Abstract
Nuclear factor κB (NF-κB) activation has been observed in human atherosclerotic plaques and is enhanced in unstable coronary plaques, but whether such activation has a protective or pathophysiological role remains to be determined. We addressed this question by developing a short-term culture system of cells isolated from human atherosclerotic tissue, allowing efficient gene transfer to directly investigate signaling pathways in human atherosclerosis. We found that NF-κB is activated in these cells and that this activity involves p65, p50, and c-Rel but not p52 or RelB. This NF-κB activation can be blocked by overexpression of IκBα or dominant-negative IκB kinase (IKK)-2 but not dominant-negative IKK-1 or NF-κB-inducing kinase, resulting in selective inhibition of inflammatory cytokines (tumor necrosis factor α, IL-6, and IL-8), tissue factor, and matrix metalloproteinases without affecting the antiinflammatory cytokine IL-10 or tissue inhibitor of matrix metalloproteinases. Our results demonstrate that the canonical pathway of NF-κB activation that involves p65, p50, c-Rel, and IKK-2 is activated in human atherosclerosis and results in selective up-regulation of major proinflammatory and prothrombotic mediators of the disease.
Nuclear factor κB (NF-κB) proteins are a family of transcription factors that play an important role in a number of physiological processes that include cell survival, proliferation, and activation. The NF-κB family comprises five members, relA (p65), relB, c-Rel, p105/p50, and p100/p52, all sharing the Rel homology domain, which allows their dimerization and translocation to the nucleus. NF-κB dimers are bound to inhibitors such as IκBα, which retain NF-κB in the cytosol. In the so-called canonical or classical activation pathway, in response to inflammatory mediators such as tumor necrosis factor α (TNFα) and IL-1, IκB kinase (IKK)-2 (IKKβ), within the multisubunit IKK complex, phosphorylates IκBα, leading to its ubiquitination and degradation, allowing NF-κB to translocate to the nucleus (1). This pathway is crucial for the activation of innate immunity and inflammation. However, an alternative or noncanonical pathway leading to NF-κB activation, under the control of NF-κB-inducing kinase (NIK) and IKK-1, recently emerged. Briefly, in response to stimuli such as lymphotoxin β, NIK has been shown to activate IKK-1, leading to inducible processing of p100 with preferential nuclear translocation of p52-RelB dimers. This noncanonical pathway is strongly suggested to have a physiological role in B cell-mediated responses and adaptive humoral immunity (2, 3).
Although NF-κB activation is essential for normal physiological processes, it also has been suggested to play a pathological role when dysregulated in a number of diseases that include rheumatoid arthritis and Crohn's disease and possibly atherosclerosis (4). Nuclear translocation of p65 has been observed in human and experimental atherosclerosis (5, 6), is enhanced in unstable coronary atherectomies (7), and colocalizes with the expression of target genes (8). However, results from animal models of atherosclerosis have been more controversial and largely limited by the lethality of p65-, IκBα-, and IKK-2-knockout mice (1). Studies in animal models showed that NF-κB blockade by drug-eluting stents (using gene therapy, small-molecule inhibitors, or RNA interference) reduces neointimal formation after interventional procedures (9–12). Deficiency of p50, which has been suggested to play a regulatory role in NF-κB activity, resulted in more inflammatory atherosclerotic lesions in low-density lipoprotein receptor (LDL-R)–/– mice (13). However, macrophage-restricted IKK-2 deletion increased atherosclerosis in LDL-R–/– mice, with more inflammatory infiltrates in early lesions and more necrosis in advanced stages of the disease (14). These observations are in line with recent studies suggesting that NF-κB may be involved not only in the onset of inflammation but in also its resolution (15).
Thus, whether NF-κB activation is protective or pathological in human atherosclerosis is still an open question and remains to be addressed in the most physiologically relevant system, namely the disease tissue itself. We report an ex vivo culture system of cells freshly isolated from human atherosclerotic plaques that models interactions taking place in vivo and permits the direct investigation of signaling pathways. We report that the canonical pathway of NF-κB activation that involves IKK-2, p65, p50, and c-Rel plays an essential role in the control of mediators regulating the thrombotic potential of human atherosclerotic plaques, namely tissue factor (TF), matrix metalloproteinases (MMPs), and inflammatory cytokines.
Methods
Patient Population and Sample Collection. Carotid and femoral endarterectomies from patients undergoing revascularization procedures for symptomatic carotid or peripheral vascular disease were obtained at St. Mary's Hospital (London). The protocol was approved by the local research ethics committee. All patients gave written, informed consent.
