Abstract
Receptor tyrosine kinases of the Eph family are up-regulated in different types of cancer. EphB4 and its ligand ephrin-B2 have been linked to breast cancer, but little is known about how this receptor–ligand complex may contribute to oncogenesis. The Eph receptors transmit forward signals via their kinase domain and reverse signals via their transmembrane ephrin-B ligands. Therefore, we used EphB4 that were lacking the kinase domain and tagged with EGFP (EphB4ΔC-EGFP) to differentiate between EphB4 and ephrin-B2 signaling. Interestingly, we found that expression of EphB4ΔC-EGFP in breast cancer cells increases tumor growth in a mouse xenograft model. Given the undetectable EphB4 activation in the tumor cells, dominant negative effects of EphB4ΔC-EGFP are unlikely to explain the increased tumor growth. Examination of the tumors revealed that ephrin-B2 is primarily expressed in the vasculature and that the EphB4ΔC-EGFP tumors have a higher blood content than control tumors, concomitant with increased size of blood vessels. In support of an effect on the vasculature, the extracellular domain of EphB4 attracts endothelial cells in vitro and stimulates endothelial cell invasion, survival, and proliferation, all crucial factors for angiogenesis. These results support a model in which EphB4 promotes tumor growth by stimulating angiogenesis through ephrin-B2.
Several Eph receptors are overexpressed in various tumor types, suggesting that they play a role in cancer progression (1). In particular, EphB4 has been implicated in breast cancer. In transgenic mouse models of mammary carcinogenesis, the level of EphB4 expression correlates with the degree of tumor malignancy (2, 3). Importantly, high transgenic expression of EphB4 in the mammary gland accelerates the growth of tumors caused by the NeuT oncogene and results in more aggressive and invasive tumors (4). In human breast cancers, EphB4 has been reported to be elevated in primary infiltrating ductal breast carcinomas with a high grade of malignancy (5). In another report (6), however, EphB4 was negatively correlated with tumor growth. Interestingly, EphB4-positive cells were often found at the tumor borders and in regions that are rich in capillaries. EphB4 is also up-regulated in endometrial hyperplasias and carcinomas, small lung carcinomas, and colon carcinomas (refs. 7, 8, and references therein).
Despite the accumulating evidence linking them to cancer, very little is known about how the Eph receptors contribute to the oncogenic process (1, 9). These receptors are a large family of transmembrane tyrosine kinases and comprise two groups: EphA and EphB. All Eph receptors have an extracellular portion (ectodomain), which contains the ligand-binding domain at the N terminus, and a cytoplasmic portion with the tyrosine kinase domain. Interaction of the Eph receptors with their ligands, the ephrins, requires cell–cell contact because both the receptor and the ligand are membrane-bound. The A-ephrins are GPI-linked and preferentially bind EphA receptors and the B-ephrins are transmembrane proteins and preferentially bind EphB receptors.
In the normal mouse mammary gland, EphB4 is predominantly localized to the myoepithelial cells that surround the luminal epithelium lining the ducts and alveoli (2). A balanced expression of ephrin-B2, the preferred ligand for EphB4 (10), in the luminal epithelium and EphB4 in the adjacent myoepithelial cells contributes to normal mammary gland morphogenesis and is regulated by estrogen (2, 4). This balance is disrupted when mammary epithelial cells become transformed: ephrin-B2 expression in the epithelial cells is lost, whereas EphB4 expression increases (2). This down-regulation of the ephrin-B2 ligand suggests that the effects of EphB4 on tumor progression may be independent of its activation by ligand. Eph receptors have both kinase-dependent and kinase-independent functions (9). The kinase-independent functions are mediated by the Eph receptor ectodomain, which binds ephrin ligands on the surface of adjacent cells. Binding to EphB4 enhances the ability of ephrin-B2 to transduce signals through its cytoplasmic domain. Signaling through ephrin-B2 in endothelial cells, for example, is critical for vascular development in the embryo (11, 12).
Consistent with a kinase-independent role of EphB4 in tumors, we found that increased expression of a signaling-defective form of EphB4 in breast cancer cells makes tumor xenografts grow more rapidly and influences the tumor vasculature, which expresses ephrin-B2. Furthermore, our in vitro experiments show that the ectodomain of EphB4 exerts an attractive effect on endothelial cells and promotes their proliferation and survival. These results suggest that EphB4 plays a role in tumor progression by promoting angiogenesis.
Materials and Methods
Cell Lines and Transfections. MDA-MB-435 cells were grown in DMEM supplemented with 10% FBS. For transfections, 6-cm plates were transfected with 5 μg of DNA and 30 μl of SuperFect (Invitrogen). Transfected cells were grown for 24 h, replated in 15-cm plates, and selected with 800 μg/ml G418. The ephrin-B2ΔC-EGFP construct consists of human ephrin-B2 amino acids 1–265 in the pEGFP-N2 vector (Clontech). The EphB4ΔC-EGFP construct is described in ref. 13, and the EGFP-F vector was obtained from Clontech. Human umbilical vein endothelial (HUVE) cells were grown in microvascular endothelial cell medium 2 (EGM-2MV; Clonetics, San Diego).
Tumor Xenografts. Transfected MDA-MB-435 cells (106 cells per mouse in 0.2 ml) were injected into the mammary fat pad region of 4- to 5-week-old female BALB/c nude mice. Equal numbers of cells from three transfected lines were mixed, except for in the experiment shown in Fig. 4C, in which individual clones and untransfected cells were injected. Tumor volumes were measured with calipers and calculated as (l × w × d)/2, where l is length, w is width, and d is diameter. Two portions of tumor tissue were removed from the opposite ends of each tumor and frozen for biochemical and PCR analyses and hemoglobin assays. The remaining tissue was fixed overnight in 4% paraformaldehyde in PBS at 4°C, divided in half and scanned to record the tumor appearance (see Fig. 5 A and B). One half of the tumor was incubated in 3% sucrose and then frozen in Tissue-Tek OCT compound (Sakura Finetek, Torrance, CA), and the other half was processed for paraffin embedding.
