Abstract
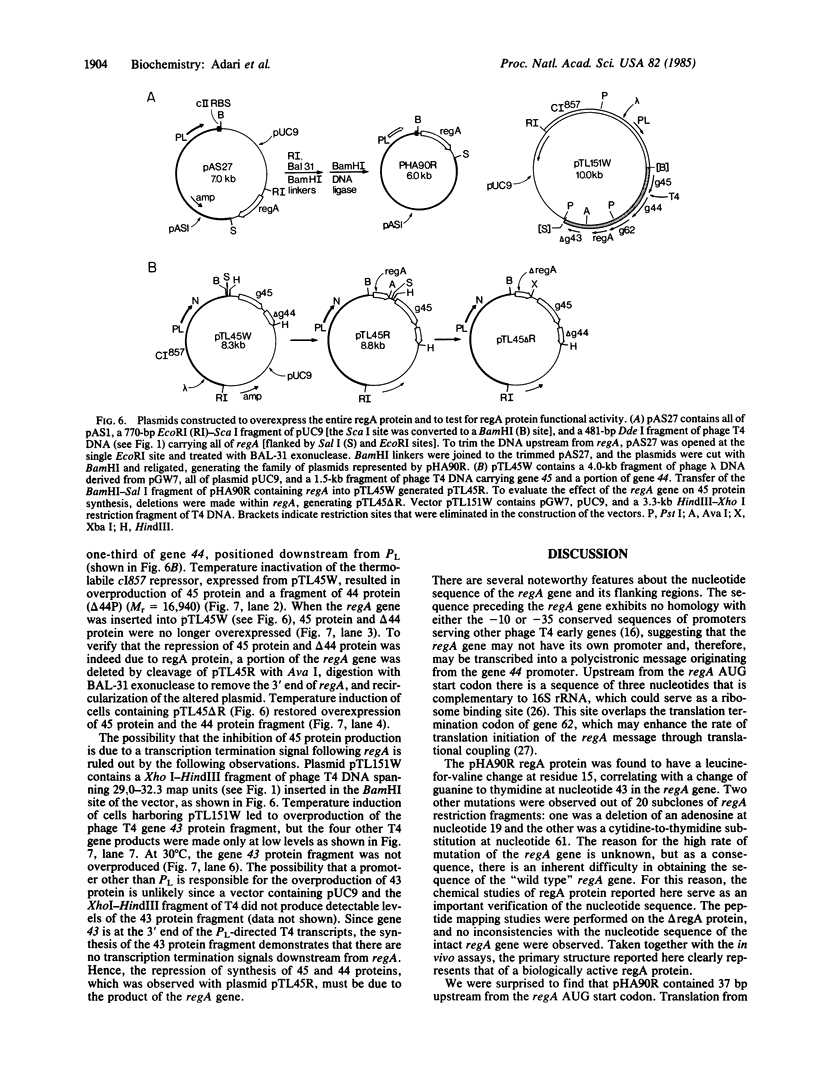
The bacteriophage T4 regA gene codes for a regulatory protein that controls the expression of a number of T4 early genes, apparently at the level of translation. Restriction fragments containing the regA structural gene have been cloned into phage M13, and the nucleotide sequence has been determined. Translation of the DNA sequence predicted that regA protein contains 122 amino acids, with a Mr of 14,620. A DNA fragment carrying 85% of the coding sequence of regA has been cloned into the phage lambda leftward promoter PL expression vector pAS1, and a high level of truncated regA protein was produced by nalidixic acid induction. Protein chemical studies of the truncated regA protein gave results consistent with the nucleotide sequence of the regA gene. Subsequently, an intact regA gene was cloned into plasmid pAS1 and overexpressed. The regA protein produced in this way regulates the level of T4 45 and 44 proteins when their corresponding genes are carried on the same plasmid as the regA gene.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baron C., Thompson T. E. Solubilization of bacterial membrane proteins using alkyl glucosides and dioctanoyl phosphatidylcholine. Biochim Biophys Acta. 1975 Mar 25;382(3):276–285. doi: 10.1016/0005-2736(75)90270-9. [DOI] [PubMed] [Google Scholar]
- Baughman G., Nomura M. Localization of the target site for translational regulation of the L11 operon and direct evidence for translational coupling in Escherichia coli. Cell. 1983 Oct;34(3):979–988. doi: 10.1016/0092-8674(83)90555-x. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Cardillo T. S., Landry E. F., Wiberg J. S. regA protein of bacteriophage T4D: identification, schedule of synthesis, and autogenous regulation. J Virol. 1979 Dec;32(3):905–916. doi: 10.1128/jvi.32.3.905-916.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karam J. D., Bowles M. G. Mutation to overproduction of bacteriophage T4 gene products. J Virol. 1974 Feb;13(2):428–438. doi: 10.1128/jvi.13.2.428-438.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karam J., Gold L., Singer B. S., Dawson M. Translational regulation: identification of the site on bacteriophage T4 rIIB mRNA recognized by the regA gene function. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4669–4673. doi: 10.1073/pnas.78.8.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L'Italien J. J., Strickler J. E. Application of high-performance liquid chromatographic peptide purification to protein microsequencing by solid-phase Edman degradation. Anal Biochem. 