Abstract
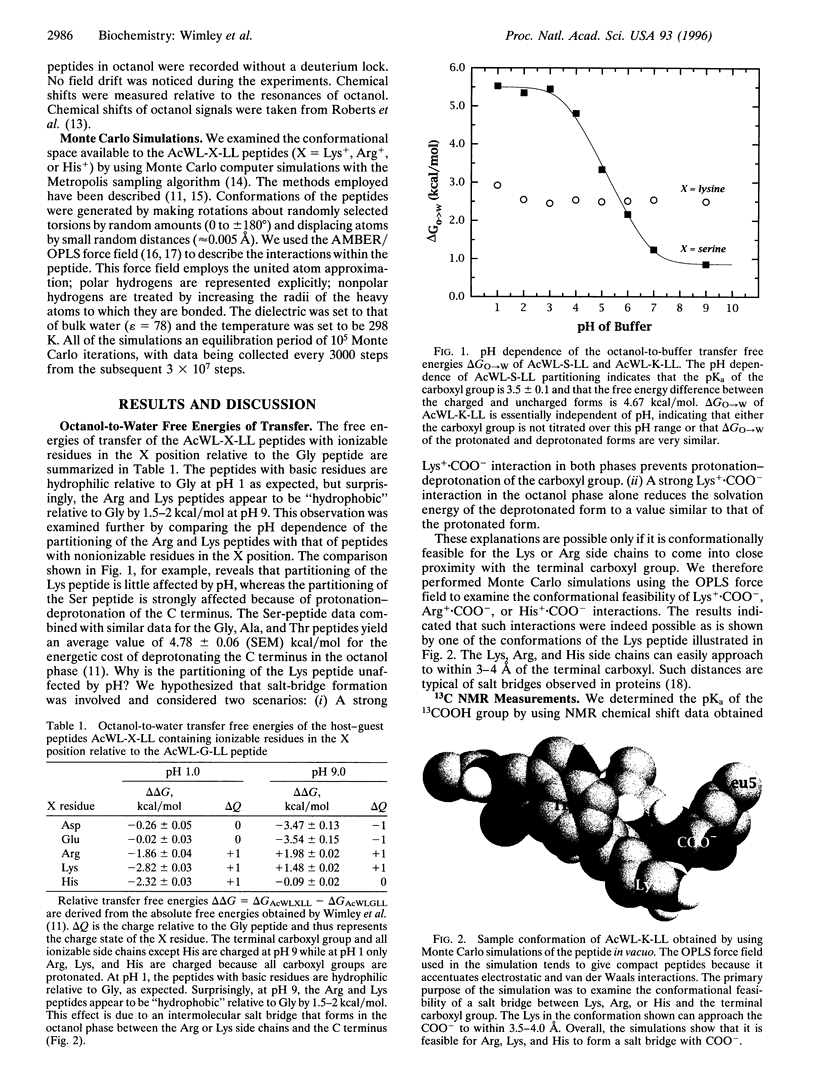
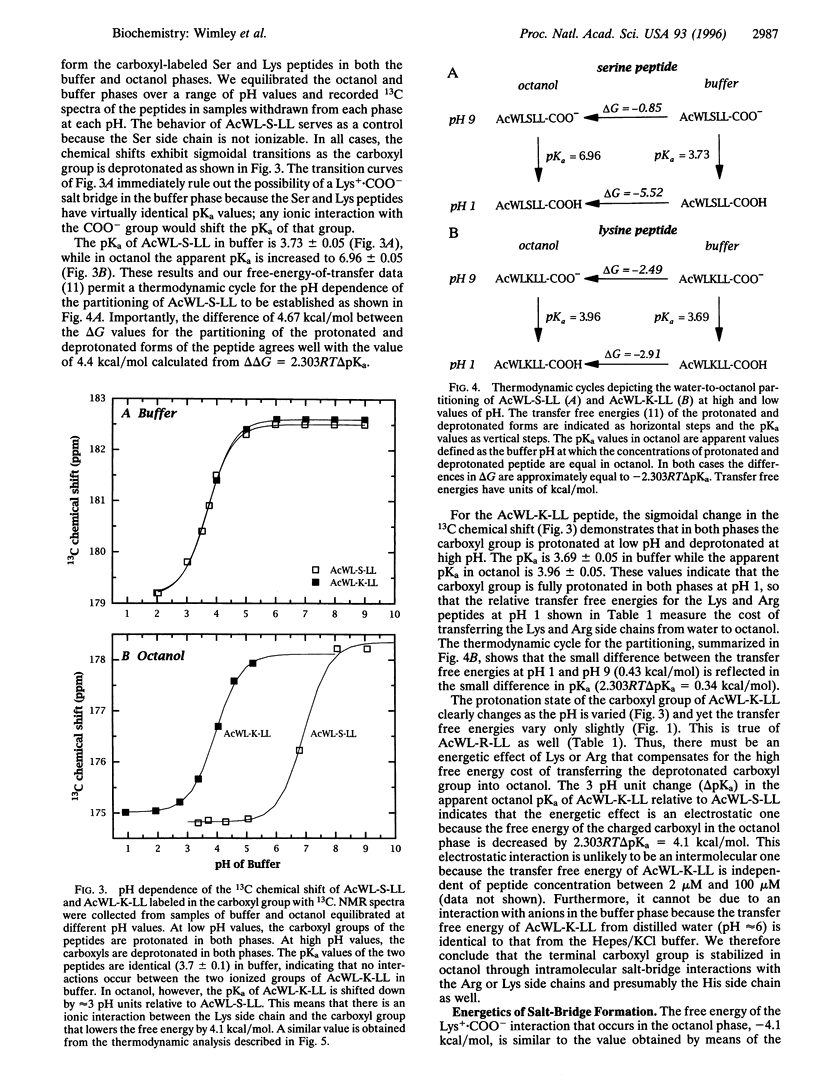
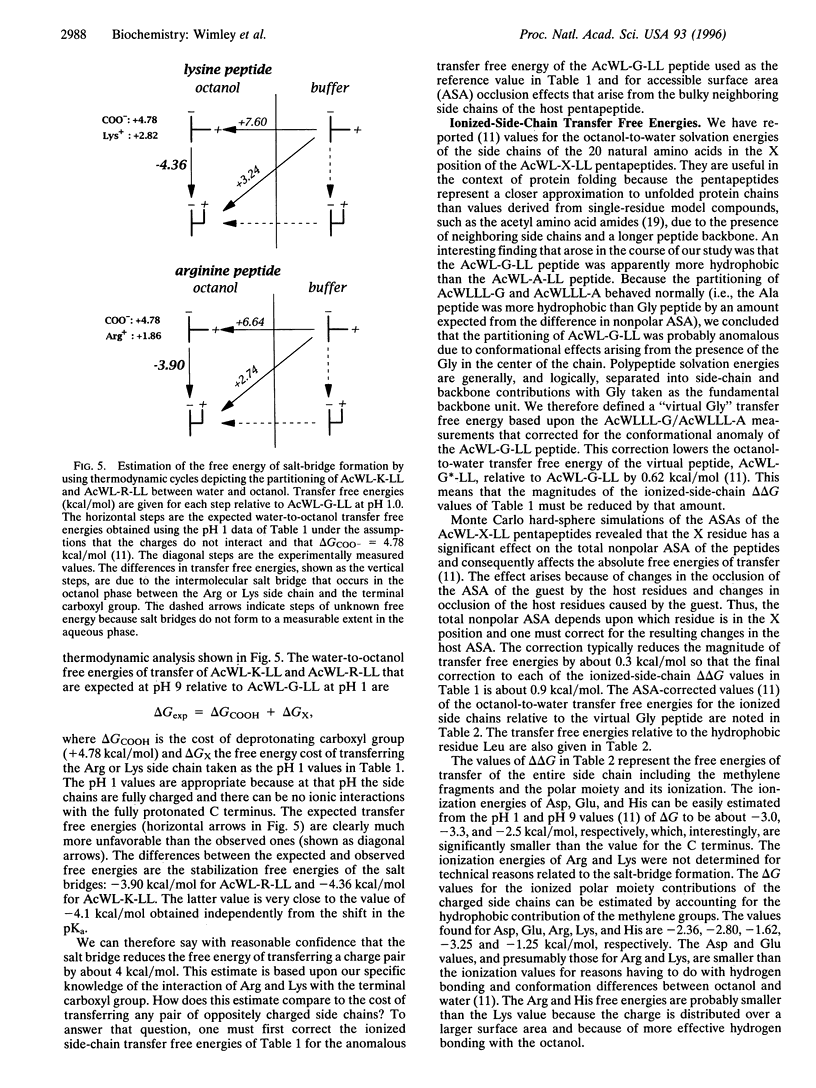
The solvation energies of salt bridges formed between the terminal carboxyl of the host pentapeptide AcWL- X-LL and the side chains of Arg or Lys in the guest (X) position have been measured. The energies were derived from octanol-to-buffer transfer free energies determined between pH 1 and pH 9. 13C NMR measurements show that the salt bridges form in the octanol phase, but not in the buffer phase, when the side chains and the terminal carboxyl group are charged. The free energy of salt-bridge formation in octanol is approximately -4 kcal/mol (1 cal = 4.184 J), which is equal to or slightly larger than the sum of the solvation energies of noninteracting pairs of charged side chains. This is about one-half the free energy that would result from replacing a charge pair in octanol with a pair of hydrophobic residues of moderate size. Therefore, salt bridging in octanol can change the favorable aqueous solvation energy of a pair of oppositely charged residues to neutral or slightly unfavorable but cannot provide the same free energy decrease as hydrophobic residues. This is consistent with recent computational and experimental studies of protein stability.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. E., Becktel W. J., Dahlquist F. W. pH-induced denaturation of proteins: a single salt bridge contributes 3-5 kcal/mol to the free energy of folding of T4 lysozyme. Biochemistry. 1990 Mar 6;29(9):2403–2408. doi: 10.1021/bi00461a025. [DOI] [PubMed] [Google Scholar]
