Abstract
Anxiety involves complex, incompletely understood interactions of genomic, environmental, and experience-derived factors, and is currently being measured by psychological criteria. Here, we report previously nonperceived interrelationships between expression variations and nucleotide polymorphisms of the chromosome 7q21–22 acetylcholinesterase-paraoxonase 1 (ACHE-PON1) locus with the trait- and state-anxiety measures of 461 healthy subjects from the Health, Risk Factors, Exercise Training, and Genetics Family Study. The AChE protein controls the termination of the stress-enhanced acetylcholine signaling, whereas the PON protein displays peroxidase-like activity, thus protecting blood proteins from oxidative stress damages. Serum AChE and PON enzyme activities were both found to be affected by demographic parameters, and showed inverse, reciprocal associations with anxiety measures. Moreover, the transient scores of state anxiety and the susceptibility score of trait anxiety both appeared to be linked to enzyme activities. This finding supported the notion of corresponding gene expression relationships. Parallel polymorphisms in the ACHE and PON1 genes displayed apparent associations with both trait- and state-anxiety scores. Our findings indicate that a significant source of anxiety feelings involves inherited and acquired parameters of acetylcholine regulation that can be readily quantified, which can help explaining part of the human variance for state and trait anxiety.
Anxiety is a ubiquitous and unavoidable experience of life, defined as a feeling of fear that is out of proportion to the nature of the threat (1). Anxiety disorders are the most prevalent of psychiatric disorders (2), which led to an extensive search of the mechanisms and/or genomic elements underlying these phenomena. Several genes were reported to be potential contributors to the genetic variance of anxiety-related traits or modifiers of the phenotypic expression of pathologic anxiety (3). However, molecular genetics and/or biochemistry have so far failed to identify variation(s) that are consistently associated with the increased psychologically measurable susceptibility to anxiety feelings in generally healthy subjects.
Variance in personality traits is often considered to involve complex interactions of environmental and experience-derived factors with several gene products and neurotransmission circuits [e.g., serotonergic (4), GABAergic, and cholinergic (5)]. Neurotransmission mediated by acetylcholine (ACh) in particular contributes to numerous physiologic functions (6) as well as to memory, learning, and panic responses (7, 8). Anxiety provokes cholinergic hyperarousal (e.g., sweating, intestinal or gastric constrictions, etc.) (9, 10). In addition, the ACh hydrolyzing enzyme acetylcholinesterase (AChE) is a target of pesticides. Human exposure to pesticides, or to the closely related chemical warfare agents, depletes both AChE and the homologous enzyme butyrylcholinesterase (BChE) (11), inducing cholinergic excitation. Polymorphisms in the corresponding ACHE and BCHE genes could hence affect both the environmental and the experience-related elements of anxiety. A third relevant enzyme is the PON protein product of the paraoxonase PON1 gene, which destroys environmental toxins that target AChE (12). PON also possesses peroxidase-like activity (13), and can directly reduce oxidative stress in macrophages and in serum (14). Polymorphisms in the ACHE, BCHE, and PON1 genes could therefore affect both the environmental and the experience-related elements of anxiety.
In mice, we observed that acute stress of even moderate intensity (e.g., confined swim) causes modulation of the genetic regulation of ACh availability in the CNS (15). This condition may be viewed as a laboratory-induced passive gene–environment interaction, demonstrating that environmental components of variance for anxiety measures are experience related, and vice versa. This gene–environment interaction involves overproduction of the readthrough monomeric AChE (AChE-R) splice variant. Such overproduction acts in the short term to reduce excess ACh after stress, but at a longer term, is associated with glucocorticoid-regulated neuronal hyperarousal and extreme sensitivity to anti-AChEs (16). Transgenic overexpression of AChE-R in mice intensifies conflict behavior (17), another phenomenon associated with anxiety (18). In view of these findings, we initiated a study aimed at testing whether genomic polymorphisms in the ACHE-PON1 locus and corresponding changes in serum AChE, BChE, and PON activities could serve as predictors of the anxiety scores of healthy humans, assisting the study of this complex phenotype.
Materials and Methods
Study Sample Description. The Health, Risk Factors, Exercise Training, and Genetics (HERITAGE) Family Study was designed to investigate the role of diverse risk factors in the genotype on responses to regular exercise (19). Measures of state and trait anxiety were obtained on a subset of the families as part of the study questionnaire. A total of 461 individuals (198 men and 263 women) from 150 two-generation families of African-American (172) or Caucasian origin (289) with complete data were available for this study.
