Abstract
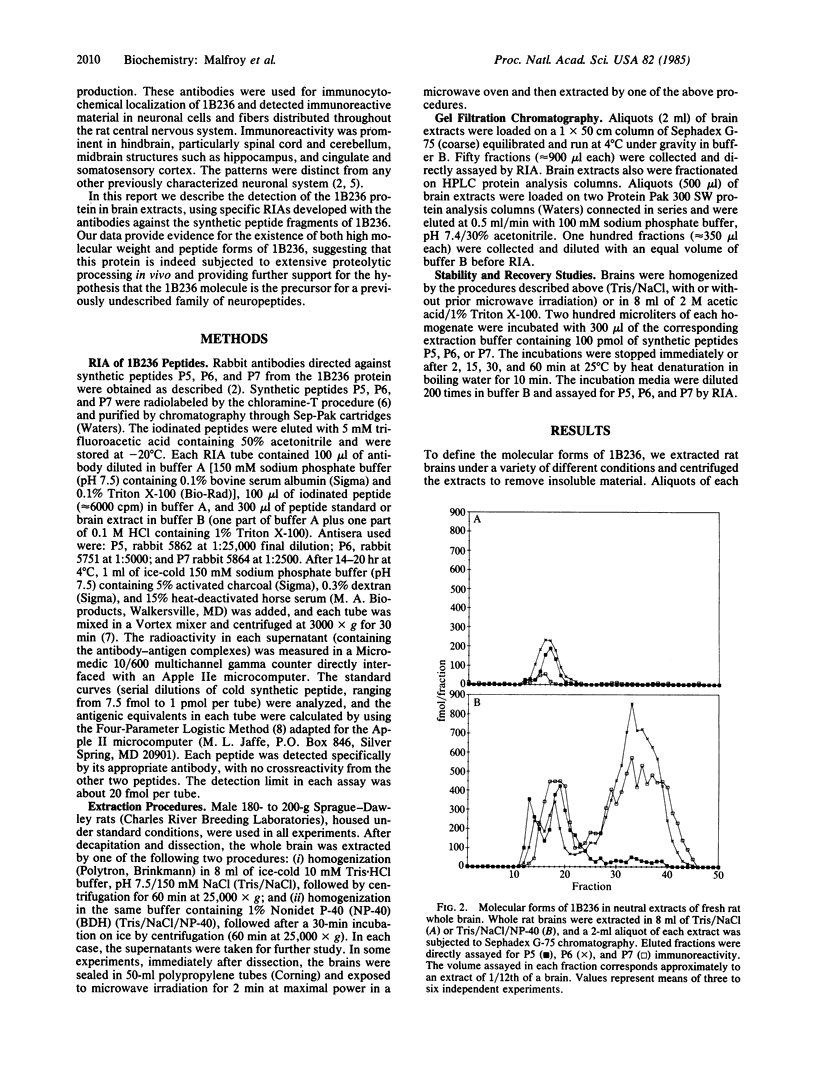
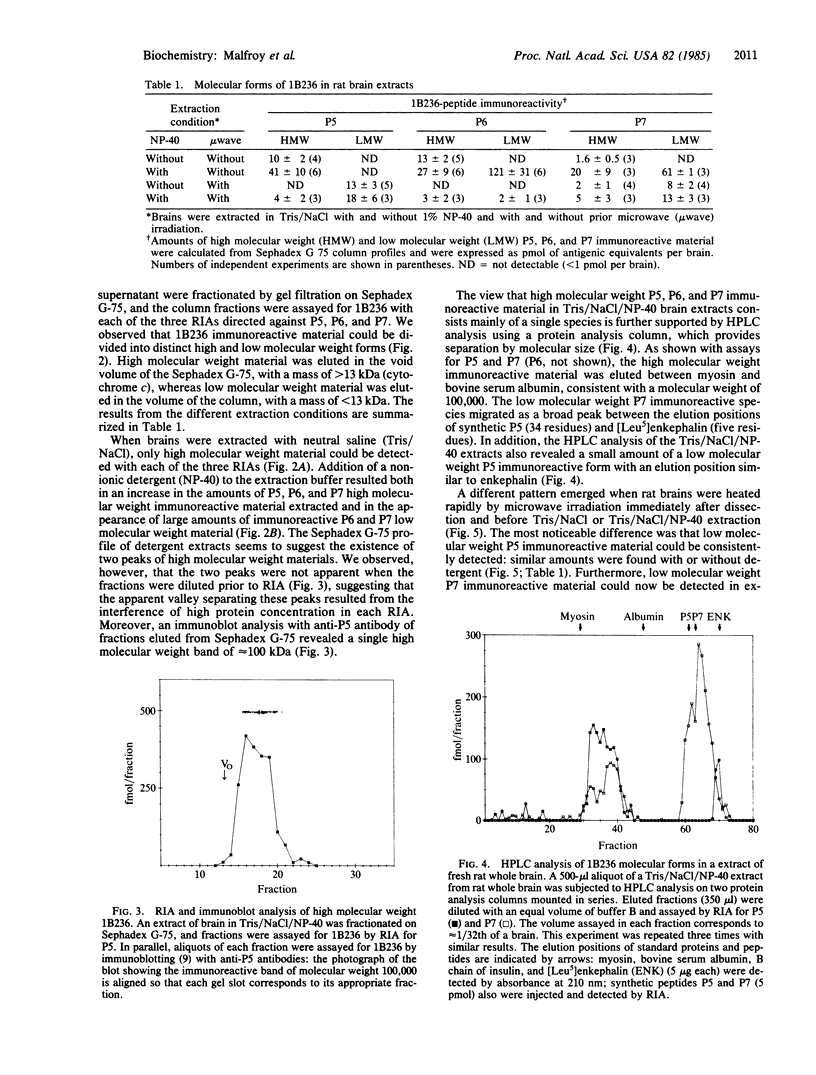
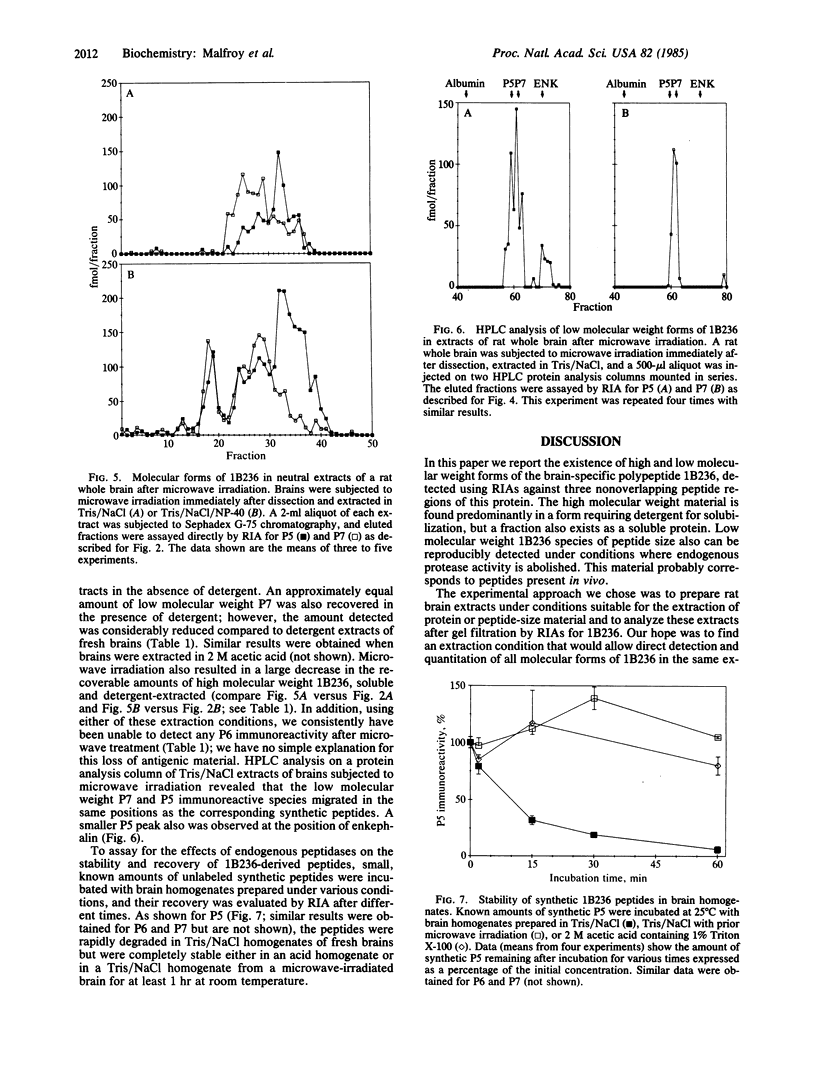
The COOH-terminal amino acid sequence of the rat brain-specific polypeptide 1B236 was previously deduced from molecular cloning and nucleotide sequence determination of its mRNA and the 1B236 protein shown to exist in the rat brain. The amino acid sequence of 1B236 contained at least three peptide sequences demarcated by pairs of basic amino acids--a structure similar to known neuropeptide and hormone precursors--which suggested that the protein might be processed in vivo to generate peptides. We have developed radioimmunoassays specific for 1B236 with antibodies against three synthetic peptides corresponding to putative cleavage products of this protein and have used these assays to define the molecular forms of 1B236 in rat brain extracts. The most abundant form is of high molecular weight (ca. 100,000) and requires detergent for solubilization; hence, it is probably membrane-bound. However, a small fraction of the high molecular weight material is soluble in the absence of detergent. In addition, several low molecular weight species are detectable in brain extracts prepared under conditions preventing proteolysis. These molecules correspond in size to two of the possible products of proteolytic processing predicted from the amino acid sequence of 1B236. The multiplicity