Summary
Background
Although the crazy-paving pattern on computed tomography is characteristic for pulmonary alveolar proteinosis (PAP), it is not specific and has not been compared between idiopathic and secondary PAPs in the large studies. The aim of this study was to determine the high resolution computed tomography (HRCT) features of idiopathic PAP.
Material/Methods
HRCT images of 35 patients (mean age: 38±14years; 54.3% male) with idiopathic PAP (proved by bronchoalveolar lavage or biopsy) were reviewed by two experienced pulmonary radiologist and detailed findings were reported.
Results
The predominant HRCT presentation of PAP was interlobular septal thickening (ILST;100%) and ground glass opacities (GGOs; 91.7%), resulting in crazy-paving pattern (83%). All patients had diffuse bilateral lung involvement that was symmetric in 97%. ILST and GGO without crazy-paving were seen in 17% and 14.7%, respectively. The overall extent of parenchymal involvement was 50 to 75% in 80% of patients. Thirty three cases (94%) had areas of geographic sparing within the affected lung. Peripheral sparing was seen in 85.7% of patients, including three patterns with some overlap: costophrenic angle (80%), apices (60%), and subpleural (57%) sparing. Other HRCT findings were: consolidation (63%), pulmonary nodules (31.4%), mediastinal and/or hilar lymphadenopathy (23%), mass-like consolidation (17%), pleural effusion (8.6%), and honey combing (5.7%). All female patients (n=16) had crazy-paving, while 13 out of 19 (68%) male patients had crazy-paving on their lung HRCT (p=0.02).
Conclusions
This study demonstrated that the predominant HRCT presentation of idiopathic PAP was interlobular septal thickening and ground glass opacities, resulting in crazy-paving pattern.
Keywords: Pulmonary Alveolar Proteinosis (PAP), Idiopathic PAP, Crazy-Paving, Computed Tomography
Background
Pulmonary alveolar proteinosis (PAP) is a rare disease characterized by alveolar deposition of surfactant-like or periodic acid-Schiff proteins [1,2]. Since the first description of PAP by Rosen et al. [3], this disease has been reported mainly in case reports or some limited studies [4].
PAP has three forms: congenital, idiopathic (primary), and secondary. Idiopathic form is the most common type accounting for about 90% of all PAPs [1]. Secondary PAP usually occurs due to exposure to some materials such as silica, cement, aluminum, titanium or nitrogen dioxide. It may also appear due to hematologic malignancy or various types of immunodeficiency disorders [1,2,5]. Indeed, PAP is the final outcome of surfactant and pulmonary immune disorders, and elevated serum granulocyte-monocyte colony-stimulating factor (GM-CSF) antibodies are seen in idiopathic PAP [6–8].
Typical radiographic appearance of PAP is bilateral symmetric opacities with sparing of apices and costophrenic angles [1]. Less frequently it may appear as multifocal asymmetric opacities or consolidations [1,2]. In some cases, especially in moderate forms, the radiographic involvement may be greater than clinical presentation (clinicoradiologic discrepancy) [9].
Computed tomography, especially high resolution computed tomography (HRCT), demonstrates more details of lung involvement. Crazy-paving pattern (network-like thickened septal lines on a background of ground-glass opacity) was first reported for PAP [10]. This pattern in PAP usually is bilateral and extensive with intervening intact geographic or lobular areas [1]. Crazy-paving has no specific zonal distribution [11–13]. Although crazy-paving is characteristic for PAP, some other diseases may result in a similar pattern, including pulmonary hemorrhage, infections, pulmonary edema, inhalation disorders, malignancies (bronchioalveolar carcinoma, lymphangitic carcinomatosis), radiation, and some drugs [1,14–16].
Because the crazy-paving pattern is not specific for PAP and related studies on this issue are lacking and also other diseases can mimic this pattern, new studies may help us to better understand the more specific radiologic presentations of idiopathic PAP. Furthermore, familiarity with various patterns of lung involvement on HRCT of patients with idiopathic PAP is essential for early diagnosis of this life-threatening disorder and thereby decreasing its morbidity and mortality.
Material and Methods
Thirty-five consecutive patients (mean age 38±14 (range: 4–68) years; 54.3% male) with proven PAP (by lung biopsy [n=10] or bronchoalveolar lavage [n=25]) from Mar. 2006 to Mar. 2011 in Masih-e-Daneshvary hospital of Tehran (a tertiary center for lung diseases) were included in the study. All patients had idiopathic PAP without any underlying diseases.