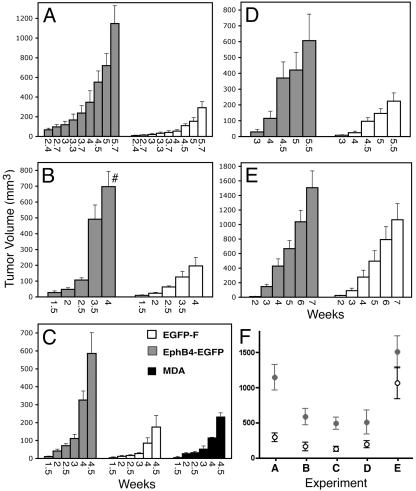
Fig. 4.
EphB4ΔC-EGFP enhances tumor growth in a xenograft model. MDA-MB-435 cells expressing EphB4ΔC-EGFP or EGFP-F and untransfected cells were injected in nude mice, and tumor sizes were measured at the indicated time points. The graphs in A–E show average tumor volumes ± SEM in groups of five to seven mice, except for the untransfected MDA cells shown in C, which were injected in two mice. #, One of the mice in this group was killed before this measurement. (F) Data used for two-way ANOVA analysis, which include one time point (the last time point for which all tumors in each group were still available) for each of the experiments shown in A–E. EphB4ΔC-EGFP increases tumor growth significantly (P < 0.001).
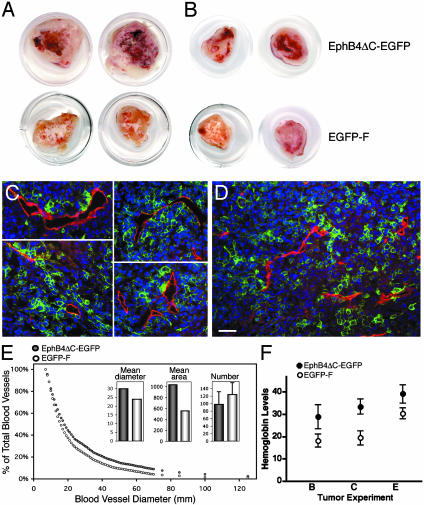
Fig. 5.
Effects of EphB4ΔC-EGFP and EGFP-F on tumor vascularization. (A and B) Tumors expressing EphB4ΔC-EGFP or EGFP-F from the experiment shown in Fig. 4A (A) and B (B). (C and D) Fluorescent images of frozen sections from EphB4ΔC-EGFP (C) or EGFP-F (D) tumors stained for CD-31 (red). Green fluorescent tumor cells are in close contact with red endothelial cells (overlap shown in yellow). (Scale bar represents 50 μm.) (E) Distribution of blood vessels with diameters larger than indicated on the abscissa. Blood vessels from EphB4ΔC-EGFP tumors are larger that those from EGFP-F tumors (P < 0.01, by Kolmogorov–Smirnov test). Mean diameter sizes (30 μm versus 24 μm, P < 0.0001 by t test), mean area sizes (1,037 μm2 versus 559 μm2; P < 0.0001 by t test), and mean numbers of blood vessels per photograph ± SD (98 ± 34 versus 124 ± 32, P < 0.01 by t test) are shown in Insets. (F) Increased hemoglobin concentration in EphB4ΔC-EGFP tumors compared with EGFP-F tumors. Means for the experiments shown in Fig. 4 B, C, and E ± SEM are shown. P < 0.002 by two-way ANOVA.
RT-PCR. mRNA isolated from human MDA-MB-435 tumor cells, tumor tissues derived from MDA-MB-435 cells, or postnatal day 1 mouse brain were reverse transcribed and amplified. For further details, see Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
Biochemistry and Immunohistochemistry. The immunoprecipitations were performed according to standard methods (14). See Supporting Materials and Methods for more details. For immunofluorescence staining, 10 μg/ml anti-CD31 rat mAb (Pharmingen) were used, followed by 10 μg/ml anti-rat Ab conjugated to Alexa Fluor 594 (Molecular Probes) in the same diluent.
Quantification of Hemoglobin and Blood Vessels in Tumors. Tumor tissue was homogenized in PBS with protease inhibitors. Hemoglobin concentration was calculated by using the Total Hemoglobin kit (Sigma) and then normalized for protein concentration. Quantification of tumor blood vessels by image analysis of CD-31-stained frozen sections is described in Supporting Materials and Methods.
Migration Assays. For cell-attraction assays, droplets containing 25,000 EphB4ΔC-EGFP or EGFP-F cells were allowed to adhere to dried fibronectin-coated coverslips for 30 min several millimeters away from droplets of HUVE cells labeled with Cell Tracker Orange (Molecular Probes). Unattached cells were washed, and growth medium was added. Coverslips were fixed after 30 h, and the HUVE cells that had migrated into the tumor cell fields were counted. For Transwell migration assays, the undersides of Transwell polycarbonate inserts (8-μm pore; Corning) were coated with 30 μg/ml collagen I, and serum-starved HUVE cells were allowed to migrate toward 4 μg/ml EphB4 Fc (R & D Systems), Fc, or 10 ng/ml basic fibroblast growth factor in serum-free medium for 3 h at 37°C. Cells that had migrated to the underside were detected by 4′,6-diamidino-2-phenylindole (DAPI) staining. For migration assays toward immobilized Fc proteins, the insert undersides were coated with 4 μg/ml EphB4 Fc or Fc for 1 h at 37°C. For cell invasion assays, QCM Collagen-Based inserts (Chemicon) were rehydrated, and 0.5 ml of growth medium supplemented with 1% FBS and 4 μg/ml EphB4 Fc, Fc, or 10 ng/ml basic fibroblast growth factor was added to the bottom chamber. After 24 h, DAPI-stained HUVE cells were counted.
