Abstract
Purpose of review
1) to outline the initial development of histotripsy, a non-invasive image guided focused ultrasound technology that mechanically homogenizes targeted tissues and 2) to describe the results of pre-clinical translational research directed towards urologic applications.
Recent Findings
Histotripsy tissue ablation is based on initiation and control of acoustic cavitation at a target point within the body. This unique mechanical mechanism of action is distinct when compared to conventional thermal ablative modalities. Features of histotripsy (non-thermal, noninvasive, high precision, real-time monitoring/feedback, tissue liquefaction) have prompted assessment of this technology as a potential ablative therapy for a number of organs and disease processes.
Summary
Ongoing research efforts to apply histotripsy to preclinical models of benign prostatic hyperplasia (BPH), prostate cancer, renal masses, and renal calculi have resulted in enhanced understanding of cavitation bioeffects, refinement of treatment systems, strategies to enhance treatment efficiency, and initiation of a pilot human clinical trial to assess the safety of histotripsy for BPH therapy.
Keywords: histotripsy, focused ultrasound, cavitation, ablation
Introduction
Histotripsy is an experimental focused ultrasound technology where acoustic energy generated by an extracorporeal transducer is focused on a target volume inside the body. Conceptually histotripsy is more similar to shockwave lithotripsy (SWL) than to high intensity focused ultrasound (HIFU). Histotripsy was initially conceived and developed at the University of Michigan; so much of the refinement and pre-clinical translation has originated from these laboratories. More recently, other groups have begun to experiment with histotripsy both along technical and translational lines of research which serves to validate the concept of histotripsy and further enrich the potential therapeutic value of this new technology.
Scientific Background
Histotripsy is defined as delivery of acoustic energy in the form of short (<50 microseconds) very high intensity pulses which induces controlled cavitation to mechanically homogenize targeted tissue [1]. Cavitation is a process that occurs when a sufficiently negative pressure is applied to a fluid (or in this case tissue) to cause microbubble formation from fluid vaporization and release of dissolved gas [2]. Once formed, the microbubbles exhibit highly dynamic patterns of oscillation and inertial collapse which impart severe stresses on surrounding cells and tissues and produces cellular and tissue disruption [3].
To characterize this process of tissue homogenization, increasing numbers of histotripsy pulses from a 750 kHz piezoelectric transducer were applied in an in-vivo rabbit model [4]. Ten pulses produced sparse, scattered focal hemorrhage containing some cellular debris. With additional pulses these small foci of homogenized tissue enlarged and merged with adjacent foci to “fill-in” the geometric focal volume ultimately producing a liquefied core of cytoplasm and fractionated cellular material surrounded by a smooth boundary [4,5]. After 1000 pulses, this process appears to be self-limited as there was no apparent difference between lesions created with 1000, 10000, or greater number of pulses [4]. The efficiency of this process can be increased using techniques to remove the bubble nuclei which persist between pulses [6].
Grossly and microscopically, the appearance of histotripsy treated tissue is distinct from thermally ablated tissue. In an ex-vivo porcine kidney model, thermocouples were placed at tissue target points treated with a wide range of acoustic parameters that included those used for histotripsy and HIFU [7]. Lesions characterized as disrupted (where liquefied material spilled out of a cavity after sectioning) corresponded to sonications where tissue temperature increase was less than 27 degrees C. Desiccated lesions were associated with sonications where tissue temperature increased 40 degrees C or greater. These results confirmed that histotripsy can be produced by selecting parameters that isolate cavitational mechanical effects and minimize thermal tissue deposition [7]. This has recently been validated with “boiling histotripsy” a related technology that induces spatially and temporally localized superheating of mm sized tissue volumes to produce microbubbles which then exhibit histotripsy behavior and produce mechanical homogenization [8,9].
The biological response to tissue treated with histotripsy is different than the response to thermal tissue coagulation. In an in-vivo rabbit model, histotripsy was applied to kidneys, which were then harvested 1 to 60 days after treatment [10]. The resultant homogenized debris was resorbed within 45–60 days with very little deposition of fibrotic tissue scar formation [10]. In separate canine studies, the liquid consistency of the tissue after prostate histotripsy facilitated drainage via the urethra and produced an effective debulking of the prostate [11–13].
The microbubble clouds responsible for tissue homogenization are excellent sound reflectors and appear as hyperechoic (bright) zones with ultrasound imaging (Figure 1)[1,4]. This feature allows accurate target co-localization and provides real-time ultrasound monitoring of treatment progression. Following histotripsy treatment, fractionated tissue appears hypoechoic as many of the structures that would have reflected US energy have been broken down and homogenized [4,11]. Ultrasound backscatter assessment and shear wave imaging have been studied as methods to better indicate the degree of tissue homogenization during histotripsy treatment [14–15].