Ex Vivo Culture of Cells Isolated from Human Atherosclerotic Plaques. Diseased intimal arterial segments were isolated under a Wild M8 dissecting microscope (Heerbrugg, Switzerland). Adherent thrombi were removed, and tissue fragments were incubated in collagenase type I (400 units/ml), elastase type III (5 units/ml), and DNase (300 units/ml), with 1 mg/ml soybean trypsin inhibitor (Sigma), 2.5 μg/ml polymixin B (Sigma), and 2 mM CaCl2, in RPMI medium 1640 (BioWhittaker) with 5% FCS (GIBCO) in a shaker at 37°C. Cells were recovered every 20 min by filtering through an 80-μm Nylon mesh (Falcon), and fresh enzymatic mixture was added, to avoid activation by collagen fragments, according to previous studies. Lipopolysaccharide content in all media, reagents, and supernatants was assessed via Limulus Amebocyte assay (BioWhittaker) and was always below the detection limit (<10 pg/ml).
Cell fractions were pooled and cultured at 106 cells per ml in RPMI medium 1640 containing 10% FCS and 10% human serum (BioWhittaker). Viability was monitored by using 3-(4,5-dimethyl-2-yl)-2,5-diphenyltetrazolium (MTT; Sigma). Apoptosis was assayed by using propidium iodide (PI) and annexin V (R & D Systems) and by cell-death ELISA (Roche Diagnostics).
Flow Cytometry and Immunostaining. Cells were phenotyped by using FITC-conjugated anti-human CD3 (Pharmingen) and anti-human CD68 (DAKO), anti-human smooth muscle cell α-actin (DAKO) with goat anti-mouse IgG FITC (Pharmingen), or isotype controls and analyzed by FACScan (Becton Dickinson) with winmdi software. Alternatively, cells were cultured onto LabTech slides (Nunc) and stained with anti-human CD68 (DAKO) followed by streptavidin-horseradish peroxidase (Amersham Pharmacia).
Adenoviral Vectors. Replication-deficient control adenoviral vectors encoding β-galactosidase (Adβ-gal) or without insert (Ad0) were provided by A. Byrnes and M. Wood (University of Oxford, Oxford). Adenovirus encoding GFP was from Quantum Biotech (Quebec, Canada). Adenoviruses encoding IκBα (AdIκBα) and dominant-negative IKK-2 (AdIKK-2dn) were provided by R. De Martin (University of Vienna, Vienna) (16, 17). Adenovirus encoding dominant-negative IKK-1 (AdIKK-1dn) was from M. Karin (University of California, San Diego). Adenovirus encoding dominant-negative NIK (AdNIKdn) was constructed by us as described (18).
Adenoviral Infection of Cells from Atherosclerotic Plaques. Freshly isolated cells were infected with AdGFP or Adβ-gal. After 36 h, GFP was analyzed by direct immunofluorescence, and intracellular β-gal was assayed by using fluorescein di-β-d-galactopyranoside (Sigma).
Alternatively, cells were infected with indicated adenoviruses at a multiplicity of infection (moi) of 200:1. After 2 h, supernatants were removed and replaced with 10% FCS plus 10% human serum. In some experiments, cells were stimulated with human recombinant soluble CD40 ligand (CD40L; Alexis, Lausanne, Switzerland) at 10 μg/ml. After 36 h, supernatants were removed. Cellular viability and/or apoptosis were assessed by MTT, cell-death ELISA, or PI and annexin V.
Western Blotting and Electrophoretic Mobility-Shift Assay. Cytosolic protein extracts were separated by SDS/PAGE on a 10% (wt/vol) polyacrylamide gel, followed by electrotransfer onto polyvinyl difluoride membrane (Millipore). IκBα, IKK-1, IKK-2, and α-tubulin were detected by using antibodies from Santa Cruz Biotechnology and horseradish peroxidase-conjugated donkey anti-rabbit antibody (Amersham Pharmacia) and visualized by enhanced chemiluminescence. Nuclear extracts were examined for NF-κB binding activity by electrophoretic mobility-shift assay as described (19). For the supershift assays, nuclear proteins were incubated with antibodies specific for known NF-κB components (p50, RelB, c-Rel, p65, and p52; Santa Cruz Biotechnology) before γ-32P-labeled NF-κB oligonucleotide was added as described (19). All viruses have been used previously in human primary cells, and successful expression was demonstrated (17, 18, 20, 21).
Analysis of Cytokine and MMP Production. Cytokine and MMP/tissue inhibitor of metalloproteinases (TIMP) production was assessed by ELISA: IL-6, IL-8, TNFα, IL-10 (Pharmingen), MMP-1, MMP-3, MMP-9, and TIMP-1 (Amersham Pharmacia).
Analysis of TF Antigen and Activity. Cells were lysed in 1% Triton X-100. TF antigen was assayed by using a TF ELISA kit (American Diagnostica, Stamford, CT) (22). To determine TF activity (Actichrome TF assay; American Diagnostica), cells were lysed in 15 mM N-octyl-β-d-glucopyranoside (22). Protein concentrations were assessed with Bradford reagent (0.1% Coomassie blue G/5% methanol/orthophosphoric acid).