Serum Analyses. Blood samples were collected at baseline in the morning after a 12-h fast. Questionnaires were completed later that morning. Serum was prepared by blood centrifugation at 2,000 × g (15 min at 4°C). Aliquots of 2 ml in cryogenic tubes were frozen at 80°C until use. Nondenaturing gel and catalytic activity measurements of AChE were as described (15). For other enzyme tests, see Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
Genotyping. Genomic DNA was prepared from permanent lymphoblastoid cells as described (20). PCR amplification by using Taq polymerase (Sigma, D-6677) was followed by Exo-Sap enzymatic purification (United States Biochemical, US78200) of the PCR product (Table 1, which is published as supporting information on the PNAS web site). Standard automated sequencing was performed by using the Big Dye Terminator cycle sequencing chemistry, ABI 3700 DNA analyzer, and data collection and sequence analysis software (Applied Biosystems). The 55L/M and 192Q/R coding sequence polymorphisms in PON1 were detected by using single-nucleotide primer extension and the SNaPshot method (SNaPshot ddNTP primer extension kit, ABI), using as probes 5′-GGCAGAAACTGG CTCTGAAGAC-3′ for 55L/M and 5′-GATCACTATTTTCTTGACCCCTACTTAC-3′for 192Q/R. After extension and calf intestine phosphatase treatment (Amersham Pharmacia Biosciences, Freiburg, Germany, E2250Y), products were electrophoresed on a 3700 ABI analyzer and results were analyzed with genescan software.
Statistics. To test whether observed genotype frequencies conformed with the Hardy–Weinberg equilibrium expectations, and to evaluate the significance of the linkage disequilibrium (LD) between each polymorphism pair, we used the χ2 test.
P values for the difference between the genotypes of the subjects in distinct trait anxiety subgroups were calculated by using the likelihood ratio test. The P value was the exact conditional tail probability given the marginal, as was assessed by 100,000 Monte Carlo simulations. P values for the differences between AChE, BChE, and PON activities were calculated by using the two-tailed Student's t test.
To measure the LD between single-nucleotide polymorphisms, we estimated the haplotype frequencies through the use of the expectation maximization algorithm and calculated Lewontin's D′ and R2 association values by using the Pearson's correlation (21, 22).
Multiple regression analysis was performed by using R statistical software (23). Classification trees were grown by using the R tree library (24). The tree function in the R software was used to define a sequence of binary partitions of the population into subsets based on age, gender, and the different enzyme activities. Classification trees were “grown” such that, at each step, the resulting subsets were the most homogeneous, with respect to the membership in the top 20% state-anxiety group. The tree was then “pruned” to a number of subsets, or “nodes,” which is determined by minimizing the misclassification error by a 20-fold crossvalidation. The process is automatic after selection of the relevant variables for the analysis.
Results
The psychological phenomenon of anxiety that is experienced by individuals at a certain time (state anxiety) differs from their general susceptibility to anxiety (trait anxiety). Both parameters are commonly measured by the self-reported questionnaires of the state-trait-anxiety inventory (STAI) (25). To investigate the role of cholinergic regulation in these measures we used sera, DNA, and STAI scores from 461 healthy individuals from the HERITAGE Family Study (19).
Mean STAI scores for the HERITAGE cohort were 35 ± 12 (range 20–80) for trait anxiety and 35 ± 9 (range 16–73) for state anxiety. Others reported similar values, albeit for far smaller groups with disease-associated anxiety symptoms (26, 27).
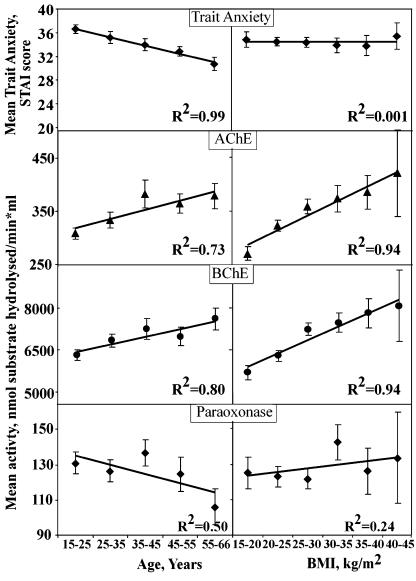
Serum Enzyme Analyses as Surrogate Measures. Our research spanned three enzymes and genes contributing to balanced ACh regulation: the closely homologous ACh-hydrolyzing enzymes, AChE and BChE (and the corresponding ACHE and BCHE genes), and the organophosphate-hydrolyzing enzyme PON (and PON1 gene). Significantly higher activities of AChE and BChE (t test, P < 0.006 and P < 0.0002, respectively), but not PON (P > 0.2), were found in females, as compared with males. Serum AChE activity was significantly higher in individuals of Caucasian origin (P < 0.002); PON activity was significantly higher in African Americans (P < 3 × 10–10), and BChE activity showed no differences between these populations (P > 0.5). AChE and BChE levels increased with age and body mass index (BMI), whereas PON activity declined with age.
