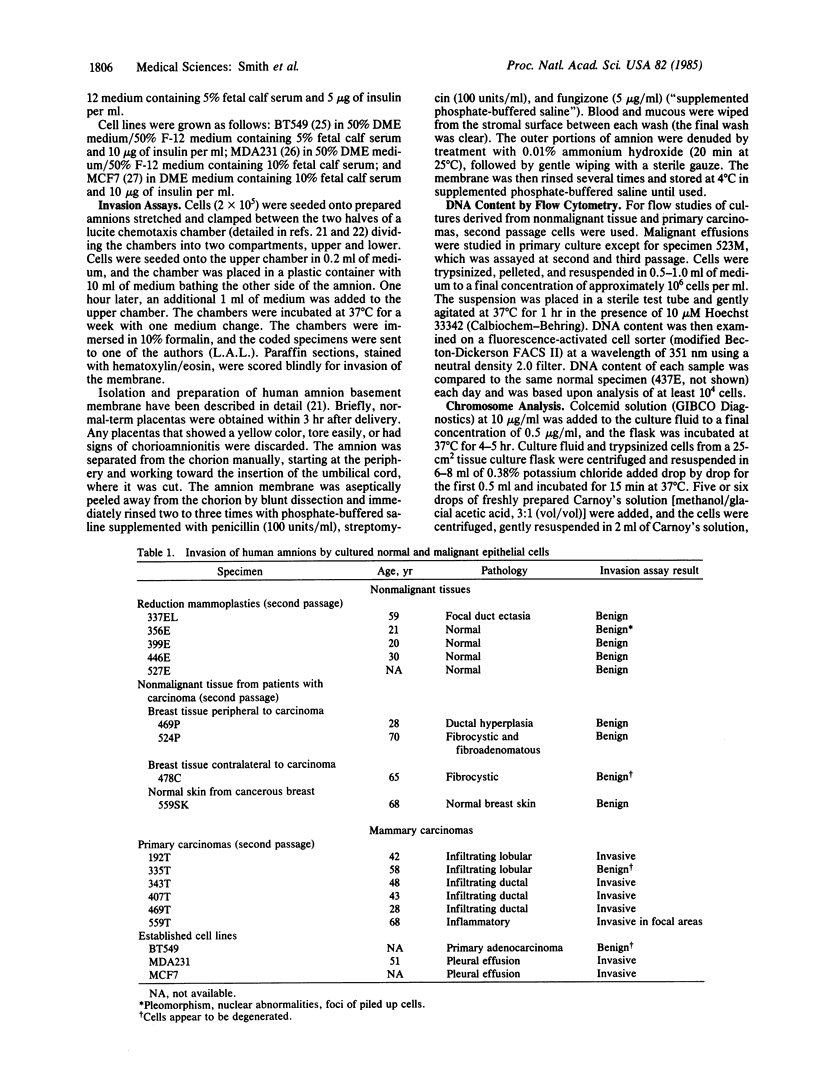
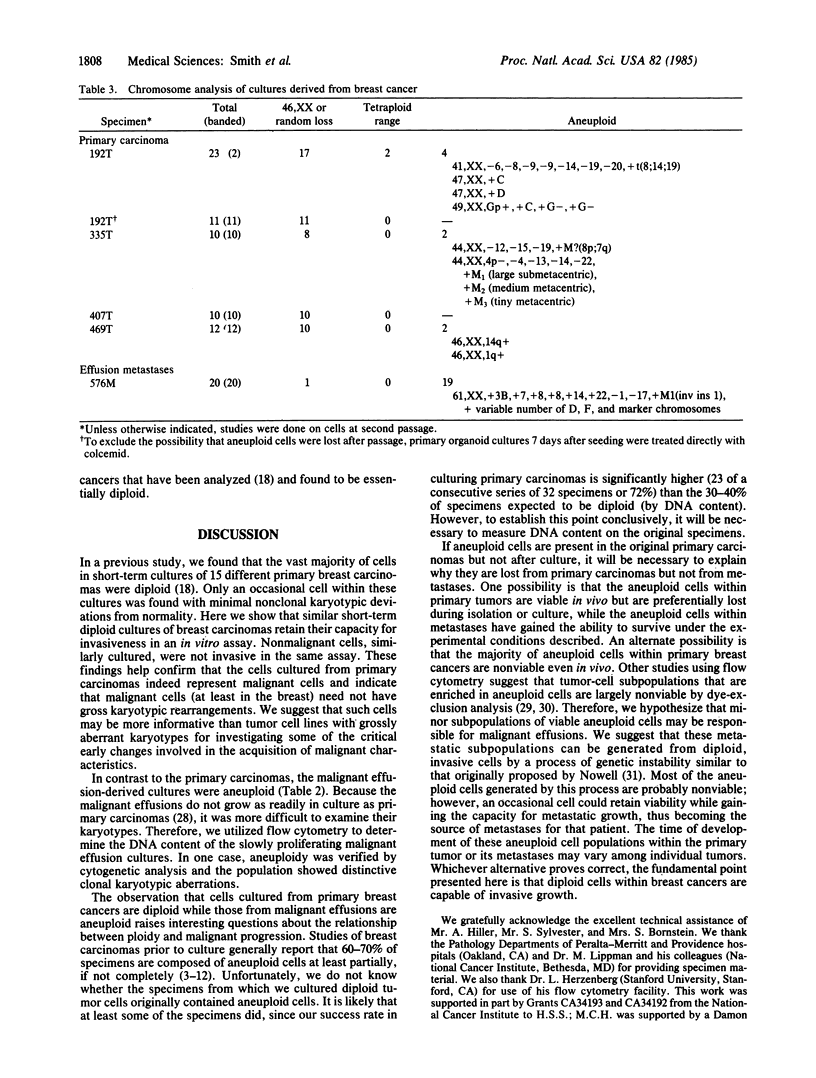
Invasiveness and ploidy were examined in cultures of human epithelial cells derived from nonmalignant breast tissue, primary breast carcinomas, and breast cancer effusion metastases. Successful short-term culture was achieved from approximately 70% of the primary breast cancers. These primary cancers were essentially diploid by flow cytometry and karyotype in contrast to the effusion metastases, which were mostly aneuploid. The diploid tumor cells retained their malignant phenotype in culture as demonstrated by invasion into a denuded human amnion basement membrane. In contrast, epithelial cells cultured from nonmalignant mammary tissue did not invade the amnion. We suggest that the diploid carcinoma cultures may be useful for investigating the essential differences between normal and malignant cells and may complement information derived from studies of tumor cell lines with grossly aberrant karyotypes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auer G. U., Caspersson T. O., Wallgren A. S. DNA content and survival in mammary carcinoma. Anal Quant Cytol. 1980 Sep;2(3):161–165. [PubMed] [Google Scholar]
- Bedrossian C. W., Raber M., Barlogie B. Flow cytometry and cytomorphology in primary resectable breast cancer. Anal Quant Cytol. 1981 Jun;3(2):112–116. [PubMed] [Google Scholar]
- Bichel P., Poulsen H. S., Andersen J. Estrogen receptor content and ploidy of human mammary carcinoma. Cancer. 1982 Nov 1;50(9):1771–1774. doi: 10.1002/1097-0142(19821101)50:9<1771::aid-cncr2820500921>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Cailleau R., Olivé M., Cruciger Q. V. Long-term human breast carcinoma cell lines of metastatic origin: preliminary characterization. In Vitro. 1978 Nov;14(11):911–915. doi: 10.1007/BF02616120. [DOI] [PubMed] [Google Scholar]
- Fosså S. D., Marton P. F., Knudsen O. S., Kaalhus O., Børmer O., Vaage S. Nuclear feulgen DNA content and nuclear size in human breast carcinoma. Hum Pathol. 1982 Jul;13(7):626–630. doi: 10.1016/s0046-8177(82)80004-x. [DOI] [PubMed] [Google Scholar]
- Fosså S. D., Thorud E., Vaage S., Shoaib M. C. DNA cytometry of primary breast cancer. Comparison of microspectrophotometry and flow cytometry, and different preparation methods for flow cytometric measurements. Acta Pathol Microbiol Immunol Scand A. 1983 Jul;91(4):235–243. [PubMed] [Google Scholar]