Statistical Analysis. One-way ANOVA with Bonferroni posttest for multiple comparisons was used.
Results
Cells Isolated from Human Atherosclerotic Plaques Produce Cytokines, MMP/TIMP-1, and TF. Viable cell yields from endarterectomy specimens were 3.8 ± 1.5 × 106 according to trypan blue exclusion, with <2% PI- and <5% annexin V-positive cells after digestion. Phenotyping of freshly isolated cells revealed the presence of CD3+ lymphocytes (6–15% in different preparations), CD68+ macrophages (30–44%), and smooth muscle cells (10–20%). A typical experiment is illustrated in Fig. 1a. Cultures on LabTech slides after 48 h showed clusters of mononuclear cells (mostly CD68+) and smooth muscle cell-like cells (Fig. 1 b and c).
Fig. 1.
Characterization of cells isolated from human atherosclerotic plaques. The percentage of CD3+, CD68+, and smooth muscle cells (α-actin+) was analyzed. Live cells were analyzed based on size and forward scatter. Results shown are from one representative experiment of eight. (a) There was no significant difference between different isotype controls. Alternatively, cells were cultured at 0.5 × 105 per ml on LabTech slides for 48 h, stained with anti-CD68 biotinylated monoclonal antibody. (Magnification: b, ×10; c, ×50.)
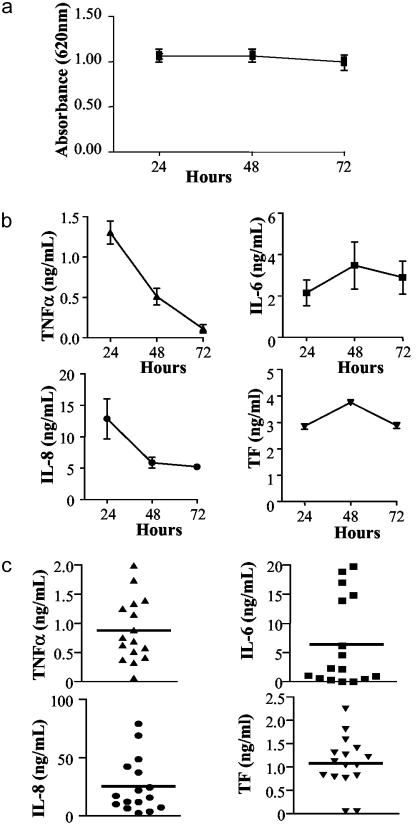
Viability of cells monitored by using MTT showed viable cells for at least 72 h of culture (Fig. 2a). TNFα production peaked at 24 h. IL-8 production showed a similar profile, whereas IL-6 and TF peaked at 48 h (Fig. 2b). Detectable production of TNFα, IL-6, and IL-8 was observed in all samples (Fig. 2c). Interestingly, the antiinflammatory cytokine IL-10 was detected in only 7 of 16 specimens (range, 29–762 pg/ml; median, 289 pg/ml). In all samples, these cells also produced TF (Fig. 2 b and c), and the majority produced MMP-1, MMP-3, MMP-9, and TIMP-1 (Table 1). No significant differences in cytokine, TF, and MMP/TIMP-1 production were observed between cells derived from different arterial sites (data not shown).
Fig. 2.
Short-term cultures of cells from human atherosclerotic plaques release cytokines and TF. (a) Cell viability was monitored by using MTT for up to 72 h of culture. (b) Kinetics of TNFα, IL-6, IL-8, and TF expression. Data are from a representative experiment and are given as mean ± SD. (c) Cells from 16 patients were cultured for 24 h, and production of TNFα, IL-6, and IL-8 in the supernatants or TF levels in cell lysates were assessed. Each point represents a mean of triplicate determinations from a single patient, and bars indicate overall mean.
Table 1. Production of MMPs and TIMP-1.