All chest HRCT images (SIEMENS SOMATOM EMOTION, Germany) were reviewed by two expert pulmonary radiologists with 17 and 10 years of experience, with almost perfect interobserver agreement (kappa=.85).
Results
All patients had diffuse bilateral lung involvement that was symmetric in 97%. One patient had bilateral multifocal asymmetric lung involvement. Interlobular septal thickening (ILST) was the most common HRCT finding which was seen in all patients. The second most common HRCT feature was ground glass opacities (GGOs) (91.7%). Crazy-paving pattern was seen in 29 (83%) patients. Indeed, the main HRCT presentation of PAP was crazy-paving which is a combination of septal thickening and GGOs (Figures 1 and 2). ILST and GGO without crazy-paving were seen in 17% and 14.7% of patients, respectively.
Figure 1.
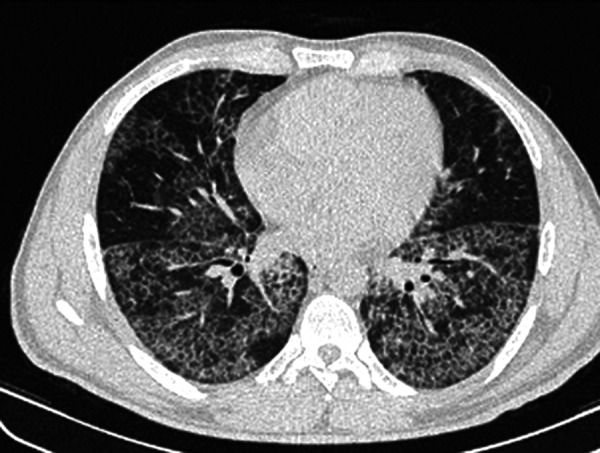
HRCT of a 38-year-old man with chronic dyspnea and cough and biopsy proved PAP demonstrating bilateral crazy-paving pattern with some geographic and subpleural sparing.
Figure 2.
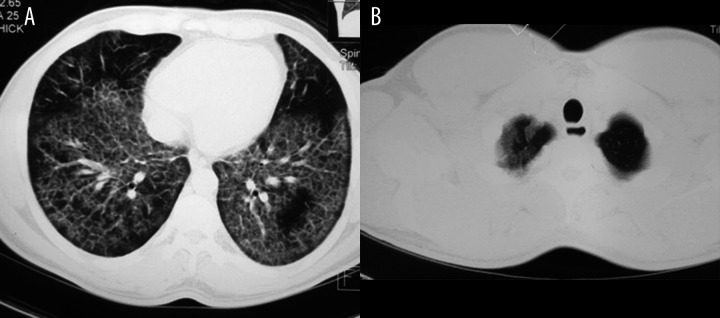
HRCT of a 30-year-old woman with idiopathic PAP presenting with chronic dyspnea and productive cough. Bilateral crazy-paving pattern is seen with subpleural (A) and left apex (B) sparing.
The overall extent of parenchymal involvement was 50 to 75% in eighty percent of patients, and 25 to 50% in twenty percent. The dominant distribution of disease was diffuse involvement of all lung zones (85.7%). Only 14.3% of patients had randomly spared upper or lower zones.
Thirty-three cases (94%) had areas of geographic sparing within the affected lung. Peripheral sparing was seen in 85.7% of patients, including three patterns with some overlap: costophrenic angle (80%), apices (60%), and subpleural (57%) sparing (Figure 3).
Figure 3.
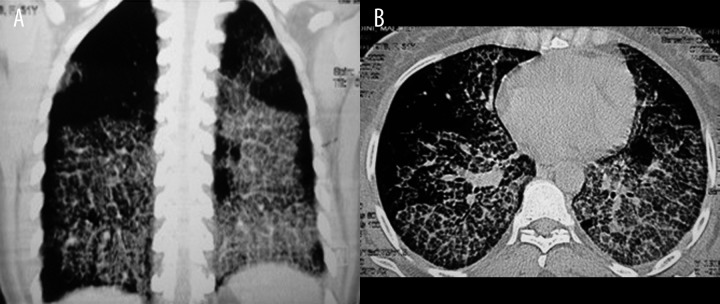
(A, B) Axial and coronal reconstructed HRCT images of a 31-year-old man with idiopathic PAP presenting with dyspnea and cough. Bilateral widespread crazy-paving pattern is seen. Costophrenic, subpleural and apical sparing is evident in coronal image.