- Frankfurt O. S., Slocum H. K., Rustum Y. M., Arbuck S. G., Pavelic Z. P., Petrelli N., Huben R. P., Pontes E. J., Greco W. R. Flow cytometric analysis of DNA aneuploidy in primary and metastatic human solid tumors. Cytometry. 1984 Jan;5(1):71–80. doi: 10.1002/cyto.990050111. [DOI] [PubMed] [Google Scholar]
- Hackett A. J., Smith H. S., Springer E. L., Owens R. B., Nelson-Rees W. A., Riggs J. L., Gardner M. B. Two syngeneic cell lines from human breast tissue: the aneuploid mammary epithelial (Hs578T) and the diploid myoepithelial (Hs578Bst) cell lines. J Natl Cancer Inst. 1977 Jun;58(6):1795–1806. doi: 10.1093/jnci/58.6.1795. [DOI] [PubMed] [Google Scholar]
- Henderson I. C., Canellos G. P. Cancer of the breast: the past decade (second of two parts). N Engl J Med. 1980 Jan 10;302(2):78–90. doi: 10.1056/NEJM198001103020203. [DOI] [PubMed] [Google Scholar]
- Krug H. The characterization of human tumours by pulse cytophotometry. Cell Mol Biol Incl Cyto Enzymol. 1977;22(3-4):309–312. [PubMed] [Google Scholar]
- Kute T. E., Muss H. B., Anderson D., Crumb K., Miller B., Burns D., Dube L. A. Relationship of steroid receptor, cell kinetics, and clinical status in patients with breast cancer. Cancer Res. 1981 Sep;41(9 Pt 1):3524–3529. [PubMed] [Google Scholar]
- Nowell P. C. The clonal evolution of tumor cell populations. Science. 1976 Oct 1;194(4260):23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- Olszewski W., Darzynkiewicz Z., Rosen P. P., Schwartz M. K., Melamed M. R. Flow cytometry of breast carcinoma: I. Relation of DNA ploidy level to histology and estrogen receptor. Cancer. 1981 Aug 15;48(4):980–984. doi: 10.1002/1097-0142(19810815)48:4<980::aid-cncr2820480421>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Owens R. B., Smith H. S., Nelson-Rees W. A., Springer E. L. Epithelial cell cultures from normal and cancerous human tissues. J Natl Cancer Inst. 1976 Apr;56(4):843–849. doi: 10.1093/jnci/56.4.843. [DOI] [PubMed] [Google Scholar]
- Raber M. N., Barlogie B., Latreille J., Bedrossian C., Fritsche H., Blumenschein G. Ploidy, proliferative activity and estrogen receptor content in human breast cancer. Cytometry. 1982 Jul;3(1):36–41. doi: 10.1002/cyto.990030109. [DOI] [PubMed] [Google Scholar]