Cell Proliferation and Apoptosis Assays. For MDA-MB-435 cell-proliferation assays, cells were plated in 24-well plates (15,000 cells per well) in growth medium supplemented with 3 μg/ml Fc or ephrin-B2 Fc clustered with 0.3 μg/ml anti-Fc Abs. Ephrin-B2 Fc and Fc were added again at day 2. Cells were collected every 24 h, stained with Trypan blue, and counted. We confirmed that the decreased numbers of ephrin-B2 Fc treated cells were not a consequence of defects in cytokinesis by counting DAPI-stained nuclei in attached cells (data not shown). For HUVE cell proliferation, 20,000 cells per well were plated in triplicate and cells were treated with 3 μg/ml clustered EphB4 Fc or Fc. Additionally, on day 3, one-fourth of the cells were replated to allow sufficient growth.
To measure apoptosis, HUVE cells were plated on fibronectin-coated coverslips overnight in growth medium. Cells were then incubated in serum-free medium supplemented with 3 μg/ml clustered EphB4 Fc or Fc for 24 h. Apoptosis was detected by using the ApopTag apoptosis detection system (Serologicals, Clarkston, GA).
Results
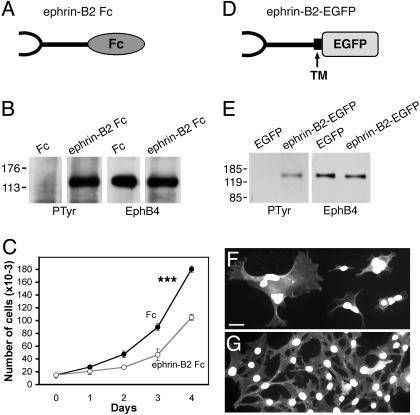
EphB4 Activation Inhibits MDA-MB-435 Cancer Cell Growth. To examine the effects of EphB4 activation in MDA-MB-435 human breast cancer cells, we treated two clonal cell lines with soluble clustered ephrin-B2 Fc (Fig. 1A). Ephrin-B2 caused EphB4 tyrosine phosphorylation and inhibited cell proliferation significantly (Fig. 1 B and C, and data not shown). Also, we made a form of ephrin-B2 that can be expressed on the cell surface and has EGFP (ephrin-B2ΔC-EGFP) in place of the cytoplasmic domain (Fig. 1D). Ephrin-B2ΔC-EGFP activated EphB4 effectively (Fig. 1E). On neomycin selection to isolate stably transfected clones, we repeatedly obtained small groups of green fluorescent cells that survived for several weeks, but we were unable to generate colonies. Many of these cells were abnormally large and multinucleated, in contrast to cells expressing EGFP-F (Fig. 1 F and G), suggesting that persistently elevated EphB4 activation causes defects in cytokinesis. These results indicate that the EphB4 receptor is competent to signal in the MDA-MB-435 cells. However, the low basal levels of EphB4 tyrosine phosphorylation in these cells (Fig. 1 B and E) and the negative effects of EphB4 activation on cell proliferation suggest that EphB4 kinase activity is not critical for tumor progression.
Fig. 1.
EphB4 activation by ephrin-B2 inhibits MDA-MB-435 cancer cell proliferation. (A) Schematic representation of ephrin-B2 Fc. (B) Soluble ephrin-B2 induces EphB4 tyrosine phosphorylation. MDA-MB-435 cells were treated with clustered ephrin-B2 Fc or Fc for 20 min. EphB4 immunoprecipitates were probed with antiphosphotyrosine (PTyr) Abs and reprobed with anti-EphB4 Abs. (C) Soluble ephrin-B2 inhibits MDA-MB-435 cell proliferation (P < 0.001; two-way ANOVA). Cells were grown in the presence of ephrin-B2 Fc or Fc and counted at the indicated time points. (D) Schematic representation of ephrin-B2ΔC-EGFP; TM, transmembrane domain. (E) Membrane-bound ephrin-B2 induces EphB4 tyrosine phosphorylation. MDA-MB-435 cells were transiently transfected with ephrin-B2ΔC-EGFP. EphB4 immunoprecipitates were probed with antiphosphotyrosine (PTyr) Abs and reprobed with anti-EphB4 Abs. (F and G) Membrane-bound ephrin-B2 causes defects in cell division. MDA-MB-435 cells transfected with ephrin-B2ΔC-EGFP (F) and EGFP-F (G) were stained with DAPI (white) to label nuclei. (Scale bar represents 30 μm.)