of 1B236 forms, together with other data, suggests that this protein undergoes extensive post-translational modification, including proteolytic processing to generate peptides that may be physiologically relevant.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayon A., Shoemaker W. J., McGinty J. F., Bloom F. Immunodetection of endorphins and enkephalins: a search for reliability. Int Rev Neurobiol. 1983;24:51–92. doi: 10.1016/s0074-7742(08)60220-2. [DOI] [PubMed] [Google Scholar]
- Douglass J., Civelli O., Herbert E. Polyprotein gene expression: generation of diversity of neuroendocrine peptides. Annu Rev Biochem. 1984;53:665–715. doi: 10.1146/annurev.bi.53.070184.003313. [DOI] [PubMed] [Google Scholar]
- Elder J. H., Alexander S. endo-beta-N-acetylglucosaminidase F: endoglycosidase from Flavobacterium meningosepticum that cleaves both high-mannose and complex glycoproteins. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4540–4544. doi: 10.1073/pnas.79.15.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazarossian V. E., Chavkin C., Goldstein A. A specific radioimmunoassay for the novel opioid peptide dynorphin. Life Sci. 1980 Jul 7;27(1):75–86. doi: 10.1016/0024-3205(80)90021-1. [DOI] [PubMed] [Google Scholar]
- Kanazawa I., Jessell T. Post mortem changes and regional distribution of substance P in the rat and mouse nervous system. Brain Res. 1976 Nov 26;117(2):362–367. doi: 10.1016/0006-8993(76)90748-4. [DOI] [PubMed] [Google Scholar]