- Russo R. G., Foltz C. M., Liotta L. A. New invasion assay using endothelial cells grown on native human basement membrane. Clin Exp Metastasis. 1983 Apr-Jun;1(2):115–127. doi: 10.1007/BF00121491. [DOI] [PubMed] [Google Scholar]
- Russo R. G., Liotta L. A., Thorgeirsson U., Brundage R., Schiffmann E. Polymorphonuclear leukocyte migration through human amnion membrane. J Cell Biol. 1981 Nov;91(2 Pt 1):459–467. doi: 10.1083/jcb.91.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slocum H. K., Pavelic Z. P., Rustum Y. M., Creaven P. J., Karakousis C., Takita H., Greco W. R. Characterization of cells obtained by mechanical and enzymatic means from human melanoma, sarcoma, and lung tumors. Cancer Res. 1981 Apr;41(4):1428–1434. [PubMed] [Google Scholar]
- Smith H. S., Hackett A. J., Lan S., Stampfer M. R. Use of an efficient method for culturing human mammary epithelial cells to study adriamycin sensitivity. Cancer Chemother Pharmacol. 1981;6(3):237–244. doi: 10.1007/BF00256976. [DOI] [PubMed] [Google Scholar]
- Smith H. S., Lan S., Ceriani R., Hackett A. J., Stampfer M. R. Clonal proliferation of cultured nonmalignant and malignant human breast epithelia. Cancer Res. 1981 Nov;41(11 Pt 1):4637–4643. [PubMed] [Google Scholar]
- Smith H. S., Wolman S. R., Hackett A. J. The biology of breast cancer at the cellular level. Biochim Biophys Acta. 1984;738(3):103–123. doi: 10.1016/0304-419x(84)90009-x. [DOI] [PubMed] [Google Scholar]
- Soule H. D., Vazguez J., Long A., Albert S., Brennan M. A human cell line from a pleural effusion derived from a breast carcinoma. J Natl Cancer Inst. 1973 Nov;51(5):1409–1416. doi: 10.1093/jnci/51.5.1409. [DOI] [PubMed] [Google Scholar]
- Stampfer M. R. Cholera toxin stimulation of human mammary epithelial cells in culture. In Vitro. 1982 Jun;18(6):531–537. doi: 10.1007/BF02810076. [DOI] [PubMed] [Google Scholar]
- Stampfer M. R., Vlodavsky I., Smith H. S., Ford R., Becker F. F., Riggs J. Fibronectin production by human mammary cells. J Natl Cancer Inst. 1981 Aug;67(2):253–261. [PubMed] [Google Scholar]
- Stampfer M., Hallowes R. C., Hackett A. J. Growth of normal human mammary cells in culture. In Vitro. 1980 May;16(5):415–425. doi: 10.1007/BF02618365. [DOI] [PubMed] [Google Scholar]