| MMP-1 | MMP-3 | MMP-9 | TIMP-1 | |
|---|---|---|---|---|
| Mean, ng/ml | 29.9 | 79.2 | 31.2 | 214.5 |
| Range, ng/ml | 5.1-67.5 | 6.0-335.7 | 3.5-120.9 | 51.6-371.4 |
| SEM | 6.9 | 26.9 | 9.4 | 37.5 |
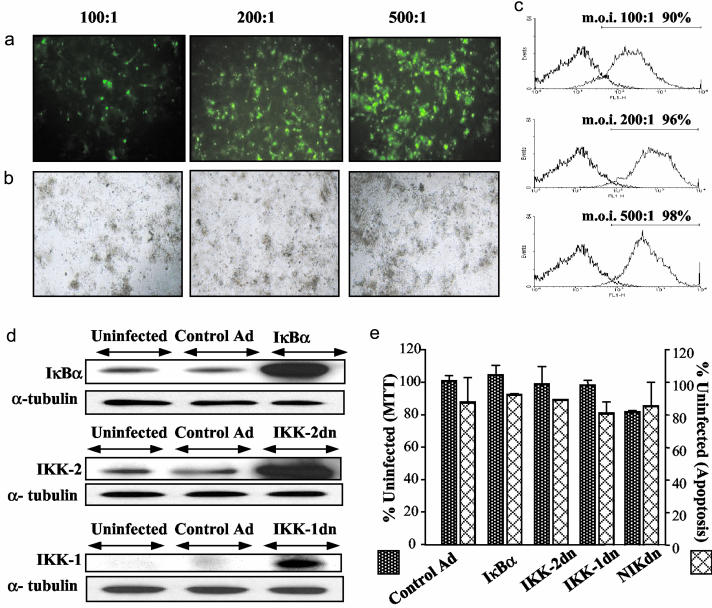
Successful Gene Transfer in Cells Isolated from Human Atherosclerotic Tissue. Successful infection was demonstrated by using AdGFP (Fig. 3 a and b) and Adβ-gal (>90% infection at an moi of 100:1 and >95% at an moi of 200:1; Fig. 3c). In subsequent experiments, an moi of 200:1 was used. Expression of the transgenes was also demonstrated by Western blotting (Fig. 3d). There was no significant difference (P > 0.05) in viability and apoptosis between uninfected and adenovirus-infected cells 36 h after infection (Fig. 3e). Similarly, there was no difference in PI-annexin V staining (<15% annexin V- and 5% PI-positive cells in all conditions; data not shown).
Fig. 3.
Efficient gene transfer into cells isolated from human atherosclerotic plaque. Cells were infected with AdGFP at indicated mois: direct immunofluorescence at ×20 magnification (a) and normal light conditions (b). (c) Di-β-d-galactopyranoside staining (gray) in Adβ-gal-infected cells compared with uninfected cells with di-β-d-galactopyranoside (black); the percentage of infected cells is indicated. (d) Endogenous and adenovirally delivered IκBα, IKK-1, and IKK-2 36 h postinfection. (e) Adenoviral infection does not induce cell death (determined by MTT) or apoptosis (determined by cell-death ELISA). Values are given as percentage relative to uninfected cells: P > 0.05 for all conditions compared with control-virus-infected cells.
The NF-κB Pathway Is Activated in Human Atheroma Cell Cultures and Has a Functional Role in Up-Regulating Inflammatory Cytokines, TF, and MMP but Not IL-10 or TIMP-1. IκBα overexpression significantly inhibited release of cytokines, TF, and MMP from human atherosclerotic plaque cells. In the case of TNFα, percentage inhibition was 66–99%. Inhibition of IL-6 production showed a broader variation, ranging from 46% to 92%, whereas release of IL-8 was almost completely abolished. AdIKK-2dn also significantly inhibited TNFα, IL-6, and IL-8. In contrast, AdIKK-1dn or AdNIKdn had no effect on the expression of these molecules. IL-10 production was not inhibited under any of the above-described adenoviral conditions, suggesting that its expression is completely NF-κB-independent (Fig. 4).
Fig. 4.
Analysis of NF-κB-dependent responses in human atherosclerotic plaque cells by adenoviral gene transfer. Cells were infected with control adenovirus, AdIκBα, AdIKK-2dn, AdIKK-1dn, or AdNIKdn, and levels of TNFα (a), IL-6 (b), IL-8 (c), IL-10 (d), and TF antigen (e) were assessed. Values are given as percentage of inhibition relative to uninfected cells: **, P < 0.01 and ***, P < 0.001 compared with control-virus-infected cells. Analysis is shown of NF-κB binding activity of adenovirus-infected cells by electrophoretic mobility-shift assay (f) and detection of specific NF-κB subunits by supershift analysis in uninfected cells (from same experiment as shown in f, representative) (g).
TF antigen expression was inhibited by AdIκBα and AdIKK-2dn but not by AdIKK-1dn or AdNIKdn (Fig. 4e). Similar results were obtained for TF activity. For example, both AdIκBα and AdIKK-2dn inhibited TF activity by >95% (data not shown). Finally, both AdIκBα and AdIKK-2dn inhibited production of MMP-1, MMP-3, and MMP-9 but not TIMP-1 (Table 2).