1982 Nov 15;127(1):198–212. doi: 10.1016/0003-2697(82)90165-8. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Mott J. E., Grant R. A., Ho Y. S., Platt T. Maximizing gene expression from plasmid vectors containing the lambda PL promoter: strategies for overproducing transcription termination factor rho. Proc Natl Acad Sci U S A. 1985 Jan;82(1):88–92. doi: 10.1073/pnas.82.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Ho Y. S., Shatzman A. The use of pKc30 and its derivatives for controlled expression of genes. Methods Enzymol. 1983;101:123–138. doi: 10.1016/0076-6879(83)01009-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauerbier W., Hercules K. Control of gene function in bacteriophage T4. IV. Post-transcriptional shutoff of expression of early genes. J Virol. 1973 Sep;12(3):538–547. doi: 10.1128/jvi.12.3.538-547.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spicer E. K., Nossal N. G., Williams K. R. Bacteriophage T4 gene 44 DNA polymerase accessory protein. Sequences of gene 44 and its protein product. J Biol Chem. 1984 Dec 25;259(24):15425–15432. [PubMed] [Google Scholar]
- Trimble R. B., Maley Level of specific prereplicative mRNA's during bacteriophage T4 regA-, 43- and T4 43- infection of Escherichia coli B. J Virol. 1976 Feb;17(2):538–549. doi: 10.1128/jvi.17.2.538-549.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojanowska M., Miller E. S., Karam J., Stormo G., Gold L. The bacteriophage T4 regA gene: primary sequence of a translational repressor. Nucleic Acids Res. 1984 Aug 10;12(15):5979–5993. doi: 10.1093/nar/12.15.5979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiberg J. S., Mendelsohn S. L., Warner V., Aldrich C., Cardillo T. S. Genetic mapping of regA mutants of bacteriophage T4D. J Virol. 1977 Jun;22(3):742–749. doi: 10.1128/jvi.22.3.742-749.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiberg J. S., Mendelsohn S., Warner V., Hercules K., Aldrich C., Munro J. L. SP62, a viable mutant of bacteriophage T4D defective in regulation of phage enzyme synthesis. J Virol. 1973 Oct;12(4):775–792. doi: 10.1128/jvi.12.4.775-792.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G. G., Tanyashin V. I., Murray N. E. Molecular cloning of fragments of bacteriophage T4 DNA. Mol Gen Genet. 1977 Nov 14;156(2):203–214. doi: 10.1007/BF00283493. [DOI] [PubMed] [Google Scholar]
- Wood W. B., Revel H. R. The genome of bacteriophage T4. Bacteriol Rev. 1976 Dec;40(4):847–868. doi: 10.1128/br.40.4.847-868.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]