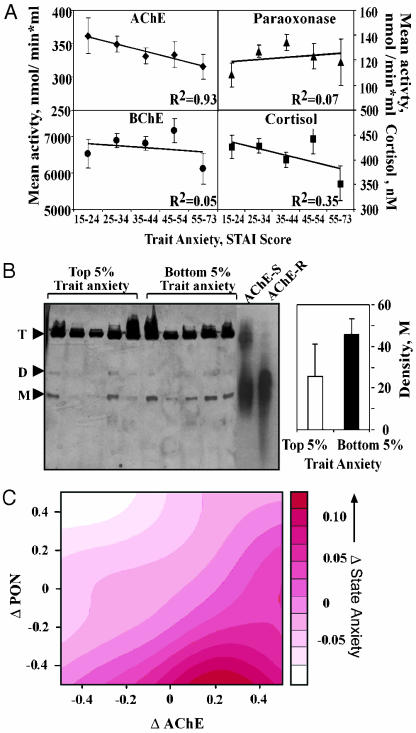
Trait-, but not state-anxiety scores decreased with age, suggesting that one's experience and/or age provide better protection from trait, but not state anxiety (Fig. 1 and data not shown). Also, state- and trait-anxiety measures showed direct, albeit weak association, both in Caucasians and in African Americans (Fig. 5, which is published as supporting information on the PNAS web site, and Supporting Materials and Methods). Compatible with these changes, an inverse correlation was found between AChE, but not BChE or PON activities, and trait anxiety, but not state anxiety (R2 = 0.93, Fig. 2A), suggesting a trait-anxiety-predictive role for serum AChE activities. Cortisol levels, however, did not correlate with trait-anxiety scores, in agreement with the apparent equivocal relationship between emotional distress and cortisol (28). Intriguingly, PON but not BChE or AChE activity, displayed an inverse association with state anxiety (R2 = 0.89), suggesting a state-anxiety-predictive role for this enzyme.
Fig. 1.
Acquired changes in measured values. Graphs show mean ± SEM for each value (n = 434). Note that trait-anxiety scores decrease with age, whereas AChE and BChE activities are elevated with both age and BMI.
Fig. 2.
Serum AChE activity is correlated with trait and state anxiety. (A) Trait-anxiety associations. Serum AChE activity, but not cortisol levels or BChE or PON activities, is inversely correlated with trait anxiety. Graphs show best-fit lines through mean ± SEM values of each parameter. (B) Overrepresented AChE monomers in the serum of subjects with low trait-anxiety scores. Nondenaturing gel electrophoresis of serum samples from subjects with the noted trait-anxiety scores was followed by enzyme activity staining. Recombinant AChE-R and synaptic AChE were similarly separated (but signals were developed for a shorter time). Note that AChE-R remains monomeric (M), whereas synaptic AChE includes a significant tetrameric (T) fraction, with minor dimers (D). (Inset) Shown is quantification of the rapidly migrating enzyme in the top and bottom 5% trait-anxiety groups. (C) Inverse association of AChE and PON variations with state anxiety. The differences between the observed and expected state-anxiety score values of each subject were plotted as a function of the corresponding differences in serum PON and AChE activities. Note the increasing tendency for anxious feelings (red) in those subjects with higher then expected serum AChE activities and the ameliorating effect on these values of higher than expected PON levels. Smoothness parameter = 0.3.
Alternative splicing of ACHE gene products yields at least three distinct proteins with AChE hydrolytic activity. Of these, the primary synaptic AChE variant forms tetramers, the erythrocytic AChE-E protein appears as glycophosphoinositide-bound dimers, and the stress-induced AChE-R variant remains monomeric (29). Nondenaturing gel electrophoresis followed by activity staining revealed, in all serum samples, active tetramers with very small amounts of dimers; however, active monomers were overrepresented in serum samples from subjects with the lowest trait-anxiety scores, as compared with those with the highest scores (Fig. 2B). Immunolabeling of serum protein blots suggested that serum AChE monomers represent AChE-R (data not shown and ref. 30), supporting the notion that individuals' capacity to respond to external stimuli by overproducing monomeric AChE-R associates with reduced trait-anxiety scores.
Biochemical Prediction of State-Anxiety Scores. Next, we addressed the difference between trait and state anxiety. In subjects with increased serum AChE activity and elevated serum AChE monomers, the ACHE gene should be close to its maximal expression capacity. This predicted limited ability for this gene to react to a changing environment by further overproducing AChE to suppress the induced stress (15). Therefore, we expected to find elevated risk for state anxiety in individuals with higher than expected serum AChE activities. The inverse association of PON levels with state-anxiety measures further suggested an opposite relationship for PON; namely, that individuals with higher then expected serum PON activities would display reduced risk for state anxiety. To test this working hypothesis, we calculated the difference between one's measured state anxiety and the expected average state anxiety, based on the demographic parameters. The differences between the measured and the predicted anxiety values were plotted as a function of the parallel differences between the measured and predicted serum AChE and PON activities. This result yielded a significant two-dimensional interaction between PON and AChE (at P < 0.004, ANOVA, Fig. 2C). These findings implied that subjects with exceptionally low PON activities may be at greater risk to develop state anxiety under conditions that fail to trigger AChE overproduction. This risk would be larger, for example, in aged subjects due to their considerably lower PON activities and the corresponding increases in AChE activities, which may explain the sustained state-anxiety measures in aged subjects. In conclusion, serum AChE activities and the reciprocal interaction with PON activities displayed distinct predictive associations with STAI scores.
