Abstract
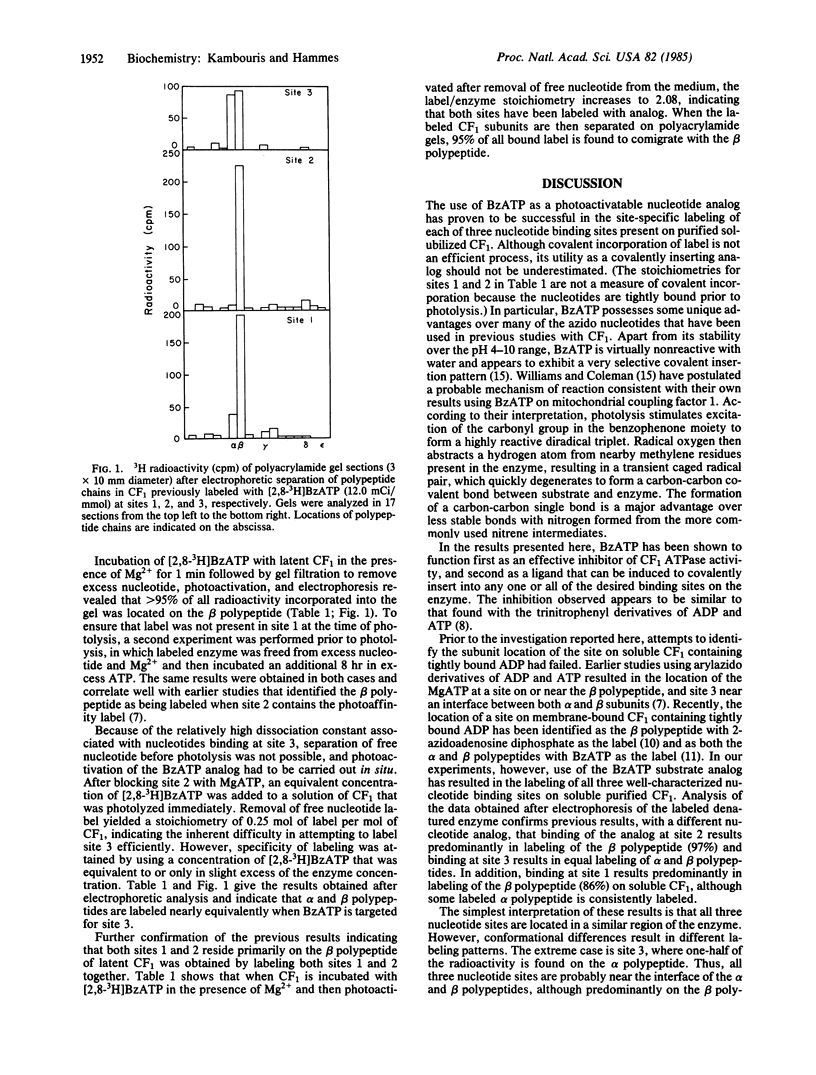
The subunit locations of each of the three nucleotide binding sites of soluble chloroplast coupling factor 1 have been studied with the photoaffinity label 3'-O-(4-benzoyl)benzoyl-ATP. This derivative is an effective inhibitor of ATPase activity. Photolysis of the radioactive label when bound to each of the three nucleotide sites on the coupling factor has been examined. For the nucleotide site that normally binds ADP very tightly, NaDodSO4/polyacrylamide gel electrophoresis after photolysis indicates that primarily the beta polypeptide chain is appreciably labeled (86%), although some labeling of the alpha polypeptide chain is found (14%). For the site that binds MgATP tightly, 97% of the radioactivity is found on the beta polypeptide chain. The alpha and beta polypeptide chains are labeled in approximately equal amounts when photolysis is carried out with the nucleotide analog bound to the third site.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binder A., Jagendorf A., Ngo E. Isolation and composition of the subunits of spinach chloroplast coupling factor protein. J Biol Chem. 1978 May 10;253(9):3094–3100. [PubMed] [Google Scholar]
- Bruist M. F., Hammes G. G. Further characterization of nucleotide binding sites on chloroplast coupling factor one. Biochemistry. 1981 Oct 27;20(22):6298–6305. doi: 10.1021/bi00525a003. [DOI] [PubMed] [Google Scholar]
- Bruist M. F., Hammes G. G. Mechanism for catalysis and regulation of adenosine 5'-triphosphate hydrolysis by chloroplast coupling factor 1. Biochemistry. 1982 Jul 6;21(14):3370–3377. doi: 10.1021/bi00257a019. [DOI] [PubMed] [Google Scholar]
- Cantley L. C., Jr, Hammes G. G. Characterization of nucleotide binding sites on chloroplast coupling factor 1. Biochemistry. 1975 Jul;14(13):2968–2975. doi: 10.1021/bi00684a027. [DOI] [PubMed] [Google Scholar]
- Carlier M. F., Hammes G. G. Interaction of nucleotides with chloroplast coupling factor 1. Biochemistry. 1979 Aug 7;18(16):3446–3451. doi: 10.1021/bi00583a002. [DOI] [PubMed] [Google Scholar]
- Colman R. F. Affinity labeling of purine nucleotide sites in proteins. Annu Rev Biochem. 1983;52:67–91. doi: 10.1146/annurev.bi.52.070183.000435. [DOI] [PubMed] [Google Scholar]
- Czarnecki J. J., Abbott M. S., Selman B. R. Photoaffinity labeling with 2-azidoadenosine diphosphate of a tight nucleotide binding site on chloroplast coupling factor 1. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7744–7748. doi: 10.1073/pnas.79.24.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresser M. J., Myers J. A., Boyer P. D. Catalytic site cooperativity of beef heart mitochondrial F1 adenosine triphosphatase. Correlations of initial velocity, bound intermediate, and oxygen exchange measurements with an alternating three-site model. J Biol Chem. 1982 Oct 25;257(20):12030–12038. [PubMed] [Google Scholar]
- Kohlbrenner W. E., Boyer P. D. Probes of catalytic site cooperativity during catalysis by the chloroplast adenosine triphosphate and the adenosine triphosphate synthase. J Biol Chem. 1983 Sep 25;258(18):10881–10886. [PubMed] [Google Scholar]
- Penefsky H. S. Reversible binding of Pi by beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1977 May 10;252(9):2891–2899. [PubMed] [Google Scholar]
- Shavit N. Energy transduction in chloroplasts: structure and function of the ATPase complex. Annu Rev Biochem. 1980;49:111–138. doi: 10.1146/annurev.bi.49.070180.000551. [DOI] [PubMed] [Google Scholar]
- VAMBUTAS V. K., RACKER E. PARTIAL RESOLUTION OF THE ENZYMES CATALYZINE PHOTOPHOSPHORYLATION. I. STIMULATION OF PHOTOPHOSPHORYLATION BY A PREPARATION OF A LATENT, CA++- DEPENDENT ADENOSINE TRIPHOSPHATASE FROM CHLOROPLASTS. J Biol Chem. 1965 Jun;240:2660–2667. [PubMed] [Google Scholar]
- Williams N., Coleman P. S. Exploring the adenine nucleotide binding sites on mitochondrial F1-ATPase with a new photoaffinity probe, 3'-O-(4-benzoyl)benzoyl adenosine 5'-triphosphate. J Biol Chem. 1982 Mar 25;257(6):2834–2841. [PubMed] [Google Scholar]