The other common HRCT finding of our patients was consolidation which was seen in 22 patients (63%) (Figure 4). Other findings included pulmonary nodules (31.4%) (Figure 5), mediastinal and/or hilar lymphadenopathy (23%), mass-like consolidation (17%), pleural effusion (8.6%) and honey combing (5.7%). All HRCT features of patients with PAP are shown in Table 1.
Figure 4.
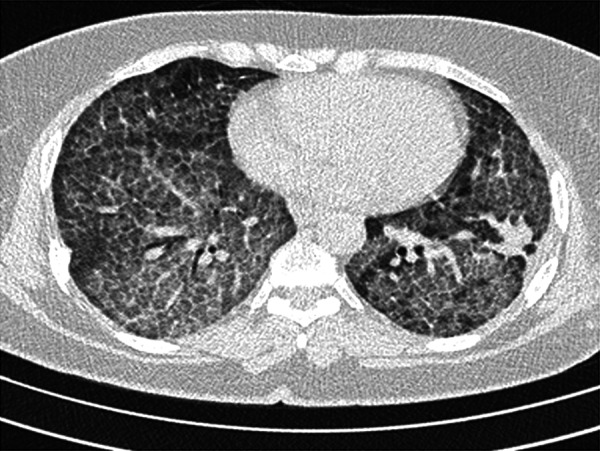
Axial HRCT image of a 40-year-old man with idiopathic PAP demonstrates bilateral widespread crazy-paving pattern and a mass-like consolidation in left lower lobe.
Figure 5.
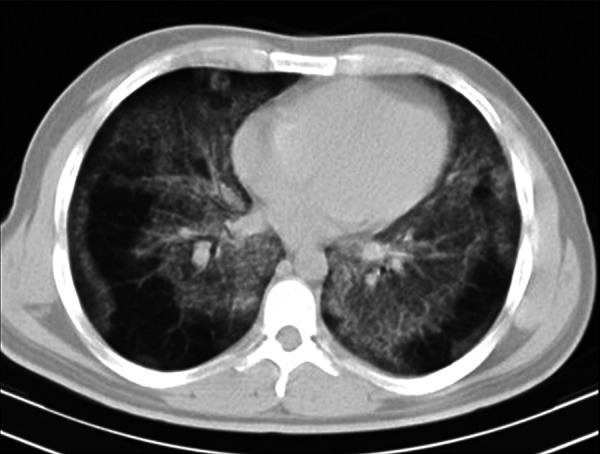
Bilateral tiny nodular infiltration and ground glass opacities along with subpleural sparing in a 41-year-old man with idiopathic PAP.
Table 1.
Frequency (%) of HRCT findings in the patients with idiopathic pulmonary alveolar proteinosis.
| HRCT findings | Sex | Age group | Total (n=35) | ||||
|---|---|---|---|---|---|---|---|
| Male (n=19) | Female (n=16) | <30 (n=7) | 30–50 (n=23) | >50 (n=5) | |||
| Interlobular septal thickenig | 100 | 100 | 100 | 100 | 100 | 100 | |
| Ground glass opacities | 95 | 100 | 86 | 100 | 100 | 91.7 | |
| Crazy-paving | 68 | 100 | 86 | 87 | 60 | 83 | |
| Sparing | Costophrenic | 57 | 50 | 78 | 48 | 80 | 80 |
| Apices | 74 | 43 | 43 | 60 | 80 | 60 | |
| Subpleural | 63 | 50 | 78 | 52 | 80 | 57 | |
| Consolidation | 58 | 69 | 71 | 70 | 20 | 63 | |
| Nodules | 42 | 19 | 71 | 17 | 40 | 31 | |
| Mass-like consolidation | 5.3 | 31 | 43 | 13 | 0 | 17 | |
| Lymphadenopathy | Mediastinal | 26.3 | 6.3 | 14.3 | 21.7 | 0.0 | 17 |
| Mediastinal/hilar | 10.5 | 0.0 | 0.0 | 0.0 | 40 | 6 | |
| Pleural effusion | Unilateral | 5.3 | 0.0 | 0.0 | 4.3 | 0.0 | 2.9 |
| Bilateral | 10.5 | 0.0 | 0.0 | 0.0 | 40 | 5.7 | |
| Honeycombing | 10.5 | 0.0 | 0.0 | 8.7 | 0.0 | 5.7 | |
The vast majority of pulmonary HRCT findings were in association with crazy-paving pattern, except for the pleural effusion; all three patients with pleural effusion had no crazy-paving.