Figure 1.
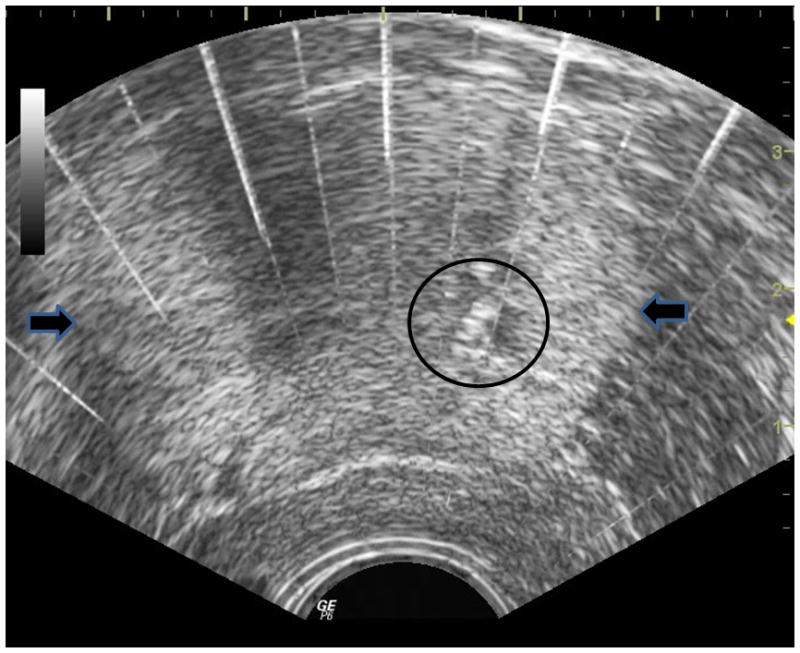
The histotripsy bubble cloud is seen (circle) within a canine prostate (lateral margins marked by arrows on this transverse transrectal ultrasound image. Histotripsy is applied from an extracorporeal therapy transducer located in a suprapubic location (beyond the top of this image.
A unique feature of histotripsy is the high precision with which tissue bioeffects can be confined within a targeted volume. Using a phantom prostate composed of red blood cells and agar, histotripsy was applied in the pattern of a “plus” sign. Cavitation activity lyses the red blood cells which change color. This color change is readily apparent and serves as a marker of histotripsy mechanical effect (Figure 2) [16]. The straight lines and right angle corners created are indicative of the precision achievable with histotripsy ablation. An even greater level of precision is possible by applying preconditioning pulses to delete bubble nuclei at the periphery of the targeted volume before delivery of histotripsy pulses [17].
Figure 2.
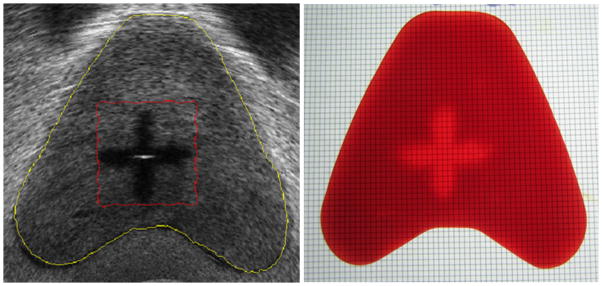
A transverse transrectal ultrasound image (left) of the red blood cell prostate phantom imaged within a human pelvic phantom after application of histotripsy in a “plus” pattern. The same prostate red blood cell phantom after removal and transverse sectioning reveals lysis of the red blood cells (lighter region) corresponding to the same pattern of cellular homogenization seen with ultrasound overlayed with a 1mm grid.