- Barlow D. J., Thornton J. M. Ion-pairs in proteins. J Mol Biol. 1983 Aug 25;168(4):867–885. doi: 10.1016/s0022-2836(83)80079-5. [DOI] [PubMed] [Google Scholar]
- Creamer T. P., Rose G. D. Alpha-helix-forming propensities in peptides and proteins. Proteins. 1994 Jun;19(2):85–97. doi: 10.1002/prot.340190202. [DOI] [PubMed] [Google Scholar]
- Dao-pin S., Anderson D. E., Baase W. A., Dahlquist F. W., Matthews B. W. Structural and thermodynamic consequences of burying a charged residue within the hydrophobic core of T4 lysozyme. Biochemistry. 1991 Dec 10;30(49):11521–11529. doi: 10.1021/bi00113a006. [DOI] [PubMed] [Google Scholar]
- Eisenberg D., McLachlan A. D. Solvation energy in protein folding and binding. Nature. 1986 Jan 16;319(6050):199–203. doi: 10.1038/319199a0. [DOI] [PubMed] [Google Scholar]
- Eriksson A. E., Baase W. A., Zhang X. J., Heinz D. W., Blaber M., Baldwin E. P., Matthews B. W. Response of a protein structure to cavity-creating mutations and its relation to the hydrophobic effect. Science. 1992 Jan 10;255(5041):178–183. doi: 10.1126/science.1553543. [DOI] [PubMed] [Google Scholar]
- Franks N. P., Abraham M. H., Lieb W. R. Molecular organization of liquid n-octanol: an X-ray diffraction analysis. J Pharm Sci. 1993 May;82(5):466–470. doi: 10.1002/jps.2600820507. [DOI] [PubMed] [Google Scholar]
- Hendsch Z. S., Tidor B. Do salt bridges stabilize proteins? A continuum electrostatic analysis. Protein Sci. 1994 Feb;3(2):211–226. doi: 10.1002/pro.5560030206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm L., Sander C. Evaluation of protein models by atomic solvation preference. J Mol Biol. 1992 May 5;225(1):93–105. doi: 10.1016/0022-2836(92)91028-n. [DOI] [PubMed] [Google Scholar]