The HRCT features of six patients without crazy-paving were: ILST (n=6), GGOs (n=5), pulmonary nodules (n=5), consolidations (n=4), hilar or mediastinal lymphadenopathy (n=3), mass-like consolidation (n=3), and pleural effusion (n=3).
There was no statistically significant difference in HRCT presentations of PAP between age groups. All female patients (n=16) had crazy-paving, while 13 out of 19 (68%) male patients had crazy-paving on their lung HRCT (p=0.02).
Discussion
Although PAP affects patients of all ages, it is more common in young patients with male predominance; male to female ratio of 2 to 4:1 [2,17,18]. In this study most of the patients were 30–50 years old and male to female ratio was 1.2:1.
As we expected, the predominant HRCT presentation of PAP was interlobular septal thickening and ground glass opacities, resulting in crazy-paving pattern (83%). Frazier et al. [1] in their study on 28 patients with PAP found the crazy-paving pattern in 75% of the patients. This pattern was seen in 71% of idiopathic PAPs in Ishii et al. study [13]. Other studies have reported relatively similar frequencies for crazy-paving with a maximum prevalence of 94% [5,19]. However, Xu et al. [2] reported significantly lower prevalence; in their systematic review of 241 Chinese PAP patients from 1965 to 2006, crazy-paving appearance was seen in 18.6% of patients. Further studies may be needed for clarifying the cause of this difference between Chinese and other populations.
Crazy-paving pattern may be absent in some patients with PAP. In our study ILST and GGOs without crazy-paving were seen in 17% and 14.7% of patients, respectively. In the Frazier et al. study [1], 25% of patients showed GGOs without crazy-paving pattern. In this situation high clinical suspicion in the presence of diffuse bilateral GGOs with or without septal thickening in the absence of other conditions (such as viral pneumonia, pneumocystis jiroveci pneumonia, etc.) should raise the likelihood of PAP.
This study demonstrates that a vast majority (86%) of the PAP patients have costophrenic angle, apices, or subpleural sparing. This feature is identical to that of similar studies. In the Ishii et al. study [13] subpleural sparing was seen in 71% of autoimmune PAP group. In the Frazier et al. study [1], costophrenic and subpleural sparing accounted for 50% and 34%, respectively, while in our study these sparing patterns were present in 80% and 57%, respectively.
Consolidation was the other common CT finding of our patients. PAP is a process of occupying alveolar space and therefore may present as pulmonary consolidation or mass-like consolidation. Other nonspecific findings including pulmonary nodules, mediastinal and/or hilar lymphadenopathy, mass-like consolidation, pleural effusion and honeycombing were not frequently seen. These results are identical to previous studies [1,2,13,19].
Holbert et al. [17] demonstrated that the dominant lung involvement pattern was geographic GGOs with uniform zonal pattern, a feature similar to our findings. The extent of the disease in the present study was 50–75% in 80% of patients, while in the Frazier et al. study [1] study only 21% had disease extent of 50–75% and 57% of them had 75–100% parenchymal involvement. It seems that the extent and zonal distribution of idiopathic PAP is higher than of secondary PAP, as some inhaled dusts may deposit based on gravity or some other conditions (such as infectious, metastatic or malignant processes) may spread to zones with higher vascular supply.
In this study we found that the prevalence of crazy-paving in females was significantly higher than in male patients. The cause of this difference is unknown and has not been reported in the previous studies.
Finally, it should be mentioned that this study was carried out only on idiopathic PAP. Further studies are needed for comparison of imaging features of idiopathic and secondary PAP.
Conclusions
This study demonstrated that the predominant HRCT presentation of idiopathic PAP was interlobular septal thickening and ground glass opacities, resulting in crazy-paving pattern.