Table 2. Inhibition of MMP but not TIMP-1 by AdIkBα and AdIKK-2dn.
| Control virus | IκBα | IKK-2dn | NIKdn | IKK-1dn | |
|---|---|---|---|---|---|
| MMP-1 | 1 ± 10 | 70 ± 5* | 78 ± 7* | 2 ± 9 | 3 ± 7 |
| MMP-3 | 0 ± 13 | 88 ± 6* | 85 ± 10* | 3 ± 6 | 0 ± 10 |
| MMP-9 | 0 ± 11 | 69 ± 18* | 69 ± 17* | 0 ± 18 | 8 ± 1 |
| TIMP-1 | 0 ± 12 | 0 ± 10 | 0 ± 10 | 4 ± 2 | 0 ± 1 |
Data are percent inhibition relative to uninfected cells: *, P < 0.001 versus control-virus-infected cells.
Accordingly, electrophoretic mobility-shift assay analysis showed inhibition of NF-κB binding activity after infection with AdIκBα and AdIKK-2dn but not AdIKK-1dn and AdNIKdn. Furthermore, supershift analysis demonstrated that NF-κB activity in atheroma cell culture consists of p65, c-Rel, and p50 but not relB or p52 (Fig. 4 f and g).
CD40L Signaling Is NF-κB-Dependent in Cells Isolated from Human Atherosclerotic Plaques. Stimulation of cells isolated from atherosclerotic plaques with soluble CD40L significantly up-regulated TF, TNFα, IL-8, and IL-6. Addition of CD40L also increased expression of MMP-1, MMP-3, and MMP-9 (Table 3). AdIκBα and AdIKK-2dn inhibited CD40L-superinduced production of TNFα, IL-6, and TF as well as MMP-1, MMP-3, and MMP-9 (Table 3).
Table 3. NF-κB blockade inhibits CD40L-induced responses.
| Control Ad
|
IκBα
|
IKK-2dn
|
||
|---|---|---|---|---|
| No stimulus | CD40L | |||
| TNFα | 0.6 ± 0.0 | 2.1 ± 0.1††† | 0.1 ± 0.0*** | 0.1 ± 0.0*** |
| IL-6 | 2.2 ± 0.3 | 8.1 ± 0.9††† | 3.2 ± 0.5*** | 3.0 ± 0.1*** |
| IL-8 | 72 ± 28 | 232 ± 24††† | 15 ± 2*** | 38 ± 3*** |
| TF | 1.6 ± 0.4 | 8.4 ± 0.2††† | 0.1 ± 0.0*** | 1.2 ± 0.7*** |
| MMP-1 | 49 ± 4 | 106 ± 21†† | 32 ± 3*** | 21 ± 4*** |
| MMP-3 | 303 ± 17 | 716 ± 32††† | 54 ± 10*** | 109 ± 26*** |
| MMP-9 | 46 ± 1 | 93 ± 37†† | 17 ± 4*** | 5 ± 1*** |
Values are ng/ml. ††, P < 0.05, †††, P < 0.001 compared with no CD40L; **, P < 0.01, ***, P < 0.001 compared with control-virus-infected cells stimulated with CD40L.
Discussion
Several molecules have emerged as leading pathophysiological contributors to atherosclerosis, including TF, the major initiator of the coagulation cascade, MMPs, which degrade collagen fibrils leading to loss of fibrous cap integrity, and proinflammatory cytokines, which promote infiltration and activation of inflammatory cells as well as inducing TF and MMP (23). Although expression of these molecules is normally tightly regulated, in atherosclerotic plaques their expression is increased for extended periods of time, promoting their pathological effects. Thus, there is marked interest in understanding the molecular mechanisms involved in up-regulation of these molecules, because their identification could lead to the definition of novel targets for therapeutic intervention.
A transcription factor that may be involved in the up-regulated expression of proinflammatory and prothrombotic factors in human atherosclerosis is NF-κB, the activation of which has been observed in human and experimental atherosclerosis (5, 6). Thus far, functional data on the role of NF-κB in atherosclerosis in animal models are conflicting. In fact, NF-κB blockade is effective in reducing neointimal formation (9–12). However, reduced NF-κB activity in macrophages increased atherosclerotic lesion size (14). Because the involvement of NF-κB in the regulation of gene expression is cell type-, stimulus-, and disease-specific (17, 20, 21, 24), we decided to investigate the involvement of NF-κB activation in the regulation of proinflammatory and prothrombotic factors directly in human atherosclerosis disease tissue, the most physiologically relevant system in terms of the human disease.
We developed a short-term culture system of dissociated cells from human atherosclerotic tissue based on a previously described and well established method of rheumatoid arthritis synovial cell culture (25, 26). This complex mixture, with macrophages, lymphocytes, and smooth muscle cells as the most abundant cells, forms cellular aggregates and produces cytokines, TF, and MMPs in the absence of extrinsic stimulation. Furthermore, these cells are responsive to CD40L, suggesting that stimulation of cells in the atherosclerotic plaque by CD40L-expressing cells during acute phases of atherosclerotic disease could lead to up-regulation of inflammatory mediators, MMPs, or TF. By using this short-term culture system, we found that NF-κB is activated in human atherosclerosis, as demonstrated by the high levels of NF-κB DNA-binding activity present in the nucleus of human atherosclerotic plaque cells. Interestingly, by using supershift analysis, we also found that this activity consists of p65, c-Rel, and p50 NF-κB subunits, with relB and p52 absent. Because p65 and c-Rel are mostly activated through the canonical pathway, whereas the relB and p52 through the noncanonical one, these results suggest that only the canonical pathway of NF-κB activation is induced in human atherosclerotic plaque cells.