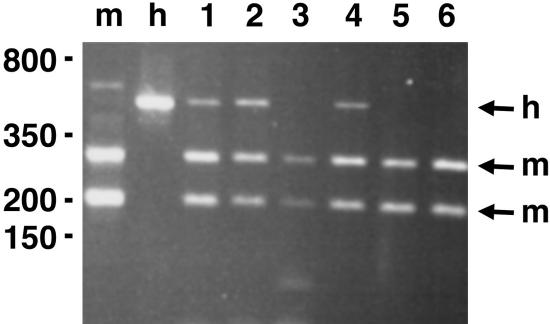
Ephrin-B2 Is Preferentially Expressed in the Blood Vessels of MDA-MB-435 Tumors. In addition to signaling through it kinase domain, EphB4 can signal through activation of ephrin-B2 on adjacent cells. Because genetic studies have shown that signaling through the ephrin-B2 cytoplasmic domain plays an essential role in angiogenesis (11, 12), we examined whether ephrin-B2 is expressed in the blood vessels of MDA-MB-435 xenografts grown in nude mice. In these tumors, ephrin-B2 is of human origin in the tumor cells and of mouse origin in the vasculature. We performed RT-PCR by using tumors derived from mixed cell populations as well as individual cell clones. By using primers that amplify both the mouse and human ephrin-B2, followed by restriction digestion to differentiate between the two, we detected predominantly mouse ephrin-B2 (Fig. 2). In fact, human ephrin-B2 was detected in only one of the four clonal tumors. Thus, ephrin-B2 expression is low and heterogeneous in the MDA-MB-435 cells. It should be noted that the human breast cancer cells constitute the majority of the tumor mass. Hence, the predominant amplification of mouse ephrin-B2 suggests that this ligand is highly expressed in the cells of mouse origin, which include vascular cells and blood cells. However, only a very small percentage of monocytes and macrophages express ephrin-B2 in vivo (10, 15). We also detected ephrin-B1 and ephrin-B3 in the tumor cells, as well as low levels of ephrin-B1 in the endothelial cells (see Fig. 7, which is published as supporting information on the PNAS web site). These ephrins, however, do not bind to EphB4 efficiently (10). These results suggest that ephrin-B2 is expressed preferentially in the blood vessels of MDA-MB-435 tumors.
Fig. 2.
Ephrin-B2 is preferentially expressed in the blood vessels of MDA-MB-435 tumor xenografts. Mouse and human ephrin-B2 were simultaneously PCR-amplified from tumors derived from mixed populations of MDA-MB-435 cells (lanes 1 and 2) or clonal cells expressing EphB4ΔC-EGFP (lanes 3 and 4) or EGFP-F (lanes 5 and 6). The control PCR products were from mouse P1 brain (m) and cultured mixed populations of MDA-MB-435 cells (h). Digestion with a restriction enzyme that recognizes only the mouse ephrin-B2 shows that in the tumors ephrin-B2 was amplified predominantly from mouse cells. Digestion with a human-specific enzyme confirmed this conclusion (data not shown).
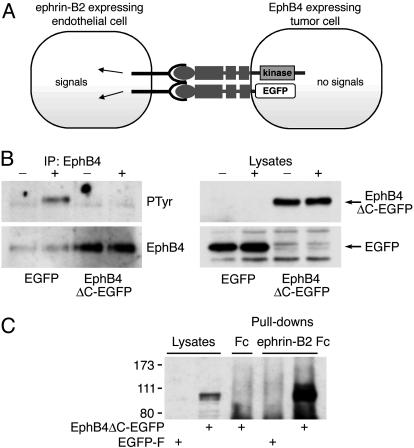
A Signaling-Defective Form of EphB4 Promotes MDA-MB-435 Tumor Growth. To assess the kinase-independent functions of EphB4 in tumor growth, we engineered a form of EphB4 containing the extracellular and transmembrane domains fused to EGFP (EphB4ΔC-EGFP) (Fig. 3A). Because it lacks the kinase domain, EphB4ΔC-EGFP cannot transduce forward signals in the tumor cells (Fig. 3A). On the contrary, it acts as a dominant negative to block EphB4 activation by ligand (Fig. 3B). However, EphB4ΔC-EGFP retains the ability to bind ephrin-B2 (Fig. 3C).
Fig. 3.
EphB4ΔC-EGFP inhibits EphB4 signaling and promotes ephrin-B2 signaling. (A) Schematic representation. (B) Stable MDA-MB-435 cells expressing EGFP-F or EphB4ΔC-EGFP were treated with clustered ephrin-B2 Fc or Fc for 20 min, and endogenous EphB4 was immunoprecipitated. The blots, probed for phosphotyrosine (PTyr) and reprobed for EphB4, show that EphB4ΔC-EGFP inhibits endogenous EphB4 activation. Lysates were probed with anti-EGFP Abs to visualize transfected proteins. (C) Ephrin-B2 Fc was used for pull-down experiments with cells transiently transfected with EphB4ΔC-EGFP or EGFP-F. The blot, probed with anti-EphB4 Abs, shows that EphB4ΔC-EGFP binds to ephrin-B2 Fc.
We prepared MDA-MB-435 cell lines stably transfected with EphB4ΔC-EGFP and, as a control, farnesylated EGFP-F. Both constructs localized to the surface of cultured cells, as expected (data not shown). Three EphB4ΔC-EGFP-expressing clones, or three EGFP-F clones, were mixed and injected in the mammary fat-pad region of nude mice to seed mammary tumors. The EphB4ΔC-EGFP tumors grew substantially faster than the EGFP-F tumors (Figs. 4A and 5A). This effect was confirmed by using mixtures of the same cell lines after FACS sorting to isolate the 20% most-fluorescent cells (Fig. 4B). Each of the three EphB4ΔC-EGFP and EGFP-F sorted cell lines were also injected individually and compared with untransfected MDA-MB-435 cell populations (Fig. 4C). The EphB4ΔC-EGFP tumors again grew faster than the EGFP-F tumors, indicating that the faster growth of the EphB4ΔC-EGFP tumors is not caused by the presence of a single fast-growing clone or a clone secreting a growth-promoting or angiogenic factor. The tumors from the EGFP-F cells grew similarly to those from untransfected cells, indicating that EGFP-F does not negatively affect tumor growth. To verify these results further, we repeated the experiment shown in Fig. 4A two more times by using mixtures of three cell lines obtained from two additional independent transfections (Fig. 4 D and E). Together, these experiments confirm a highly significant effect of EphB4ΔC-EGFP on tumor growth (Fig. 4F; P < 0.001).