- Loh Y. P., Brownstein M. J., Gainer H. Proteolysis in neuropeptide processing and other neural functions. Annu Rev Neurosci. 1984;7:189–222. doi: 10.1146/annurev.ne.07.030184.001201. [DOI] [PubMed] [Google Scholar]
- Milner R. J., Sutcliffe J. G. Gene expression in rat brain. Nucleic Acids Res. 1983 Aug 25;11(16):5497–5520. doi: 10.1093/nar/11.16.5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G., Milner R. J., Bloom F. E. Cellular localization and function of the proteins encoded by brain-specific mRNAs. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 2):477–484. doi: 10.1101/sqb.1983.048.01.052. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G., Milner R. J., Shinnick T. M., Bloom F. E. Identifying the protein products of brain-specific genes with antibodies to chemically synthesized peptides. Cell. 1983 Jul;33(3):671–682. doi: 10.1016/0092-8674(83)90010-7. [DOI] [PubMed] [Google Scholar]
- Yanagishita M., Rodbard D. Computerized optimization of radioimmunoassays for hCG and estradiol: an experimental evaluation. Anal Biochem. 1978 Jul 15;88(1):1–19. doi: 10.1016/0003-2697(78)90393-7. [DOI] [PubMed] [Google Scholar]
- Yang H. Y., Hong J. S., Costa E. Regional distribution of LEU and MET enkephalin in rat brain. Neuropharmacology. 1977 Apr;16(4):303–307. doi: 10.1016/0028-3908(77)90112-5. [DOI] [PubMed] [Google Scholar]