This in vitro model system is easily amenable to genetic modification by recombinant adenoviruses, a powerful tool for dissecting intracellular pathways in vitro in normal human cells (including nondividing cells such as macrophages) and cells derived from pathological tissues (20, 24) that avoids the use of chemical inhibitors, which can be limited by their availability or specificity. We found that adenovirus-mediated overexpression of IκBα or IKK-2dn, inhibitors of the canonical pathway of NF-κB induction, significantly reduced NF-κB DNA-binding activity in the nucleus of cells from atherosclerotic plaques. This finding was accompanied by reduced production of prothrombotic and proinflammatory mediators, namely TF, TNFα, IL-6, IL-8, MMP-1, MMP-3, and MMP-9. In terms of the regulation of MMP-1, MMP-3, and MMP-9, although activator protein-1 has been proposed as the dominant transcription factor (27), NF-κB may also be required for MMP-1, MMP-3, and MMP-9 secretion in certain cell types (20, 28–30), in accordance with our data. However, NF-κB blockade in human macrophages and rabbit foam cells from s.c. granulomas inhibited MMP-1 and MMP-3 but not MMP-9 (31), which was somewhat surprising because the MMP-9 gene has an NF-κB binding site in its proximal promoter (30). This difference from our data using human atherosclerotic tissue may be due to a species difference or different cellular composition.
Unexpectedly, however, we found that overexpression of either IκBα or IKK-2dn had no effect on production of the antiinflammatory cytokine IL-10 or the inhibitor of MMP activity TIMP-1, suggesting that NF-κB may be selectively involved in the expression of pathological mediators in human atherosclerosis. These results are reminiscent of studies in human rheumatoid arthritis tissue in which NF-κB was also found to be selectively involved in the regulation of proinflammatory but not antiinflammatory mediators (32). However, our results using human disease tissue are in disagreement with a recent study in mice in which deficiency of IKK-2 in macrophages resulted in inhibition of both proinflammatory cytokines and IL-10. Because IL-10 is a cytokine with an antiatherosclerotic effect (33), down-regulation of IL-10 could explain, at least partially, the increase in atherosclerotic lesions, inflammatory infiltrates, and necrosis observed by these authors (14). Differences between human and murine cells in terms of signaling should be taken into account to explain these discrepancies. In human macrophages, expression of IL-10 has been shown to be NF-κB-independent (32), whereas in mouse macrophages, IL-10 has been shown to require NF-κB (14, 34). Moreover, in contrast to mouse cells, in human macrophages, IKK-2 blockade did not inhibit lipopolysaccharide-induced proinflammatory cytokine production but was able to reduce CD40L-induced cytokine production (17). Furthermore, atherosclerotic lesions in humans and animal models differ in a number of features, which may also contribute to differences between our study and the results from LDL-R–/– mice. Such differences between human and animal systems should always be taken into account when evaluating the entirety of the data that have been gathered in the fast-moving field of NF-κB pathway regulation in atherosclerosis.
Finally, overexpression of IKK-1dn or NIKdn, two inhibitors of the noncanonical pathway of NF-κB activation, had no effect on either the nuclear DNA-binding activity of NF-κB or production of TF, proinflammatory cytokines, and MMPs. Collectively, these data suggest that the canonical rather than noncanonical pathway is activated in human atherosclerosis and has a functional role in up-regulating proinflammatory and prothrombotic mediators involved in disease pathogenesis.
CD40L signaling also has been proposed to be important in the pathogenesis of atherosclerosis. Indeed, CD40L expression has been detected in human atherosclerotic plaques, and CD40L-expressing cells during acute phases of atherosclerotic disease are likely to lead to up-regulation of inflammatory mediators, MMP, or TF. Furthermore, disruption of CD40–CD40L signaling has been shown to reduce atherosclerotic lesion development in experimental models (35). We recently showed that, although IKK-2 is not required for lipopolysaccharide-induced NF-κB activation in human macrophages (17, 21), IKK-2 function is essential for NF-κB activation in response to CD40L. These observations held true in human atherosclerotic tissue, because overexpression of IκBα and IKK-2dn inhibited CD40L-induced production of cytokines, TF, and MMP, confirming the requirement for IKK-2.