Histotripsy for BPH
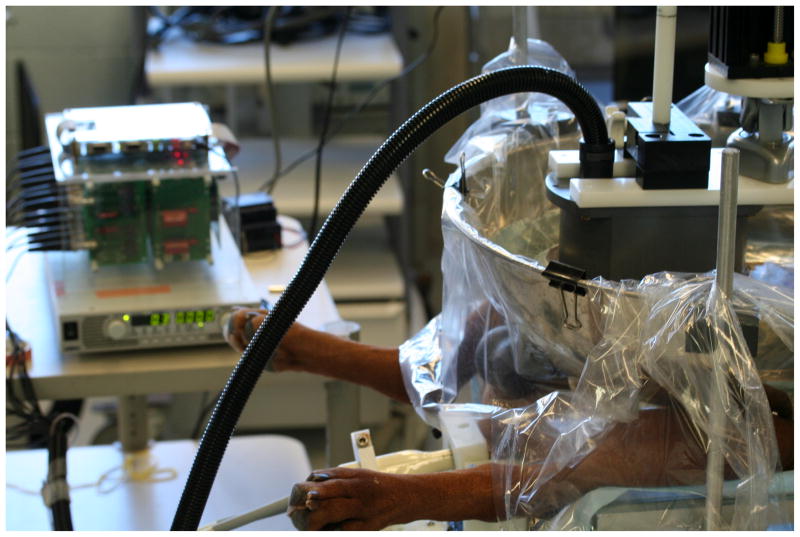
Histotripsy is an intriguing technology to apply to BPH. Potential advantages include energy application from an extracorporeal source with real-time transrectal ultrasound (TRUS) image-guidance, non-thermal tissue homogenization and liquefaction which facilitates drainage of debris via the urethra, and rapid debulking of the prostate. To explore and understand the potential of histotripsy as a therapy for BPH, a canine model (intact older males) was utilized for its anatomic similarity to the human prostate and urethra. Histotripsy was applied transabdominally by positioning anesthetized canine subjects supine and placing a water bolus over the suprapubic region. The histotripsy transducer was coupled to the canine through the water bolus and its focal volume targeted on the prostate. A 10 MHz transrectal ultrasound imager provided high resolution images of the prostate for targeting and observation of the bubble cloud (Figure 3). Volume ablation of glandular and prostatic urethral tissue was accomplished by moving the bubble cloud (by changing the position of the histotripsy transducer) along a pre-prescribed grid pattern or by manipulation of joystick controls guided by TRUS image feedback.
Figure 3.
Experimental histotripsy apparatus in use during canine prostate treatment. A power supply and driving electronics (left) supply power to the histotripsy transducer which is suspended over the suprapubic region of this canine subject in a bath of degassed water. The histotripsy transducer is positioned to locate the focal volume within the prostate. A transrectal ultrasound probe is inserted in the rectum to provide imaging of the prostate and bubble cloud during treatment.
Once the feasibility of prostate histotripsy was established in acute subjects [11], it became apparent that glandular tissue was more easily homogenized than periurethral prostatic tissue (28000 pulses/cm3 versus 270000 pulses/cm3) [12]. In a subsequent study, the endoscopic appearance of the prostatic urethra was assigned a score immediately after histotripsy. This score was found to correlate with the probability of disintegration of the prostatic urethra by 14 days which allowed drainage of homogenate from the treatment cavity via the urethra [18]. An alternative treatment strategy, targeting a modest 1–2 cm3 volume of glandular tissue with histotripsy and sparing periurethral tissue, produced pools of homogenized debris that generally resorbed over 8 weeks. Prostate volume was decreased 12% at harvest and most treatment cavities contained simple fluid. There was no evidence of abscess or increased chronic inflammation in the prostates [19].
In all of the previous experiments very little hemorrhage was observed. To further explore this finding, nine canine subjects were administered warfarin (international normalized ratio 1.2–11.3) and then aggressive histotripsy of the prostate was performed to create a TURP-like defect [20]. Mild hematuria without clots was observed during the first 48 hours after treatment. Serial assessment of hemoglobin did not demonstrate significant change, suggesting that the mechanical action of histotripsy may also be inducing hemostatic effects even in the setting of anticoagulation with warfarin.
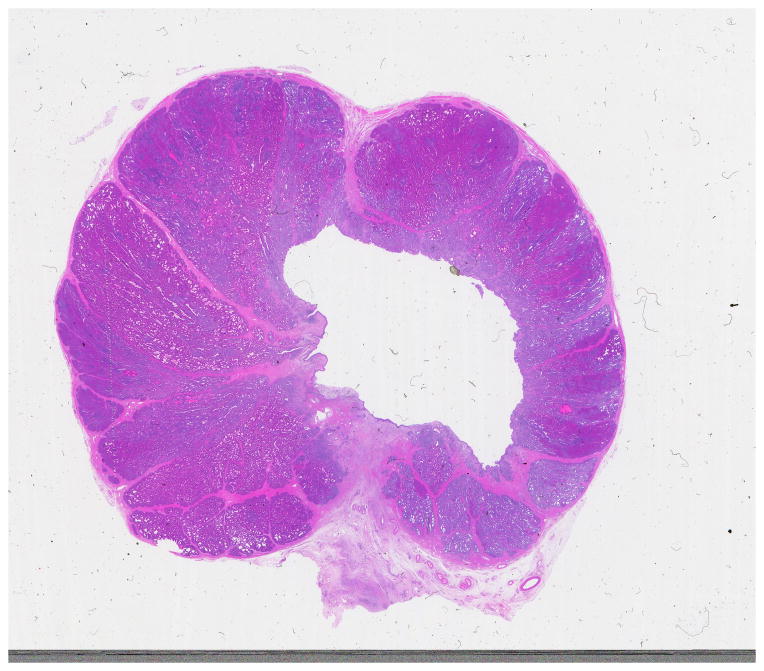
Local and systemic effects of histotripsy were studied in eighteen canine subjects that underwent treatment of at least a 4 cm3 volume of prostate tissue encompassing glandular tissue and prostatic urethra. Prostates were harvested at time points between 0 and 56 days after treatment and in each case tissue homogenization or a drained treatment cavity were apparent. Mild post-treatment discomfort resolved with catheter removal. When the rectum was inadvertently treated on several occasions, tissue effects were apparent at harvest and in one case a prostatorectal fistula developed. Otherwise, only minimal hematuria and transient post-treatment abnormalities in blood tests were observed [13]. Urothelialization of the treatment cavity with minimal residual debris was seen 28 days after treatment (Figure 4). Based on this study it appeared that histotripsy when properly targeted in the prostate was safe and well tolerated in the canine model.