- Honig B. H., Hubbell W. L. Stability of "salt bridges" in membrane proteins. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5412–5416. doi: 10.1073/pnas.81.17.5412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellis J. T., Jr, Nyberg K., Fersht A. R. Energetics of complementary side-chain packing in a protein hydrophobic core. Biochemistry. 1989 May 30;28(11):4914–4922. doi: 10.1021/bi00437a058. [DOI] [PubMed] [Google Scholar]
- Komine S., Yoshida K., Yamashita H., Masaki Z. Voiding dysfunction in patients with human T-lymphotropic virus type-1-associated myelopathy (HAM). Paraplegia. 1989 Jun;27(3):217–221. doi: 10.1038/sc.1989.32. [DOI] [PubMed] [Google Scholar]
- Marian A. J., Roberts R. Recent advances in the molecular genetics of hypertrophic cardiomyopathy. Circulation. 1995 Sep 1;92(5):1336–1347. doi: 10.1161/01.cir.92.5.1336. [DOI] [PubMed] [Google Scholar]
- Matsumura M., Becktel W. J., Matthews B. W. Hydrophobic stabilization in T4 lysozyme determined directly by multiple substitutions of Ile 3. Nature. 1988 Aug 4;334(6181):406–410. doi: 10.1038/334406a0. [DOI] [PubMed] [Google Scholar]
- Matthews B. W. Can proteins be turned inside-out? Nat Struct Biol. 1995 Feb;2(2):85–86. doi: 10.1038/nsb0295-85. [DOI] [PubMed] [Google Scholar]
- Pace C. N. Contribution of the hydrophobic effect to globular protein stability. J Mol Biol. 1992 Jul 5;226(1):29–35. doi: 10.1016/0022-2836(92)90121-y. [DOI] [PubMed] [Google Scholar]
- Richards F. M. Areas, volumes, packing and protein structure. Annu Rev Biophys Bioeng. 1977;6:151–176. doi: 10.1146/annurev.bb.06.060177.001055. [DOI] [PubMed] [Google Scholar]