The measured effects were largely independent of family links. Of the whole data set, 92 informative families with at least two siblings in each family were further analyzed. Differences between the serum and anxiety variables of two randomly chosen siblings from each family revealed low correlations within families both for anxiety and for serum variables, indicating a major contribution of environmental and experience-derived factors.
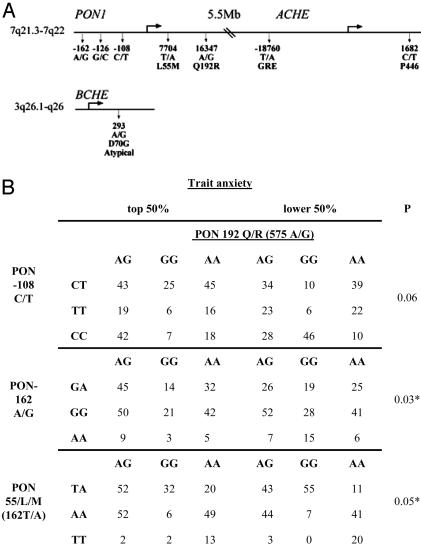
The ACHE-PON1 Locus as a Candidate Site. To evaluate the genomic contribution toward the observed biochemical differences, we analyzed single-nucleotide polymorphisms in the ACHE, BCHE, and PON1 genes. The extended human ACHE promoter includes a functional glucocorticoid response element (GRE), suggesting overexpression under stress. Israeli subjects frequently carry at this region an activating deletion associated with constitutively elevated blood AChE activity and acute anti-AChE hypersensitivity (31). Because this deletion is exceedingly infrequent in the U.S. population (0.34% vs. 3.64% allele frequency in Israelis), we focused as a linkage marker on ACHE's biochemically ineffective P446 polymorphism, with 12% frequency in the HERITAGE subjects, which is compatible with findings of others (32).
In the PON1 coding region, we genotyped the substitutions (indicated by amino acid number and symbol) of L55M (TTG into ATG), reducing PON protein and mRNA levels and Q192R (CAA into CGA), which affects PON′s catalytic efficiency (33). We also genotyped three known PON1 promoter polymorphisms (indicated by distance in nucleotides from the translation start site at 0): –108C/T, –162A/G, –126G/C, contributing to 22.4%, 2.4%, and none of the variation in PON1 expression, respectively (33). Of the numerous BCHE mutations, we genotyped the D70G substitution yielding the “atypical” BChE variant, with enzymatic activity 30% lower than the wild-type enzyme (34). Homozygous carriers of this polymorphism display extreme anxiety after exposure to anti-AChEs (35). Genotypes at all seven positions did not deviate from Hardy–Weinberg equilibrium expectations. The PON1 and ACHE genes both map to the long arm of chromosome 7, with 5.5 Mb separating between them, whereas the BCHE gene is located on the long arm of chromosome 3 (Fig. 3A). Therefore, interactions between PON1 and ACHE, but not BCHE polymorphisms, could reflect cis effects.
Fig. 3.
The analyzed genotypes and their frequencies. (A). Shown are the chromosome positions and the polymorphic sites that were studied in the PON1 (GenBank accession no. AF539592), ACHE (GenBank accession no. AF002993), and BCHE (GenBank accession no. NM000055) genes. Nucleotide numbers begin at the translation start sites at 0. (B) Distributions and joint contributions of tested polymorphism pairs with significant effects on trait-anxiety scores.
Population and Family Considerations. Because of the presumed multigenic origin of anxiety phenotypes (1, 2), and the modest differences between the anxiety symptoms in healthy individuals, we did not expect drastic genotype-phenotype associations. Nevertheless, significantly different genotype frequencies were found in part of the analyzed sites between subjects with a trait- but not state-anxiety scores in the highest and lowest decile. Members of the high trait-anxiety group included significantly more subjects heterozygous for the P446 polymorphism in ACHE (15 vs. 4 subjects, P < 0.03) and significantly more subjects homozygous to the Alzheimer's disease-predictive PON192 variant (13 vs. 5 subjects, P < 0.05; ref. 36), whereas lower trait-anxiety group members included significantly more subjects heterozygous for the PON-108 polymorphism (23 vs. 11 subjects, P < 0.03), and for the PON-126 polymorphism (six vs. one subject, P < 0.05, χ2 test).