Immunoblotting experiments confirmed that EphB4ΔC-EGFP and EGFP-F were expressed in tumor tissue and EphB4ΔC-EGFP was present at much higher levels than endogenous EphB4 (see Fig. 8A, which is published as supporting information on the PNAS web site). Furthermore, the transfected proteins were readily detectable on the tumor cell surface by fluorescence microscopy (shown for EphB4ΔC-EGFP in Fig. 8B).
An advantage of marking tumor cells with a fluorescent protein is that metastatic cells can be readily identified (see Fig. 9, which is published as supporting information on the PNAS web site). Many lung metastases were detected at 9 weeks in the control mice bearing EGFP-F tumors, consistent with reports (16) that MDA-MB-435 tumors metastasize to the lungs. EphB4ΔC-EGFP slightly increased the number of larger metastases, but the difference did not reach significance.
The EphB4 Ectodomain Promotes Tumor Vascularization. Examination of the transfected cell clones revealed that expression of EphB4ΔC-EGFP does not affect MDA-MB-435 cell proliferation in culture (see Fig. 10, which is published as supporting information on the PNAS web site). Thus, the differential in vitro and in vivo effects of EphB4ΔC-EGFP on breast cancer cells are consistent with the hypothesis that EphB4 promotes tumor growth in vivo by means of effects on ephrin-B2-positive tumor endothelial cells.
We noticed that the EphB4ΔC-EGFP tumors from the experiment in Fig. 4A contained more blood than the EGFP-F tumors (Fig. 5A). Quantitation of CD31-stained sections by image analysis revealed that the blood vessels in the outer regions of the EphB4ΔC-EGFP tumors had slightly lower density but larger size than those in the EGFP tumors (Fig. 5 C–E, and see Fig. 11, which is published as supporting information on the PNAS web site). Interestingly, the fractional area occupied by blood vessels in the tumor sections was 1.4-fold higher in the EphB4ΔC-EGFP tumors than in the EGFP-F tumors (3.8% versus 2.7%, respectively). However, it is not known whether these vascular differences could depend on the large difference in tumor sizes because, in this experiment, all of the tumors were collected at the same time. Therefore, in the later experiments, we collected the tumors when they reached a volume of ≈1cm3, regardless of the time from the injection. The EphB4ΔC-EGFP tumors that were of a similar size to EGFP-F tumors still appeared to contain more blood (Fig. 5B). By using a quantitative assay to measure the average hemoglobin content in the tumor tissue from the experiments in Figs. 4 B, C, and E, we found a 1.5-fold increase in the amount of hemoglobin in the EphB4ΔC-EGFP versus EGFP-F tumors (Fig. 5F). Thus, these tumors contain more blood, consistent with the larger size of their blood vessels.
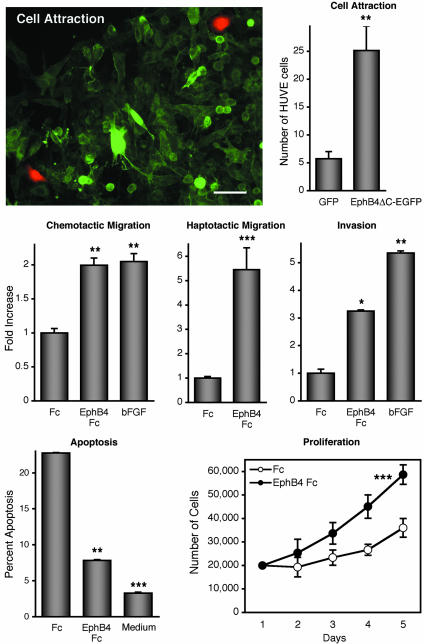
The EphB4 Ectodomain Promotes Endothelial Cell Migration, Survival, and Proliferation. To examine the effects of EphB4 on ephrin-B2-expressing endothelial cells, we performed in vitro assays with HUVE cells (17) (Fig. 6). Interestingly, in a lateral migration assay, tumor cells with EphB4ΔC-EGFP on their surface attracted HUVE cells significantly better than EGFP-F cells. To verify the effects of EphB4 and to eliminate possible confounding effects of chemoattractants that may be secreted by the EphB4ΔC-EGFP cells, we also performed Transwell migration assays. In these assays, the soluble EphB4 ectodomain fused to human Fc (EphB4 Fc) exerted a chemoattractive effect on the endothelial cells. EphB4 Fc also had an attractive effect when immobilized on the surface on which the cells migrated, therefore mimicking its association with the cell surface. In addition, EphB4 Fc stimulated endothelial cell invasion through a collagen gel, suggesting that EphB4 expression on tumor cells promotes colonization of the tumor mass by endothelial cells initiating new blood vessels. Furthermore, EphB4 Fc increased the proliferation of HUVE cells and their survival under conditions of low nutrients (serum-free medium), such as those that may be found in the tumor environment. These data support the notion that the EphB4 ectodomain promotes angiogenesis through ephrin-B2 in endothelial cells.
Fig. 6.
The ectodomain of EphB4 promotes endothelial cell migration, invasion, survival, and proliferation. (Top) In a lateral migration assay, EphB4ΔC-EGFP tumor cells attract more HUVE cells (red) than EGFP-F cells. (Scale bar represents 50 μM.) HUVE cells that had migrated into the areas of green tumor cells were quantified. (Middle) Transwell or collagen-based invasion assays were used to measure HUVE cell migration or invasion toward soluble or immobilized EphB4 Fc, Fc, or basic fibroblast growth factor. (Bottom Left) HUVE cells were serum-starved in the presence of EphB4 Fc or Fc. Percentage of apoptosis was calculated by counting the number of apoptotic cells per DAPI-stained nuclei. The experiments described above were performed in triplicate and repeated at least three times. Data represent the mean ± SEM. *, P < 0.05; **, P < 0.01; and ***, P < 0.001, compared with control by one-way ANOVA and Tukey's post hoc test. (Bottom Right) HUVE cells grown in medium supplemented with EphB4 Fc or Fc were counted, and the mean ± SD is shown for one of four independent experiments. P < 0.001 by two-way ANOVA.