References
- 1.Frazier AA, Franks TJ, Cooke EO, et al. From the Archives of the AFIP, Pulmonary Alveolar Proteinosis. Radiographics. 2008;28:883–99. doi: 10.1148/rg.283075219. [DOI] [PubMed] [Google Scholar]
- 2.Xu Z, Jing J, Wang H, et al. Pulmonary alveolar proteinosis in China: A systematic review of 241 cases. Respirology. 2009;14:761–66. doi: 10.1111/j.1440-1843.2009.01539.x. [DOI] [PubMed] [Google Scholar]
- 3.Rosen SH, Castleman B, Liebow AA. Pulmonary alveolar proteinosis. N Engl J Med. 1958;258:1123–42. doi: 10.1056/NEJM195806052582301. [DOI] [PubMed] [Google Scholar]
- 4.Seymour JF, Presneill JJ. Pulmonary alveolar proteinosis: progress in the first 44 years. Am J Respir Crit Care Med. 2002;166:215–35. doi: 10.1164/rccm.2109105. [DOI] [PubMed] [Google Scholar]
- 5.Souza CA, Marchiori E, Gonçalves LP, et al. Comparative study of clinical, pathological and HRCT findings of primary alveolar proteinosis and silicoproteinosis. Eur J Radiol. 2012;81(2):371–78. doi: 10.1016/j.ejrad.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Ioachimescu OC, Kavuru MS. Pulmonary alveolar proteinosis. Chron Respir Dis. 2006;3:149–59. doi: 10.1191/1479972306cd101rs. [DOI] [PubMed] [Google Scholar]
- 7.Lin FC, Chang GD, Chern MS, et al. Clinical significance of anti-GM-CSF antibodiesin idiopathic pulmonary alveolar proteinosis. Thorax. 2006;61:528–34. doi: 10.1136/thx.2005.054171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uchida K, Beck DC, Yamamoto T, et al. GM-CSF autoantibodies and neutrophil dysfunction in pulmonary alveolar proteinosis. N Engl J Med. 2007;356:567–79. doi: 10.1056/NEJMoa062505. [DOI] [PubMed] [Google Scholar]
- 9.Lee KN, Levin DL, Webb WR, et al. Pulmonary alveolar proteinosis: high-resolution CT, chest radiographic, and functional correlations. Chest. 1997;111:989–95. doi: 10.1378/chest.111.4.989. [DOI] [PubMed] [Google Scholar]
- 10.Johkoh T, Itoh H, Muller NL, et al. Crazy-paving appearance at thin-section CT: spectrum of disease and pathologic findings. Radiology. 1999;211(1):155–60. doi: 10.1148/radiology.211.1.r99ap10155. [DOI] [PubMed] [Google Scholar]
- 11.Albafouille V, Sayegh N, De Coudenhove S, et al. CT scan patterns of pulmonary alveolar proteinosis in children. Pediatr Radiol. 1999;29(3):147–52. doi: 10.1007/s002470050560. [DOI] [PubMed] [Google Scholar]
- 12.Takiguchi Y, Uchiyama T, Nagao K. Computed tomographic appearances of pulmonary alveolar proteinosis. Nihon Kyobu Shikkan Gakkai Zasshi. 1994;32(3):233–38. [Abstract] [PubMed] [Google Scholar]
- 13.Ishii H, Trapnell BC, Tazawa R, et al. Comparative study of high-resolution CT findings between autoimmune and secondary pulmonary alveolar proteinosis. Chest. 2009;136(5):1348–55. doi: 10.1378/chest.09-0097. [DOI] [PubMed] [Google Scholar]
- 14.Maimon N, Heimer D. The crazy-paving pattern on computed tomography. CMAJ. 2010;182(14):1545. doi: 10.1503/cmaj.091422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossi SE, Erasmus JJ, Volpacchio M, et al. “Crazy-paving” pattern at thin section CT of the lungs: radiologic-pathologic overview. Radiographics. 2003;23:1509–19. doi: 10.1148/rg.236035101. [DOI] [PubMed] [Google Scholar]
- 16.Lee CH. The crazy-paving sign. Radiology. 2007;243:905–6. doi: 10.1148/radiol.2433041835. [DOI] [PubMed] [Google Scholar]
- 17.Holbert JM, Costello P, Li W, et al. CT features of pulmonary alveolar proteinosis. Am J Roentgenol. 2001;176(5):1287–94. doi: 10.2214/ajr.176.5.1761287. [DOI] [PubMed] [Google Scholar]
- 18.McCook TA, Kirk DR, Merten DF, et al. Pulmonary alveolar proteinosis in children. Am J Roentgenol. 1981;137:1023–27. doi: 10.2214/ajr.137.5.1023. [DOI] [PubMed] [Google Scholar]
- 19.Briens E, Delaval P, Mairesse MP, et al. Pulmonary alveolar proteinosis. Rev Mal Respir. 2002;19(2):166–82. [PubMed] [Google Scholar]