Figure 4.
Hematoxylin and eosin stained slide of a prostate harvested four weeks after histotripsy treatment. The cuboidal histotripsy treatment volume is appreciated here as the roughly rectangular empty cavity encompassing both glandular and prostatic urethral tissue.
Although histotripsy can create precise lesions in the ideal setting (phantom), targeting errors or patient motion in the in-vivo setting may expose adjacent structures to histotripsy and potential injury. It was apparent in earlier research that thresholds for damage from histotripsy vary based on tissue characteristics [12,21]. To establish the thresholds for damage of critical periprostatic structures, two studies [22,23] were conducted applying histotripsy (1000, 10000, or 100000 pulses) directly to the urinary sphincter, neurovascular bundle, rectum, and 750,000 pulses to the bladder trigone, and ureteral orifices. The rectum exhibited the greatest susceptibility for damage with 10000 pulses causing moderate collagen disruption and focal mucosal homogenization. The other structures however appeared quite resilient to histotripsy damage. After 100000 pulses the urinary sphincter was structurally intact and exhibited minimal histologic muscle fiber disruption. Arteries, veins, and nerves within the neurovascular bundles appeared intact, though extensive homogenization was apparent in surrounding loose connective tissue [22]. Cystoscopy after histotripsy revealed edema of the bladder trigone. One of 12 renal units exhibited transient hydronephrosis in follow-up, though at harvest all ureteral orifices were cannulated and found to be patent [23]. Histologically only localized pockets of mild fibrosis were evident and deemed to be clinically insignificant.
To determine the best acoustic path to the human prostate, abdominal pelvic computed tomography data sets from 17 human subjects were analyzed. Three dimensional reconstructions of the pelvic bony anatomy and prostate were used to back project from the prostate to the therapy transducer located in either a perineal or suprapubic location. The perineal approach was superior, allowing an average unblocked transducer surface area of 77.0%, 94.4%, and 99.6% when targeting the base, middle, and apex of the prostate [24]. A transrectal approach is used by commercial prostate HIFU systems and would also simplify targeting and energy deliver with histotripsy. This is not yet feasible with current piezoelectric technology as the transducer surface area needed to initiate acoustic cavitation is larger than can be practically inserted in the rectum. However future evolution of piezoelectric technology will likely make transrectal histotripsy practical.
In order to translate histotripsy from the research lab to human use, HistoSonics, Inc. was formed in December 2009. Subsequently a human prototype device (VortxRX™) was created for treatment of BPH and in May 2013 the US Food and Drug Administration approved a human pilot trial.
Histotripsy for Prostate Cancer
Much of the pre-clinical work applying histotripsy to the prostate as reviewed in the previous section is also applicable to development of histotripsy as a focal therapy modality for prostate cancer treatment. To further explore histotripsy treatment of prostate cancer a unique canine ACE-1 prostate cancer model with metastatic potential was developed [25]. Utilizing this ACE-1 prostate tumor model, ten canine subjects were implanted with tumor cells [26]. Discrete tumors were identified by TRUS two weeks later in all subjects. In seven canine subjects, histotripsy was applied and created a bubble cloud which was translated through a portion of the targeted tumors within the prostate using real-time TRUS feedback. Four prostates were harvested acutely and three at 2–3 weeks after treatment. Tumor homogenization was apparent in all acute subjects while histology from chronic subjects revealed necrosis and hemorrhage. Metastases were apparent in all three tumor implanted controls, while none of the histotripsy treated chronic subjects exhibited metastases [26].
This work demonstrated initial feasibility of histotripsy treatment of implanted ACE-1 tumor in canine prostate and suggests that real-time TRUS-guided histotripsy may have utility as a prostate focal therapy. Additional larger studies are being designed to more extensively examine completeness of local tumor ablation, systemic response, and further characterization of metastatic burden after histotripsy.