Discussion
Here, we report that a kinase-defective form of the EphB4 receptor promotes the growth of MDA-MB-435 tumor xenografts. We propose that tumor cells displaying EphB4 on their surface function as support cells for ephrin-B2-positive vascular cells and promote the formation of blood vessels that drive increased tumor growth. The blood vessels in the EphB4ΔC-EGFP tumors have lower density but contain more blood than those in the EGFP-F tumors. Similar to its role in the embryonic vasculature, ephrin-B2 signaling may promote remodeling of immature tumor vascular networks, with the pruning of some vessels and enlargement of others (11, 12). This type of vascular changes was observed also in the ear vasculature of transgenic mice overexpressing ephrin-B2 in endothelial cells (18). Interestingly, other angiogenic factors that promote blood vessel remodeling and maturation, such as angiopoietin 1, also preferentially increase the size rather than the density of blood vessels (19, 20). Indeed, the effects of ephrin-B2 may be at least in part mediated by up-regulation of the angiopoietin 1 receptor Tie2 (12). Thus, we propose a role for ephrin-B2 in promoting more efficient tumor vascularization. It will be interesting to determine whether EphB receptor-expressing tumors have a less chaotic blood flow and lower interstitial fluid pressure, which may make them more accessible to delivered drugs (21).
The role of ephrin-B2 signaling in blood vessels is in contrast to the effects of VEGF overexpression, which has been shown to increase blood vessel numbers disproportionately to the increase in tumor mass, resulting in higher vascular densities (ref. 22 and references therein). VEGF-induced blood vessels are typically immature, tortuous, and leaky, and they require additional factors to become fully functional (20). Interestingly, VEGF up-regulates ephrin-B2 in endothelial cells (23), which may account for the presence of ephrin-B2 in the vasculature of the MDA-MB-435 tumors as well as other tumors (24–26). Therefore, ephrin-B2 may work in concert with other factors, such as VEGF, to promote angiogenesis when EphB receptors on surrounding cells enable ephrin-B2 signaling.
Our data suggest that endothelial ephrin-B2 promotes the formation of new blood vessels in EphB4-positive tumor tissue, in addition to promoting vascular remodeling. This effect is not reflected in increased vessel density, however, because of the even greater increase in tumor cell growth. Mural cells, such as smooth muscle cells and pericytes, and other surrounding cells, such as astrocytes in the nervous system, have been proposed to guide and stabilize newly formed blood vessels (20, 27). In at least some cases, these changes in vascularization may involve an ephrin-B-EphB interaction between endothelial cells and surrounding cells (28). Endothelial ephrin-B2 is indeed required for vascularization of the EphB receptor-positive neural tube (11). Further supporting the idea that EphB4 on cells surrounding endothelial cells can affect blood vessels, vascular abnormalities have been noted in the mammary gland and kidney of MMTV-EphB4 transgenic mice (29). By expressing on their surface attractive and adhesive molecules for endothelial cells, tumor cells may mediate endothelial cell penetration during invasive angiogenesis and stabilize newly formed blood vessels.
Several mechanisms could contribute to the proangiogenic effects of ephrin-B2. First, our in vitro migration assays show that endothelial cells are attracted toward the EphB4-expressing tumor cells. Ephrin-B2-mediated endothelial cell adhesion to EphB4 and increased cell–extracellular matrix adhesion in response to ephrin-B2 signaling (30, 31) could contribute to this effect. Second, ephrin-B1 activates Jun N-terminal kinase (JNK) (30, 32) and ephrin-B2 likely shares similar signaling mechanisms. Thus, activation of JNK by ephrin-B2 could up-regulate the expression of proteases that degrade the extracellular matrix to allow penetration of endothelial cells into the tumor (33). These findings are consistent with our data that ephrin-B2 activation increases endothelial cell invasion into a collagen gel. Ephrin-B2-mediated activation of JNK and the PI3 kinase pathway may also contribute to the increased endothelial cell survival that we observed in response to serum starvation in the presence of EphB4 Fc (34). Third, we found that stimulating ephrin-B2 signaling increases HUVE cell proliferation, in agreement with recent data on retinal endothelial cells (34). Fourth, Src family kinases are important for ephrin-B mediated angiogenesis in vitro (35) and may also play an important role in tumor angiogenesis. In agreement with our observations, other evidence also supports a role for EphB receptors in promoting an angiogenic response in ephrin-B-positive endothelial cells. For instance, EphB3 Fc and EphB4 Fc induce robust endothelial cell sprouting in vitro (12, 31, 35) and EphB1 Fc presented in the stroma induces vascularization of the mouse cornea (30).
Despite the low overall EphB4 activation in MDA-MB-435 cells, localized activation presumably occurs where tumor cells are in contact with the ephrin-B2-positive endothelial cells. Therefore, the dominant negative effects of EphB4ΔC-EGFP may also have contributed to increasing tumor growth by inhibiting forward signaling of endogenous EphB4 and other EphB receptors that may be present in the MDA-MB-435 cells. We found that activation of EphB4 signaling inhibits MDA-MB-435 cell proliferation, at least in vitro. This finding is consistent with reports (17, 36) that activation of EphB4 inhibits cell proliferation and decreases MAPK activity.