Next, we jointly considered polymorphism pairs in subjects from the entire analyzed population, divided evenly into high or low trait-anxiety scores. Significant contributions to the trait-anxiety score emerged within the PON1 gene for PON55 with PON192 (P < 0.05) and for PON-126 with PON-162 (P < 0.03; Fig. 3B). Both the average trait anxiety and the allele frequencies differed significantly between populations (African Americans, 36.1 ± 9.5; Caucasians, 33.7 ± 8.4; t test, P < 0.006 for trait anxiety and P < 1.3 × 10–5, 2.5 × 10–8, 0.02, 0.01, 1.1 × 10–6 for ACHE P446, PON108, PON162, PON55, and PON192 respectively, χ2 test). Therefore, we considered the possibility of false positives due to population stratification combined with trait and allele differences. Significant differences in the PON1 gene between the top and bottom trait-anxiety deciles were, however, maintained in African-American subjects, despite the considerably smaller group size, supporting the relevance of this analysis. Thus, polymorphisms in the ACHE-PON1 locus appeared to be significant, albeit ethnic origin-dependent predictors of trait anxiety, either due to the modified phenotype they caused or because of LD to other polymorphisms.
Linkages Between the Biochemical and Genetic Data. LD score analysis of the tested polymorphisms reflected similar patterns to those described in previous studies (37). In addition to the predictably high scores within PON1, we found a relatively high and significant score between the ACHE and PON1 genes (Fig. 4). However, the corresponding r2 correlation coefficients (Fig. 4B) were high within the PON1 gene but low between ACHE and PON1 polymorphisms, suggesting that the high LD scores were mainly due to the large differences in the incidence of the studied polymorphisms.
Fig. 4.
Combined genotype-phenotype predictions of trait and state anxiety. Shown is the LD analysis of the tested polymorphisms in the healthy population, presented as absolute D′ (A) and R2 correlation coefficient values (B) in parallel matrices. ns, nonsignificant LD. (C) A prediction tree of state-anxiety values within the population subset with top 20% trait-anxiety scores based on genotype, age, gender, AChE, BChE, and PON activities. The numbers at the terminal nodes represent the number of subjects, and below, the probability of their belonging to the top 20% state-anxiety score group.
To better evaluate the implications of the above correlations, we tested the value of combined individual biochemical/genetic polymorphism data for prediction of individuals at risk for significantly distinct anxiety measures. Regression analysis normalized for gender, age, ethnic origin, and BMI showed a clear effect of the genotyped markers on the serum activity level of PON (P < 2.2 × 10–16). A smaller, but significant effect was observed on BChE activity (P < 0.04). The effect of the PON1 genotype was also significant when normalized for family relations (based on the differences between the measurements of different family members).
We next wished to test whether the genotyped polymorphisms and/or the examined serum enzyme activities affect trait-anxiety scores. To this end, we applied a general linear model (38), again after normalizing for the demographic effects, to compare the most anxious individuals (with the top 20% state-anxiety scores) against the remaining population. Significant effects emerged for both the genotyped polymorphisms (P < 0.06) and cumulative serum enzyme activities (P < 0.005) on the trait-anxiety score. The population subset with the top 20% trait-anxiety scores was then classified by using the regression tree method (24), testing the probability of specific individuals to belong to the top 20% state-anxiety score group (Fig. 4C). Thus, for example an African-American female younger than 33.4 years of age with serum AChE activity <237.6 but >194.2 nmol of hydrolyzed substrate per min·ml would have a 0% chance of belonging to the 20% top state-anxiety group (Fig. 4C, note the numbers at the end of the tree branches). Similar regressions could be translated into straightforward equations predicting serum enzyme activities and anxiety scores (Tables 1–5, which are published as supporting information on the PNAS web site, and Supporting Materials and Methods).
Discussion
Our findings identified previously nonperceived interactions between anxiety, serum AChE, BChE, and PON activities, and their corresponding genotypes. STAI scores were affected by demographic parameters, and also were significantly associated with inherited genotype properties combined with the corresponding enzyme activities. The state anxiety capacity to respond to changing conditions was best reflected by higher than expected serum AChE levels. These scores are less likely to appear in subjects with high basal activity of serum AChE (e.g., aged or overweight individuals), which is compatible with the prediction of a maximal expression level for this gene that does not depend on demographic parameters. We have further found a reciprocal association of the differences between the observed and predicted AChE and PON activity values, which implies that PON activity may determine the requirement for AChE overproduction. These interrelationships predict STAI scores distinctly and significantly, which may open these psychological parameters for genetic and biochemical tests.
Our laboratory findings correlated significantly with the psychologically examined anxiety scores of the HERITAGE Family Study subjects. Furthermore, these correlations were common for individuals of diverse ethnic origins. This finding supports the notion that these biochemical and genetic factors are causally related to anxiety: either they reflect inherited elements which cause or mediate anxiety, or, when enzyme activities are involved, anxiety causes them. Our biochemical findings, based on self-reported anxiety measures, should be confirmed by a diagnostic interview and replicated in an independent sample. More importantly, replication in a clinically documented anxious population, compared with a normal population, would contribute significantly to the clinical value and implications of this study.