Histotripsy for Renal Masses
Research is also being conducted applying histotripsy for treatment of small renal masses. Feasibility of extracorporeal histotripsy ablation of renal tissue was confirmed in an in-vivo rabbit model with rapid resorption of the homogenized debris and minimal scar formation [4,10]. To assess treatment effect on malignant tissue, 2 mm3 cubes of VX-2 tumor from a donor rabbit were implanted below the renal capsule in 15 rabbits. Two weeks after implantation, malignant masses were discernable with transcutaneous ultrasound imaging and treated by translating the bubble cloud through a portion of the tumor mass under ultrasound guidance. Histology from acute specimens demonstrated pools of homogenized tumor, while kidneys harvested at 24 hours after treatment also exhibited an acute inflammatory response [27]. This study confirmed malignant tissue in the kidney could be homogenized with histotripsy and led to the next set of experiments to measure the metastatic burden after histotripsy.
Concerns have previously been raised (based on studies of other modalities with cavitation potential) that application of acoustic energy to malignant tissues could disrupt the structure of a tumor and its cellular attachments and increase downstream metastases. To assess whether these same concerns apply to histotripsy, aggressive VX-2 tumor was implanted in the left kidneys of New Zealand White rabbits [28]. Histotripsy was applied to a portion of the implanted tumors 13 days after implantation, followed by nephrectomy the following day, and necropsy 1 week later. Successful treatment was confirmed by presence of homogenized material adjacent and within residual implanted tumor in all cases. No statistical difference in total lung metastases or metastatic density was found between treated and control rabbits [28]. Similar results have been seen in a murine model using mechanical HIFU, a form of ultrasound therapy where both thermal and cavitational effects are induced [29]. Research is ongoing to more explicitly measure circulating tumor cells and time course of metastatic implants following histotripsy therapy in several other aggressive tumor models.
Histotripsy for Renal Stones
Histotripsy therapy is based on the isolation and control of cavitation in order to homogenize targeted tissue. Cavitation is also known to play a role in SWL treatment of urinary stones. The feasibility of using histotripsy for treatment of renal stones was tested in a tank study with Ultracal-30 model stones. Histotripsy was found to erode stones at a rate of 88 mg/min compared to 110 mg/min achieved with a piezoelectric lithotripter [30,31]. However, histotripsy produced particles no larger than 100 micrometers compared to the range of fragment sizes produced by SWL [30,31]. These results highlight a distinction between two methods of stone comminution. Histotripsy (pure cavitation) is efficient at erosion while SWL predominantly produces a progressive stone subdivision. This led to the hypothesis that potential synergies could be exploited between SWL and histotripsy to enhance treatment of urinary stones. An apparatus was constructed that incorporated both an electrohydraulic shock wave source and a histotripsy transducer co-localized on model stones. Application of both histotripsy and SWL pulses produced more efficient stone comminution and a fragment distribution shifted toward smaller sizes than SWL alone [32]. Histotripsy appeared to pit the stone surface which enhanced SWL induced crack propagation while the sequential stone subdivision resulting from SWL provided a greater surface area for histotripsy stone erosion [32]. Further studies are underway to control and/or eliminate residual bubble nuclei which act to shield the stone from incoming acoustic energy. Better control of bubble behavior adjacent to the stone may allow substantial improvement in efficiency of histotripsy and SWL pulses. These optimizations, if validated in animal models, are envisioned to shorten therapy time, allow treatment of larger stones, and minimize size and volume of stone fragments.
Other Histotripsy Applications
Other urologic applications of histotripsy include erosion of ureteroceles in an ex-vivo tissue model [33] and in-vitro histotripsy destruction of Escherichia Coli biofilms that can frequently coat indwelling urinary catheters and stents [34]. Histotripsy has also been evaluated as a non-invasive therapy for other non-urologic diseases. Transcutaneous liver ablation in an in-vivo porcine model has been shown feasible and appears promising for treatment of hepatocellular carcinoma and other liver malignancies [35]. Creation of intracardiac ventricular septal communications, initially conceived as a method to repair or palliate congenital heart defects, has been successful in a porcine model without deleterious effects up to 1 month after treatment [36]. Fetal intervention with histotripsy has been evaluated in a fetal sheep model. Intrauterine histotripsy ablation of renal and liver tissue was successful and appeared to have no developmental impact when necropsy examination was performed after birth [37]. This raises a number of possibilities for in utero treatment of urologic conditions including posterior urethral valves and ureteropelvic junction obstructions. Histotripsy has been utilized to homogenize deep venous thrombosis and re-establish venous flow without damaging the walls of femoral veins in an in-vivo porcine model [38]. Additionally, histotripsy has been used to create an acoustic embolus trap in phantom blood vessel models which could prove useful in conjunction with acoustic or other forms of thrombolysis [39].