Repulsive EphB4 signaling could favor the dissemination of metastatic cells by decreasing cell–cell and cell–matrix adhesion (4, 37). In MMTV-NeuT transgenic mice, EphB4 ectopic expression in mammary epithelial cells induces formation of lung metastases, suggesting that EphB4 can promote a more aggressive and invasive tumor phenotype (4). This increase in metastasis may occur by means of localized EphB4 activation in tumor cells contacting blood vessels, leading to their detachment from the tumor mass and release into the circulation. However, EphB4ΔC-EGFP, which inhibits EphB4 signaling, did not significantly decrease the metat-static dissemination of MDA-MB-435 cells to the lungs. We observed only a slight increase in the larger lung metastases in mice with EphB4ΔC-EGFP tumors, possibly because EphB4ΔC-EGFP promotes vascularization and growth of the metastases.
Another Eph receptor, EphA2, is up-regulated in various human cancers and mouse cancer models, and overexpression of inactive EphA2 causes transformation of mammary epithelial cells (3, 38). Activation of EphA2, however, decreases cell proliferation and the growth and invasiveness of malignant breast and prostate cancer cells (38, 39). Although VEGF up-regulates endothelial expression of ephrin-A1 (a ligand for EphA2), EphA2 is present in tumor endothelial cells and can promote angiogenic responses by forward signaling (13, 40). Hence, it will be interesting to investigate similarities and differences in the activities of EphA and EphB receptors in cancer progression.
In conclusion, our studies support a proangiogenic role for EphB4 in tumor progression. We propose that the interplay between the EphB4 ectodomain on tumor cells and ephrin-B2 in the vasculature promotes blood vessel formation and remodeling. Thus, disrupting EphB4-ephrin-B2 binding represents a potential avenue for antiangiogenic cancer therapies. On the other hand, a more efficient vasculature due to increased ephrin-B2 signaling may facilitate delivery of anticancer drugs to tumor tissue. Studies in the future may reveal the therapeutic advantages of stimulating versus inhibiting ephrin-B2 signaling in tumor blood vessels.
Supplementary Material
Acknowledgments
We thank E. Ruoslahti for helpful comments on the manuscript; J. Nickel and R. Newlin for the immunohistochemistry; D. T. Scadden for the EphB4 cDNA; and C. A. Hauser for MDA-MB-435 lines. This work was supported by University of California Breast Cancer Program Grants 5JB-0086 and 7WB-0090 (to E.B.P.), National Institutes of Health Grant CA82713 (to E.B.P.), and a Department of Defense postdoctoral fellowship (to M.K.).
Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; HUVE cells, human umbilical vein endothelial cells.
References
- 1.Dodelet, V. C. & Pasquale, E. B. (2000) Oncogene 19, 5614–5619. [DOI] [PubMed] [Google Scholar]
- 2.Nikolova, Z., Djonov, V., Zuercher, G., Andres, A. C. & Ziemiecki, A. (1998) J. Cell Sci. 111, 2741–2751. [DOI] [PubMed] [Google Scholar]
- 3.Andres, A. C., Reid, H. H., Zurcher, G., Blaschke, R. J., Albrecht, D. & Ziemiecki, A. (1994) Oncogene 9, 1461–1467. [PubMed] [Google Scholar]
- 4.Munarini, N., Jager, R., Abderhalden, S., Zuercher, G., Rohrbach, V., Loercher, S., Pfanner-Meyer, B., Andres, A.-C. & Ziemiecki, A. (2002) J. Cell Sci. 115, 25–37. [DOI] [PubMed] [Google Scholar]
- 5.Berclaz, G., Andres, A. C., Albrecht, D., Dreher, E., Ziemiecki, A., Gusterson, B. A. & Crompton, M. R. (1996) Biochem. Biophys. Res. Commun. 226, 869–875. [DOI] [PubMed] [Google Scholar]
- 6.Berclaz, G., Flutsch, B., Altermatt, H. J., Rohrbach, V., Djonov, V., Ziemiecki, A., Dreher, E. & Andres, A. C. (2002) Oncol. Rep. 9, 985–989. [PubMed] [Google Scholar]
- 7.Liu, W., Ahmad, S. A., Jung, Y. D., Reinmuth, N., Fan, F., Bucana, C. D. & Ellis, L. M. (2002) Cancer 94, 934–939. [DOI] [PubMed] [Google Scholar]
- 8.Berclaz, G., Karamitopoulou, E., Mazzucchelli, L., Rohrbach, V., Dreher, E., Ziemiecki, A. & Andres, A. C. (2003) Ann. Oncol. 14, 220–226. [DOI] [PubMed] [Google Scholar]
- 9.Murai, K. K. & Pasquale, E. B. (2003) J. Cell Sci. 116, 2823–2832. [DOI] [PubMed] [Google Scholar]
- 10.Sakano, S., Serizawa, R., Inada, T., Iwama, A., Itoh, A., Kato, C., Shimizu, Y., Shinkai, F., Shimizu, R., Kondo, S., et al. (1996) Oncogene 13, 813–822. [PubMed] [Google Scholar]
- 11.Wang, H. U., Chen, Z. F. & Anderson, D. J. (1998) Cell 93, 741–753. [DOI] [PubMed] [Google Scholar]