This ex vivo model system of short-term culture of atheroma cells described here is not without potential limitations. The technique used to isolate and culture cells from atherosclerotic lesions, although based on the well established culture of synovial cells from the joints of patients with rheumatoid arthritis, could possibly lead to some degree of cell activation. There is also the limitation of the lack of control tissue, without which correlations between cytokine levels and disease state can be of limited value only. Nevertheless, the potential limitations are outweighed by far by the advantages of the methodology presented in this study, which allows analysis of signal transduction in live human cells derived from small specimens of diseased tissue and could be used broadly in the future for screening new therapeutic targets for atherosclerosis and possibly other diseases.
In summary, our data directly analyze the functional role of NF-κB in human atherosclerotic tissue. In addition, we have demonstrated that the canonical pathway of NF-κB activation, rather than the noncanonical one, is activated and plays a major role in the regulation of proinflammatory and prothrombotic responses in human atherosclerotic plaques. Our study is timely because recently a deeper understanding of NF-κB and the signaling pathways regulating its activity is fuelling significant drug-development efforts from pharmaceutical companies (3). Furthermore, our study is a step forward toward specific identification of components of the NF-κB pathway involved in disease, which possibly could guide the future development of clinically useful inhibitors.
Acknowledgments
C.M. dedicates this article to her mother, Rita. This work was funded by the European Society of Cardiology and Fondazione per il cuore Onlus, Italy. The Kennedy Institute of Rheumatology receives a core grant from arc.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: TNFα, tumor necrosis factor α; IKK, IκB kinase; NIK, NF-κB-inducing kinase; TF, tissue factor; MMP, matrix metalloproteinase; MTT, 3-(4,5-dimethyl-2-yl)-2,5-diphenyltetrazolium; PI, propidium iodide; Ad, adenovirus; β-gal, β-galactosidase; dn, dominant negative; moi, multiplicity of infection; CD40L, CD40 ligand; TIMP, tissue inhibitor of metalloproteinases.
References
- 1.Karin, M. & Ben-Neriah, Y. (2000) Annu. Rev. Immunol. 18, 621–663. [DOI] [PubMed] [Google Scholar]
- 2.Pomerantz, J. L. & Baltimore, D. (2002) Mol. Cell 10, 693–695. [DOI] [PubMed] [Google Scholar]
- 3.Karin, M., Yamamoto, Y. & Wang, Q. M. (2004) Nat. Rev. Drug Discov. 3, 17–26. [DOI] [PubMed] [Google Scholar]
- 4.Monaco, C. & Paleolog, E. (2004) Cardiovasc. Res. 61, 671–682. [DOI] [PubMed] [Google Scholar]
- 5.Brand, K., Page, S., Rogler, G., Bartsch, A., Brandl, R., Knuechel, R., Page, M., Kaltschmidt, C., Baeuerle, P. A. & Neumeier, D. (1996) J. Clin. Invest. 97, 1715–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hajra, L., Evans, A. I., Chen, M., Hyduk, S. J., Collins, T. & Cybulsky, M. I. (2000) Proc. Natl. Acad. Sci. USA 97, 9052–9057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson, S. H., Best, P. J., Edwards, W. D., Holmes, D. R., Jr., Carlson, P. J., Celermajer, D. S. & Lerman, A. (2002) Atherosclerosis 160, 147–153. [DOI] [PubMed] [Google Scholar]
- 8.Brand, K., Page, S., Walli, A. K., Neumeier, D. & Baeuerle, P. A. (1997) Exp. Physiol. 82, 297–304. [DOI] [PubMed] [Google Scholar]
- 9.Cejna, M., Breuss, J. M., Bergmeister, H., de Martin, R., Xu, Z., Grgurin, M., Losert, U., Plenk, H., Jr., Binder, B. R. & Lammer, J. (2002) Radiology (Easton, Pa.) 223, 702–708. [DOI] [PubMed] [Google Scholar]
- 10.Breuss, J. M., Cejna, M., Bergmeister, H., Kadl, A., Baumgartl, G., Steurer, S., Xu, Z., Koshelnick, Y., Lipp, J., De Martin, R., et al. (2002) Circulation 105, 633–638. [DOI] [PubMed] [Google Scholar]