Conclusion
Histotripsy is a unique image-guided, focused ultrasound non-thermal ablative technology that relies upon initiation of acoustic cavitation to mechanically homogenize targeted tissue. Histotripsy research originated from the University of Michigan but has now spread to other academic and research institutes as well. The extracorporeal delivery of energy, homogenization of tissue, and real-time monitoring of the ablation process are unique features with potential impact on improved patient tolerability and immediate confirmation of treatment adequacy. Pre-clinical exploration is ongoing for a number of urologic and non-urologic applications and has progressed to a human pilot trial for treatment of BPH.
Key Points.
Histotripsy is an image-guided non-invasive non-thermal focused ultrasound therapy that induces and controls cavitation to homogenize targeted tissue within the body.
The non-thermal mechanism of action of histotripsy allows precise ablation with conversion of tissue to a liquefied homogenate and real-time ultrasound monitoring of the bubble cloud confirms targeting and progression of treatment
Preclinical translation research has been published on histotripsy treatment of BPH, prostate cancer, renal masses, renal stones, and ureteroceles.
Histotripsy is a platform technology with many additional potential therapeutic applications within and beyond urology.
Acknowledgments
Funding: NIH DK 087871
Abbreviations
- BPH
benign prostatic hyperplasia
- HIFU
high intensity focused ultrasound
- kHz
kilohertz
- MHz
megahertz
- SWL
shockwave lithotripsy
- TRUS
transrectal ultrasound
Footnotes
Disclosure: WWR has equity, royalty, and consulting interests in HistoSonics, Inc. He is the principal investigator on a sponsored research grant from HistoSonics, Inc.
Conflicts of Interest: WWR has equity, royalty, and consulting interests in HistoSonics, Inc. He is the principal investigator on a sponsored research grant from HistoSonics, Inc.
References
- 1.Parsons JE, Cain CA, Abrams GD, Fowlkes JB. Pulsed cavitational ultrasound therapy for controlled tissue homogenization. Ultrasound in Med & Biol. 2006;32:115–29. doi: 10.1016/j.ultrasmedbio.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Maxwell AD, Wang T, Cain CA, et al. Cavitation clouds created by shock scattering from bubbles during histotripsy. J Acoustic Soc Am. 2011;130:1888–98. doi: 10.1121/1.3625239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu Z, Raghavan M, Hall TL, et al. Evolution of bubble clouds induced by pulsed cavitational ultrasound therapy – histotripsy. IEEE Trans Ultrason Ferroelectr Freq Control. 2008;55:1122–32. doi: 10.1109/TUFFC.2008.764. [DOI] [PubMed] [Google Scholar]
- 4.Roberts WW, Hall TL, Ives K, et al. Pulsed cavitational ultrasound: A noninvasive technology for controlled tissue ablation (histotripsy) in the rabbit kidney. J Urol. 2006;175:734–8. doi: 10.1016/S0022-5347(05)00141-2. [DOI] [PubMed] [Google Scholar]
- 5.Winterroth F, Xu Z, Wang T, et al. Examining and analyzing subcellular morphology of renal tissue treated by histotripsy. Ultrasound in Med & Biol. 2011;37:78–86. doi: 10.1016/j.ultrasmedbio.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang T, Xu Z, Hall TL, et al. An efficient treatment strategy for histotripsy by removing cavitation memory. Ultrasound Med Biol. 2012;38:753–66. doi: 10.1016/j.ultrasmedbio.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kieran K, Hall TL, Parsons JE, et al. Refining histotripsy: Defining the parameter space for the creation of non-thermal lesions with high intensity pulsed ultrasound in the in vitro kidney. J Urol. 2007;178:672–6. doi: 10.1016/j.juro.2007.03.093. [DOI] [PubMed] [Google Scholar]
- 8*.Wang Y, Khokhlova T, Bailey M, et al. Histological and biochemical analysis of mechanical and thermal bioeffects in boiling histotripsy lesions induced by high intensity focused ultrasound. Ultrasound Med Biol. 2013;39:424–38. doi: 10.1016/j.ultrasmedbio.2012.10.012. This study validates previous work correlating lesion morphology with use of specific acoustic parameters. It demonstrates that mechanical bioeffects can be isolated from thermal ablation when using specific parameter ranges. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simon JC, Sapozhnikov OA, Khokhlova VA, et al. Ultrasonic atomization of tissue and its role in tissue fractionation by high intensity focused ultrasound. Phys Med Biol. 2012;57:8061–78. doi: 10.1088/0031-9155/57/23/8061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall TL, Kieran K, Ives K, et al. Histotripsy of rabbit renal tissue in vivo: Temporal histologic trends. J Endourol. 2007;21:1159–66. doi: 10.1089/end.2007.9915. [DOI] [PubMed] [Google Scholar]