In the HERITAGE cohort, we also found anxiety to be affected by polymorphisms in the PON1 and BCHE genes, both having an AChE-protective function. The state-anxiety-predictive value of the difference between observed and expected AChE activity is further compatible with our recent findings that the suppression of the stress-induced AChE-R variant obliterates conflict behavior (17) and the consolidation of fearful memories (39). In mammals, responses to anxiogenics can be modulated by cholinergic agents (40, 41), and subacute intoxication with organophosphate insecticides induces anxiogenic effects (42). Altogether, this finding strengthens the significance of our observations, emphasizes the role of AChE in environmental challenges, and adds cholinergic regulation and the ACHE-PON1 locus to the findings of others of genetic components of anxiety (3). The implications of the cholinergic system as an important area of investigation may thus extend to human anxiety disorders, in addition to state and trait measures.
A measured trait of anxiety increases substantially when tested after stress (43). This change in heritability estimates is generally thought of as an example of passive gene–environment interaction, and the consequence of differential expression of several polymorphic genes before and after stress. Our current findings support the notion that the ACHE, BCHE, and PON1 polymorphisms can be thought of as part of this polygenic background, and act as revealers of between, individual differences after stress or along one's lifetime. Because both AChE and PON are targets of organophosphates (PON hydrolyzes them and AChE is inhibited by them) (44), subjects with high PON activity may less frequently need to overproduce AChE (e.g., under exposure to anticholinesterases in fresh crops) (45) than those with low PON levels. Also, PON hydrolyzes peroxides, thus lowering the oxidative stress load and protecting other proteins (13), and AChE is particularly sensitive to oxidative stress (46).
At the expression level, the observed contribution of AChE regulation to the anxiety scores of otherwise healthy subjects may be relevant to the recently reported role of ACh in limiting the production of proinflammatory cytokines (47). Increased serum AChE, and consequently decreased ACh, would hence elevate the release of proinflammatory cytokines by macrophages. Therefore, serum AChE levels may potentially serve as predictor of one's risk of inflammatory responses. Anxiety-associated roles of such cytokines (48) likely contribute to their reported effects in autoimmune and atherosclerosis diseases. This finding, in turn, is compatible with the higher risk for inflammatory diseases with increasing age and BMI (49, 50). Anxiety scores, in generally healthy subjects, may hence be relevant both for psychological and physiological symptoms. Evaluation of anxiety by genomic and biochemical measures reflecting the cholinergic balance in the circulation provides a previously unforeseen approach for studying and perhaps controlling human anxiety.
At the applied level, anxiety disorders afflict approximately one of four individuals in the U.S. at some point in their lives, imposing both an individual and a social burden reaching a total cost of $42.3 billion in the U.S. in 1990 (2). A large part of these costs may reflect inappropriate or inefficient treatment of undiagnosed and misdiagnosed sufferers, highlighting the value of extensive recognition and effective diagnosis and treatment. The importance of finding valid measures and diagnostic tools to properly diagnose and treat anxiety patients lies in the hope to relieve this individual and social burden. In an era when pharmacogenomics became a reality (51), and gene-targeted therapies develop rapidly (52), anxiety disorders present a reachable challenge.
Supplementary Material
Acknowledgments
We thank Drs. Yoram Ben-Shaul, Ariel Darvasi, David Glick, and Sagiv Shifman (The Hebrew University, Jerusalem), for critically reviewing this manuscript, and all of the contributors to this study. The HERITAGE Consortium thanks those families whose participation made these data possible. This work was supported by U.S. Army Medical Research and Materiel Command Grant DAMD 17-99-1-9547; Defense Advance Research Project Agency Grant N66001-01-C-8015; German–Israeli Foundation Grant 673; the Israel Center for Neuronal Computation Lionel Perez award (to H.S); National Heart, Lung, and Blood Institute Grants HL45670 (to C.B.), HL47323 (to A.S.L.), HL47317 (to D.C.R.), HL47327 (to J.S.S.), and HL47321 (to J.H.W.). A.S.L. is partially supported by the Henry L. Taylor Endowed Professorship in Exercise Science and Health Enhancement. C.B. is partially supported by the George Bray Chair in Nutrition. E.H.S. was the incumbent of a Lichtenstein Travel Award from Jerusalem to Baton Rouge.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ACh, acetylcholine; AChE, acetylcholinesterase; AChE-R, readthrough AChE; PON, paraoxonase; BChE, butyrylcholinesterase; HERITAGE, Health, Risk Factors, Exercise Training, and Genetics; LD, linkage disequilibrium; STAI, state-trait-anxiety inventory; BMI, body mass index.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database [accession nos. AF539592 (PON1), AF002993 (ACHE), and NM000055 (BCHE)].