- 11.Yoshimura, S., Morishita, R., Hayashi, K., Yamamoto, K., Nakagami, H., Kaneda, Y., Sakai, N. & Ogihara, T. (2001) Gene Ther. 8, 1635–1642. [DOI] [PubMed] [Google Scholar]
- 12.Zuckerbraun, B. S., McCloskey, C. A., Mahidhara, R. S., Kim, P. K., Taylor, B. S. & Tzeng, E. (2003) J. Vasc. Surg. 38, 812–819. [DOI] [PubMed] [Google Scholar]
- 13.Kanters, E., Gijbels, M. J., Van Der Made, I., Vergouwe, M. N., Heeringa, P., Kraal, G., Hofker, M. H. & De Winther, M. P. (2004) Blood 103, 934–940. [DOI] [PubMed] [Google Scholar]
- 14.Kanters, E., Pasparakis, M., Gijbels, M. J., Vergouwe, M. N., Partouns-Hendriks, I., Fijneman, R. J., Clausen, B. E., Forster, I., Kockx, M. M., Rajewsky, K., et al. (2003) J. Clin. Invest. 112, 1176–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lawrence, T., Gilroy, D. W., Colville-Nash, P. R. & Willoughby, D. A. (2001) Nat. Med. 7, 1291–1297. [DOI] [PubMed] [Google Scholar]
- 16.Foxwell, B., Browne, K., Bondeson, J., Clarke, C., de Martin, R., Brennan, F. & Feldmann, M. (1998) Proc. Natl. Acad. Sci. USA 95, 8211–8215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andreakos, E., Smith, C., Kiriakidis, S., Monaco, C., De Martin, R., Brennan, F. M., Paleolog, E., Feldmann, M. & Foxwell, B. (2003) Arthritis Rheum. 48, 1901–1912. [DOI] [PubMed] [Google Scholar]
- 18.Smith, C., Andreakos, E., Crawley, J. B., Brennan, F. M., Feldmann, M. & Foxwell, B. M. (2001) J. Immunol. 167, 5895–5903. [DOI] [PubMed] [Google Scholar]
- 19.Ciesielski, C. J., Andreakos, E., Foxwell, B. M. & Feldmann, M. (2002) Eur. J. Immunol. 32, 2037–2045. [DOI] [PubMed] [Google Scholar]
- 20.Bondeson, J., Foxwell, B., Brennan, F. & Feldmann, M. (1999) Proc. Natl. Acad. Sci. USA 96, 5668–5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiriakidis, S., Andreakos, E., Monaco, C., Foxwell, B., Feldmann, M. & Paleolog, E. (2003) J. Cell Sci. 116, 665–674. [DOI] [PubMed] [Google Scholar]
- 22.Monaco, C., Andreakos, E., Young, S., Feldmann, M. & Paleolog, E. (2002) J. Leukocyte Biol. 71, 659–668. [PubMed] [Google Scholar]
- 23.Libby, P. (2001) Circulation 104, 365–372. [DOI] [PubMed] [Google Scholar]
- 24.Conron, M., Andreakos, E., Pantelidis, P., Smith, C., Beynon, H. L., Dubois, R. M. & Foxwell, B. M. (2002) Am. J. Respir. Crit. Care Med. 165, 996–1004. [DOI] [PubMed] [Google Scholar]
- 25.Brennan, F. M., Chantry, D., Jackson, A., Maini, R. & Feldmann, M. (1989) Lancet 2 (8657), 244–247. [DOI] [PubMed] [Google Scholar]
- 26.Feldmann, M. (2002) Nat. Rev. Immunol. 2, 364–371. [DOI] [PubMed] [Google Scholar]
- 27.Benbow, U. & Brinckerhoff, C. E. (1997) Matrix Biol. 15, 519–526. [DOI] [PubMed] [Google Scholar]
- 28.Vincenti, M. P., Coon, C. I. & Brinckerhoff, C. E. (1998) Arthritis Rheum. 41, 1987–1994. [DOI] [PubMed] [Google Scholar]
- 29.Bond, M., Chase, A. J., Baker, A. H. & Newby, A. C. (2001) Cardiovasc. Res. 50, 556–565. [DOI] [PubMed] [Google Scholar]
- 30.Sato, H. & Seiki, M. (1993) Oncogene 8, 395–405. [PubMed] [Google Scholar]
- 31.Chase, A. J., Bond, M., Crook, M. F., Newby, A. C., Bond, M., Chase, A. J., Baker, A. H. & Newby, A. C. (2002) Arterioscler. Thromb. Vasc. Biol. 22, 765–771. [DOI] [PubMed] [Google Scholar]
- 32.Bondeson, J., Browne, K. A., Brennan, F. M., Foxwell, B. M. & Feldmann, M. (1999) J. Immunol. 162, 2939–2945. [PubMed] [Google Scholar]
- 33.Mallat, Z., Besnard, S., Duriez, M., Deleuze, V., Emmanuel, F., Bureau, M. F., Soubrier, F., Esposito, B., Duez, H., Fievet, C., et al. (1999) Circ. Res. 85, e17–e24. [DOI] [PubMed] [Google Scholar]
- 34.Platzer, C., Meisel, C., Vogt, K., Platzer, M. & Volk, H. D. (1995) Int. Immunol. 7, 517–523. [DOI] [PubMed] [Google Scholar]
- 35.Mach, F., Schonbeck, U., Sukhova, G. K., Atkinson, E. & Libby, P. (1998) Nature 394, 200–203. [DOI] [PubMed] [Google Scholar]