- 12.Adams, R. H., Diella, F., Hennig, S., Helmbacher, F., Deutsch, U. & Klein, R. (2001) Cell 104, 57–69. [DOI] [PubMed] [Google Scholar]
- 13.Ogawa, K., Pasqualini, R., Lindberg, R. A., Kain, R., Freeman, A. L. & Pasquale, E. B. (2000) Oncogene 19, 6043–6052. [DOI] [PubMed] [Google Scholar]
- 14.Noren, N. K., Liu, B. P., Burridge, K. & Kreft, B. (2000) J. Cell Biol. 150, 567–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu, G., Luo, H., Wu, Y. & Wu, J. (2003) J. Immunol. 171, 106–114. [DOI] [PubMed] [Google Scholar]
- 16.Price, J. E., Polyzos, A., Zhang, R. D. & Daniels, L. M. (1990) Cancer Res. 50, 717–721. [PubMed] [Google Scholar]
- 17.Kim, I., Ryu, Y. S., Kwak, H. J., Ahn, S. Y., Oh, J. L., Yancopoulos, G. D., Gale, N. W. & Koh, G. Y. (2002) FASEB J. 16, 1126–1128. [DOI] [PubMed] [Google Scholar]
- 18.Oike, Y., Ito, Y., Hamada, K., Zhang, X.-Q., Miyata, K., Arai, F., Inada, T., Araki, K., Nakagata, N., Takeya, M., et al. (2002) Blood 100, 1326–1333. [PubMed] [Google Scholar]
- 19.Thurston, G., Suri, C., Smith, K., McClain, J., Sato, T. N., Yancopoulos, G. D. & McDonald, D. M. (1999) Science 286, 2511–2514. [DOI] [PubMed] [Google Scholar]
- 20.Yancopoulos, G. D., Davis, S., Gale, N. W., Rudge, J. S., Wiegand, S. J. & Holash, J. (2000) Nature 407, 242–248. [DOI] [PubMed] [Google Scholar]
- 21.Jain, R. K. (2003) Nat. Med. 9, 685–693. [DOI] [PubMed] [Google Scholar]
- 22.Guo, P., Xu, L., Pan, S., Brekken, R. A., Yang, S. T., Whitaker, G. B., Nagane, M., Thorpe, P. E., Rosenbaum, J. S., Su Huang, H. J., et al. (2001) Cancer Res. 61, 8569–8577. [PubMed] [Google Scholar]
- 23.Mukouyama, Y. S., Shin, D., Britsch, S., Taniguchi, M. & Anderson, D. J. (2002) Cell 109, 693–705. [DOI] [PubMed] [Google Scholar]
- 24.Shin, D., Garcia-Cardena, G., Hayashi, S. I., Gerety, S., Asahara, T., Stavrakis, G., Isner, J., Folkman, J., Gimbrone, M. A. & Anderson, D. J. (2001) Dev. Biol. 230, 139–150. [DOI] [PubMed] [Google Scholar]
- 25.Gale, N. W., Baluk, P., Pan, L., Kwan, M., Holash, J., DeChiara, T. M., McDonald, D. M. & Yancopoulos, G. D. (2001) Dev. Biol. 230, 151–160. [DOI] [PubMed] [Google Scholar]
- 26.Papoutsi, M., Othman-Hassan, K., Christ, B., Patel, K. & Wilting, J. (2002) Histochem. Cell. Biol. 118, 241–249. [DOI] [PubMed] [Google Scholar]
- 27.Dorrell, M. I., Aguilar, E. & Friedlander, M. (2002) Invest. Ophthalmol. Vis. Sci. 43, 3500–3510. [PubMed] [Google Scholar]
- 28.Adams, R. H., Wilkinson, G. A., Weiss, C., Diella, F., Gale, N. W., Deutsch, U., Risau, W. & Klein, R. (1999) Genes Dev. 13, 295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andres, A. C., Munarini, N., Djonov, V., Bruneau, S., Zuercher, G., Loercher, S., Rohrbach, V. & Ziemiecki, A. (2003) Mech. Dev. 120, 511–516. [DOI] [PubMed] [Google Scholar]
- 30.Huynh-Do, U., Vindis, C., Liu, H., Cerretti, D. P., McGrew, J. T., Enriquez, M., Chen, J. & Daniel, T. O. (2002) J. Cell. Sci. 115, 3073–3081. [DOI] [PubMed] [Google Scholar]
- 31.Fuller, T., Korff, T., Kilian, A., Dandekar, G. & Augustin, H. G. (2003) J. Cell Sci. 116, 2461–2470. [DOI] [PubMed] [Google Scholar]
- 32.Xu, Z., Lai, K. O., Zhou, H. M., Lin, S. C. & Ip, N. Y. (2003) J. Biol. Chem. 278, 24767–24775. [DOI] [PubMed] [Google Scholar]
- 33.Benbow, U. & Brinckerhoff, C. E. (1997) Matrix Biol. 15, 519–526. [DOI] [PubMed] [Google Scholar]
- 34.Steinle, J. J., Meininger, C. J., Chowdhury, U., Wu, G. & Granger, H. J. (2003) Cell. Signalling 15, 1011–1017. [DOI] [PubMed] [Google Scholar]
- 35.Palmer, A., Zimmer, M., Erdmann, K. S., Eulenburg, V., Porthin, A., Heumann, R., Deutsch, U. & Klein, R. (2002) Mol. Cell 9, 725–737. [DOI] [PubMed] [Google Scholar]
- 36.Hamada, K., Oike, Y., Ito, Y., Maekawa, H., Miyata, K., Shimomura, T. & Suda, T. (2003) Arterioscler. Thromb. Vasc. Biol. 23, 190–197. [DOI] [PubMed] [Google Scholar]
- 37.Zou, J. X., Wang, B., Kalo, M. S., Zisch, A. H., Pasquale, E. B. & Ruoslahti, E. (1999) Proc. Natl. Acad. Sci. USA 96, 13813–13818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walker-Daniels, J., Hess, A. R., Hendrix, M. J. C. & Kinch, M. S. (2003) Am. J. Pathol. 162, 1037–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miao, H., Burnett, E., Kinch, M., Simon, E. & Wang, B. (2000) Nat. Cell. Biol. 2, 62–69. [DOI] [PubMed] [Google Scholar]
- 40.Cheng, N., Brantley, D. M., Liu, H., Lin, Q., Enriquez, M., Gale, N., Yancopoulos, G., Cerretti, D. P., Daniel, T. O. & Chen, J. (2002) Mol. Cancer Res. 1, 2–11. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.