- 11.Lake AM, Hall TL, Kieran K, et al. Histotripsy: minimally invasive technology for prostatic tissue ablation in an in vivo canine model. Urology. 2008;72:682–6. doi: 10.1016/j.urology.2008.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall TL, Hempel CR, Wojno K, et al. Histotripsy of the prostate: dose effects in a chronic canine model. Urology. 2009;74:932–7. doi: 10.1016/j.urology.2009.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13**.Hempel CR, Hall TL, Cain CA, et al. Histotripsy fractionation of prostate tissue: local effects and systemic response in a canine model. J Urol. 2011;185:1484–9. doi: 10.1016/j.juro.2010.11.044. This study demonstrated that histotripsy could be safely applied for homogenization of the prostate and was well tolerated in the canine model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang T, Xu Z, Winterroth F, et al. Quantitative ultrasound backscatter for pulsed cavitational ultrasound therapy – histotripsy. IEEE Trans Ultrason Ferroelectr Freq Control. 2009;56:995–1005. doi: 10.1109/tuffc.2009.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang T, Hall TL, Xu Z, et al. Imaging feedback of histotripsy treatments using ultrasound shear wave elastography. IEEE Trans Ultrason Ferroelec Freq Control. 2012;59:1167–81. doi: 10.1109/tuffc.2012.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maxwell AD, Wang T, Yuan L, et al. A tissue phantom for visualization and measurement of ultrasound-induced cavitation damage. Ultrasound Med Biol. 2010;36:2132–43. doi: 10.1016/j.ultrasmedbio.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang T, Xu Z, Hall TL, et al. Active focal zone sharpening for high-precision treatment using histotripsy. IEEE Trans Ultrason Ferroelec Freq Control. 2011;58:305–15. doi: 10.1109/TUFFC.2011.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schade GR, Styn NR, Hall TL, Roberts WW. Endoscopic assessment and prediction of prostate urethral disintegration after histotripsy treatment in a canine model. 2012;26:183–9. doi: 10.1089/end.2011.0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19*.Schade GR, Hall TL, Roberts WW. Urethral-sparing histotripsy of the prostate in a canine model. Urology. 2012;80:730–5. doi: 10.1016/j.urology.2012.05.027. This study explored histotripsy of glandular prostate without treatment of the periurethral tissue and demonstrated effective homogenization and decrease in prostate volume. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wheat JC, Hall TL, Hempel CR, et al. Prostate histotripsy in an anticoagulated model. Urology. 2010;75:207–11. doi: 10.1016/j.urology.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lake AM, Xu Z, Cain CA, et al. Renal ablation by histotripsy: Does it spare collecting system? J Urol. 2008;179:1150–4. doi: 10.1016/j.juro.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 22.Styn N, Hall TL, Fowlkes JB, et al. Histotripsy homogenization of the prostate: thresholds for cavitation damage of periprostatic structures. J Endourol. 2011;25:1531–5. doi: 10.1089/end.2010.0648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23*.Allam C, Wilkinson JE, Cheng X, et al. Histotripsy effects on the bladder trigone: functional and histologic consequences in the canine model. J Endourol. 2013 Jun 3; doi: 10.1089/end.2013.0234. [Epub ahead of print]. In this study direct targeting of bladder trigone with supratherapeutic doses of histotripsy produced minimal injury. There is little risk of collateral injury to the bladder trigone and ureteral orifices when treating prostate adenomatous tissues. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hall TL, Hempel CR, Sabb BJ, Roberts WW. Acoustic access to the prostate for extracorporeal ultrasound ablation. J Endourol. 2010;24:1875–81. doi: 10.1089/end.2009.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keller JM, Schade GR, Ives K, et al. A novel canine model for prostate cancer. Prostate. 2013;73:952–9. doi: 10.1002/pros.22642. [DOI] [PubMed] [Google Scholar]
- 26**.Schade GR, Keller J, Ives K, et al. Histotripsy focal ablation of implanted prostate tumor in an ACE-1 canine cancer model. J Urol. 2012;188:1957–64. doi: 10.1016/j.juro.2012.07.006. Utilizing a novel canine prostate cancer model, this study demonstrated the feasibility of treating prostate cancer with histotripsy in the canine model. The precision and imaging feedback with histotripsy will be beneficial when applying this technology as a focal therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Styn NR, Wheat JC, Hall TL, Roberts WW. Histotripsy of VX-2 tumor implanted in a renal rabbit model. J Endourol. 2010;24:1145–50. doi: 10.1089/end.2010.0123. [DOI] [PubMed] [Google Scholar]