- Schueler O., Margalit H. Conservation of salt bridges in protein families. J Mol Biol. 1995 Apr 21;248(1):125–135. doi: 10.1006/jmbi.1995.0206. [DOI] [PubMed] [Google Scholar]
- Serrano L., Horovitz A., Avron B., Bycroft M., Fersht A. R. Estimating the contribution of engineered surface electrostatic interactions to protein stability by using double-mutant cycles. Biochemistry. 1990 Oct 9;29(40):9343–9352. doi: 10.1021/bi00492a006. [DOI] [PubMed] [Google Scholar]
- Shirley B. A., Stanssens P., Hahn U., Pace C. N. Contribution of hydrogen bonding to the conformational stability of ribonuclease T1. Biochemistry. 1992 Jan 28;31(3):725–732. doi: 10.1021/bi00118a013. [DOI] [PubMed] [Google Scholar]
- Shortle D., Stites W. E., Meeker A. K. Contributions of the large hydrophobic amino acids to the stability of staphylococcal nuclease. Biochemistry. 1990 Sep 4;29(35):8033–8041. doi: 10.1021/bi00487a007. [DOI] [PubMed] [Google Scholar]
- Sun D. P., Sauer U., Nicholson H., Matthews B. W. Contributions of engineered surface salt bridges to the stability of T4 lysozyme determined by directed mutagenesis. Biochemistry. 1991 Jul 23;30(29):7142–7153. doi: 10.1021/bi00243a015. [DOI] [PubMed] [Google Scholar]
- Waldburger C. D., Schildbach J. F., Sauer R. T. Are buried salt bridges important for protein stability and conformational specificity? Nat Struct Biol. 1995 Feb;2(2):122–128. doi: 10.1038/nsb0295-122. [DOI] [PubMed] [Google Scholar]
- de Prat Gay G., Johnson C. M., Fersht A. R. Contribution of a proline residue and a salt bridge to the stability of a type I reverse turn in chymotrypsin inhibitor-2. Protein Eng. 1994 Jan;7(1):103–108. doi: 10.1093/protein/7.1.103. [DOI] [PubMed] [Google Scholar]