References
- 1.Weinberger, D. R. (2001) N. Engl. J. Med. 344, 1247–1249. [DOI] [PubMed] [Google Scholar]
- 2.Lepine, J. P. (2002) J. Clin. Psychiatry 63, Suppl. 14, 4–8. [PubMed] [Google Scholar]
- 3.Hariri, A. R., Mattay, V. S., Tessitore, A., Kolachana, B., Fera, F., Goldman, D., Egan, M. F. & Weinberger, D. R. (2002) Science 297, 400–403. [DOI] [PubMed] [Google Scholar]
- 4.Stein, D. J., Westenberg, H. G. & Liebowitz, M. R. (2002) J. Clin. Psychiatry 63, Suppl. 6, 12–19. [PubMed] [Google Scholar]
- 5.Degroot, A. & Treit, D. (2003) Neuroscience 117, 493–501. [DOI] [PubMed] [Google Scholar]
- 6.Borovicka, J., Schwizer, W., Mettraux, C., Kreiss, C., Remy, B., Asal, K., Jansen, J. B., Douchet, I., Verger, R. & Fried, M. (1997) Am. J. Physiol. 273, G374–G380. [DOI] [PubMed] [Google Scholar]
- 7.Everitt, B. J. & Robbins, T. W. (1997) Annu. Rev. Psychol. 48, 649–684. [DOI] [PubMed] [Google Scholar]
- 8.Battaglia, M. (2002) Mol. Psychiatry 7, 239–246. [DOI] [PubMed] [Google Scholar]
- 9.Pohjavaara, P. (2003) Nord. J. Psychiatry 57, 55–60. [DOI] [PubMed] [Google Scholar]
- 10.Mayer, E. A., Craske, M. & Naliboff, B. D. (2001) J. Clin. Psychiatry 62, Suppl. 8, 28–37. [PubMed] [Google Scholar]
- 11.Taylor, P. (1996) in Goodman and Gilman's The Pharmacological Basis of Therapeutics, eds. Hardman, J. G., Limbird, L. E., Molinoff, P. B. & Ruddon, R. W. (McGraw–Hill, New York), pp. 177–197.
- 12.Davies, H. G., Richter, R. J., Keifer, M., Broomfield, C. A., Sowalla, J. & Furlong, C. E. (1996) Nat. Genet. 14, 334–336. [DOI] [PubMed] [Google Scholar]
- 13.Aviram, M., Rosenblat, M., Bisgaier, C. L., Newton, R. S., Primo-Parmo, S. L. & La Du, B. N. (1998) J. Clin. Invest. 101, 1581–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rozenberg, O., Rosenblat, M., Coleman, R., Shih, D. M. & Aviram, M. (2003) Free Radical Biol. Med. 34, 774–784. [DOI] [PubMed] [Google Scholar]
- 15.Kaufer, D., Friedman, A., Seidman, S. & Soreq, H. (1998) Nature 393, 373–377. [DOI] [PubMed] [Google Scholar]
- 16.Meshorer, E., Erb, C., Gazit, R., Pavlovsky, L., Kaufer, D., Friedman, A., Glick, D., Ben-Arie, N. & Soreq, H. (2002) Science 295, 508–512. [DOI] [PubMed] [Google Scholar]
- 17.Birikh, K. R., Sklan, E. H., Shoham, S. & Soreq, H. (2003) Proc. Natl. Acad. Sci. USA 100, 283–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gray, J. A. (2000) The Neuropsychology of Anxiety: An Inquiry into the Functions of the Septo-Hippocampal System (Oxford Univ. Press, Oxford).
- 19.Bouchard, C., Leon, A. S., Rao, D. C., Skinner, J. S., Wilmore, J. H. & Gagnon, J. (1995) Med. Sci. Sports Exercise 27, 721–729. [PubMed] [Google Scholar]
- 20.Rankinen, T., Rice, T., Leon, A. S., Skinner, J. S., Wilmore, J. H., Rao, D. C. & Bouchard, C. (2002) Physiol. Genomics 8, 151–157. [DOI] [PubMed] [Google Scholar]
- 21.Devlin, B. & Risch, N. (1995) Genomics 29, 311–322. [DOI] [PubMed] [Google Scholar]
- 22.Slatkin, M. & Excoffier, L. (1996) Heredity 76, 377–383. [DOI] [PubMed] [Google Scholar]
- 23.Ihaka, R. & Gentleman, R. (1996) J. Comput. Graph. Stat. 5, 299–314. [Google Scholar]
- 24.Breiman, L., Friedman, J. H., Olshen R, A. & Stone, C. J. (1984) Wardsworth (Belmont, CA).
- 25.Spielberger, C. (1972) Anxiety: Current Trends in Theory and Research (Academic, New York).