- 28*.Styn NR, Hall TL, Fowlkes JB, et al. Histotripsy of renal implanted VX-2 tumor in a rabbit model: investigation of metastases. Urology. 2012;80:724–9. doi: 10.1016/j.urology.2012.06.020. This paper demonstrated no statistical difference in metastatic burden in histotripsy treated subjects compared to controls. Although, further work is needed to validate these findings, it is reassuring that a large increase in metastases was not seen after mechanical homogenization of tumor. [DOI] [PubMed] [Google Scholar]
- 29.Xing Y, Lu X, Pua EC, Zhong P. The effect of high intensity focused ultrasound treatment on metastases in a murine melanoma model. Biochemical and Biophysical Research Communications. 2008;31:645–50. doi: 10.1016/j.bbrc.2008.08.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duryea AP, Hall TL, Maxwell AD, et al. Histotripsy Erosion of Model Urinary Calculi. J Endourol. 2011;25:341–4. doi: 10.1089/end.2010.0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duryea A, Maxwell A, Roberts W, et al. In vitro comminution of model renal calculi using histotripsy. IEEE Trans Ultrason Ferroelectr Freq Control. 2011;58:971–80. doi: 10.1109/TUFFC.2011.1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32*.Duryea AP, Roberts WW, Cain CA, Hall TL. Controlled cavitation to augment SWL stone comminution: mechanistic insights in-vitro. IEEE Trans Ultrason Ferroelectr Freq Control. 2013;60:301–9. doi: 10.1109/TUFFC.2013.2566. This paper demonstrated the synergism possible when integrating histotripsy and conventional shock wave lithotripsy pulses for stone comminution. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33*.Maxwell AD, His RS, Bailey MR, et al. Pulsed focused ultrasound as a method for noninvasive treatment of ureteroceles. abstract Engineering & Urology Society Annual Meeting; 2013. This study demonstrated feasibility of using histotripsy to perforate a ureterocele in a tissue model. [Google Scholar]
- 34.Bigelow TA, Northagen T, Hill TM, Sailer FC. Ultrasound histotripsy and the destruction of Escherichia Coli biofilms. Proceedings of the 30th Annual International IEEE EMBS Conference; 2008. pp. 4467–70. [DOI] [PubMed] [Google Scholar]
- 35*.Vlaisavljevich E, Kim Y, Allen S, et al. Image-guided non-invasive ultrasound liver ablation using histotripsy: feasibility study in an in vivo porcine model. Ultrasound Med Biol. 2013;39:1398–409. doi: 10.1016/j.ultrasmedbio.2013.02.005. This study demonstrated the feasibility of transcutaneous histotripsy liver ablation in a porcine model. These encouraging results will prompt further study of histotripsy treatment of malignancy within the liver. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36**.Owens GE, Miller RE, Owens ST, et al. Intermediate-term effects of intracardiac communications created noninvasively by therapeutic ultrasound (histotripsy) in a porcine model. Pediatr Cardiol. 2012;33:83–9. doi: 10.1007/s00246-011-0094-6. Histotripsy erosion through the ventricular septum is found to be feasible in this chronic study in a porcine model. Histotripsy, a non-invasive technology, has potential to replace current invasive, morbid procedures in the neonate. [DOI] [PubMed] [Google Scholar]
- 37**.Kim Y, Fifer CG, Gelehrter SK, et al. Developmental impact and lesion maturation of histotripsy-mediated non-invasive tissue ablation in a fetal sheep model. Ultrasound Med Biol. 2013;39:1047–55. doi: 10.1016/j.ultrasmedbio.2012.12.014. The non-invasive application of histotripsy has potential for fetal interventions. In this study ablation of liver and kidney tissue in fetal sheep was performed and found to have no deleterious effect on organ development. [DOI] [PubMed] [Google Scholar]
- 38.Maxwell AD, Owens G, Gurm HS, et al. Noninvasive treatment of deep venous thrombosis using pulsed ultrasound cavitation therapy (histotripsy) in a porcine model. J Vasc Interv Radiol. 2011;22:369–77. doi: 10.1016/j.jvir.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39*.Park S, Maxwell AD, Owens GE, et al. Non-invasive embolus trap using histotripsy – an acoustic parameter study. Ultrasound Med Biol. 2013;39:611–9. doi: 10.1016/j.ultrasmedbio.2012.11.026. This study demonstrates the ability of histotripsy to trap emboli in a model vessel with flow. This may be an important adjunct when histotripsy is applied for thrombolysis. [DOI] [PMC free article] [PubMed] [Google Scholar]