- 26.Seki, E., Watanabe, Y., Sunayama, S., Iwama, Y., Shimada, K., Kawakami, K., Sato, M., Sato, H., Mokuno, H. & Daida, H. (2003) Circ. J. 67, 73–77. [DOI] [PubMed] [Google Scholar]
- 27.Wolf, D. L., Desjardins, P. J., Black, P. M., Francom, S. R., Mohanlal, R. W. & Fleishaker, J. C. (2003) J. Clin. Psychopharmacol. 23, 51–57. [DOI] [PubMed] [Google Scholar]
- 28.Vedhara, K., Miles, J., Bennett, P., Plummer, S., Tallon, D., Brooks, E., Gale, L., Munnoch, K., Schreiber-Kounine, C., Fowler, C., et al. (2003) Biol. Psychol. 62, 89–96. [DOI] [PubMed] [Google Scholar]
- 29.Soreq, H. & Seidman, S. (2001) Nat. Rev. Neurosci. 2, 294–302. [DOI] [PubMed] [Google Scholar]
- 30.Brenner, T., Hamra-Amitay, Y., Evron, T., Boneva, N., Seidman, S. & Soreq, H. (2003) FASEB J. 17, 214–222. [DOI] [PubMed] [Google Scholar]
- 31.Shapira, M., Tur-Kaspa, I., Bosgraaf, L., Livni, N., Grant, A. D., Grisaru, D., Korner, M., Ebstein, R. P. & Soreq, H. (2000) Hum. Mol. Genet. 9, 1273–1281. [DOI] [PubMed] [Google Scholar]
- 32.Bartels, C. F., Zelinski, T. & Lockridge, O. (1993) Am. J. Hum. Genet. 52, 928–936. [PMC free article] [PubMed] [Google Scholar]
- 33.Costa, L. G., Cole, T. B., Jarvik, G. P. & Furlong, C. E. (2003) Annu. Rev. Med. 54, 371–392. [DOI] [PubMed] [Google Scholar]
- 34.Neville, L. F., Gnatt, A., Padan, R., Seidman, S. & Soreq, H. (1990) J. Biol. Chem. 265, 20735–20738. [PubMed] [Google Scholar]
- 35.Loewenstein-Lichtenstein, Y., Schwarz, M., Glick, D., Norgaard Pedersen, B., Zakut, H. & Soreq, H. (1995) Nat. Med. 1, 1082–1085. [DOI] [PubMed] [Google Scholar]
- 36.Dantoine, T. F., Drouet, M., Debord, J., Merle, L., Cogne, M. & Charmes, J. P. (2002) Ann. N.Y. Acad. Sci. 977, 239–244. [DOI] [PubMed] [Google Scholar]
- 37.Brophy, V. H., Jampsa, R. L., Clendenning, J. B., McKinstry, L. A., Jarvik, G. P. & Furlong, C. E. (2001) Am. J. Hum. Genet. 68, 1428–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCullagh, P. & Nelder, J. A. (1989) Generalized Linear Models (Chapman & Hall, London).
- 39.Nijholt, I., Farchi, N., Kye, M., Sklan, E. H., Shoham, S., Verbeure, B., Owen, D., Hochner, B., Spiess, J., Soreq, H. & Blank, T. (2003) Mol Psychiatry, 9, 174–183. [DOI] [PubMed] [Google Scholar]
- 40.File, S. E., Gonzalez, L. E. & Andrews, N. (1998) Behav. Neurosci. 112, 352–359. [DOI] [PubMed] [Google Scholar]
- 41.Dazzi, L., Vacca, G., Ladu, S., Pisu, M. G., Serra, M. & Biggio, G. (2001) Neuropharmacology 41, 229–237. [DOI] [PubMed] [Google Scholar]
- 42.Sanchez-Amate, M., Flores, P. & Sanchez-Santed, F. (2001) Behav. Pharmacol. 12, 285–292. [DOI] [PubMed] [Google Scholar]
- 43.Kendler, K. S. (1993) Arch. Gen. Psychiatry 50, 905–915. [DOI] [PubMed] [Google Scholar]
- 44.Furlong, C. E., Li, W. F., Brophy, V. H., Jarvik, G. P., Richter, R. J., Shih, D. M., Lusis, A. J. & Costa, L. G. (2000) Neurotoxicology 21, 581–587. [PubMed] [Google Scholar]
- 45.McGehee, D. S., Krasowski, M. D., Fung, D. L., Wilson, B., Gronert, G. A. & Moss, J. (2000) Anesthesiology 93, 510–519. [DOI] [PubMed] [Google Scholar]
- 46.Weiner, L., Kreimer, D., Roth, E. & Silman, I. (1994) Biochem. Biophys. Res. Commun. 198, 915–922. [DOI] [PubMed] [Google Scholar]
- 47.Tracey, K. J. (2002) Nature 420, 853–859. [DOI] [PubMed] [Google Scholar]
- 48.Anisman, H. & Merali, Z. (2003) Ann. Med. 35, 2–11. [DOI] [PubMed] [Google Scholar]
- 49.Saito, I., Yonemasu, K. & Inami, F. (2003) Circ. J. 67, 323–329. [DOI] [PubMed] [Google Scholar]
- 50.Roubenoff, R. (2003) Curr. Opin. Clin. Nutr. Metab. Care 6, 295–299. [DOI] [PubMed] [Google Scholar]
- 51.Evans, W. E. & McLeod, H. L. (2003) N. Engl. J. Med. 348, 538–549. [DOI] [PubMed] [Google Scholar]
- 52.Opalinska, J. B. & Gewirtz, A. M. (2002) Nat. Rev. Drug Discov. 1, 503–514. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.