Abstract
pp32r1 (ANP32C) is oncogenic and has been shown to be overexpressed in tumors of the breast, prostate, and pancreas. In this work we show that pp32 family proteins are able to bind to the sphingosine analog FTY720 (Finguimod). Molecular docking studies highlight that a conserved residue F136 is likely to be a key determinant of the FTY720 binding site on the pp32 leucine-rich repeat domain. Transduction of the renal carcinoma cell line ACHN or cervical cancer cell line HeLa with lentivirus expressing the oncogenic family member pp32r1 or a pp32r1Y140H functional mutant illustrated an enhanced resistance to FTY720 induced apoptosis. These findings highlight that certain cancers overexpressing pp32r1 or pp32r1 mutants are likely to demonstrate enhanced resistance to FTY720 treatment.
Keywords: pp32, cell cycle, FTY720, cancer
Introduction
The acidic nuclear phosphoproteins (ANP32A-H) are an evolutionarily conserved family of proteins.1 The pp32 (ANP32A) protein is the most characterized member of the family and has been assigned a variety of names based on the identified function including; pp32 (phosphoprotein 32)2 and ANP32A (acidic nuclear phosphoprotein 32A).3 Little is known about the cellular functions of other family members; however some functionality “sharing” is likely to occur between the pp32 family members (ANP32A-H)1 since pp32 knockout mice have no distinct phenotype.4-6
Interestingly, the pp32 (ANP32A) protein acts as a tumor suppressor while its close homolog pp32r1 (ANP32C) is oncogenic and found to be overexpressed in poorly differentiated tumors, but not in normal cells.7,8 The functional role of pp32r1 at present is unclear; however despite the high sequence homology between pp32 and pp32r1 (~87%), pp32r1 is unable to substitute for pp32 at least in some situations as is demonstrated by its inability to associate with retinoblastoma protein.9
The pp32 family of proteins belongs to the superfamily of leucine-rich repeat (LRR) containing proteins. The LRR is a short motif of 20–29 residues in length that is present in tandem arrays in a variety of cytoplasmic, membrane, and extracellular proteins.10 Structurally the pp32 proteins contain several functional domains; a capped LRR motif at the N-terminus11 a central domain (amino acids 150–174) containing the region required for cellular transformation7 and a highly acidic C-terminus containing ~70% aspartic and glutamic acid residues.12 Several ANP32 proteins (A, B, and E) also contain a nuclear localization signal (NLS) with a conserved motif (KRKR) at the C-terminus of their protein sequence.11 These family members are thought to be predominantly localized in the nucleus, although it has been recently shown that pp32 is able to shuttle between nucleus and cytoplasm alongside HuR.13 In contrast the sequences of ANP32C, D, F, and H contain either no localization sequence or an incomplete NLS containing a truncated sequence (KRK) suggesting cytoplasmic localization. We have recently confirmed the cytoplasmic localization of pp32r1 by confocal microscopy using C-terminal GFP tagged pp32r1 constructs as well as Immunofluorescence microscopy using a pp32r1 specific antibody.14 The observed differential localization between pp32 family proteins suggests that some of the opposing functions of these proteins (e.g., pp32, tumor suppressor vs. pp32r1, oncogene) might originate from their differential locations and thus their alternate interaction partners and diverse cellular functions.
A functional mutation in pp32r1 comprising a Y140H substitution was previously identified in the prostate cancer cell line PC-3 and found to be associated with a hyperproliferative phenotype when transfected into cells.15 We have recently demonstrated that this increase in proliferation upon overexpression of pp32r1 or pp32r1Y140H occurs through the dysregulation of (CHD4)-mediated cell cycle control and appears to be confined to p53wt cells, since cells with mutated p53 are not hyperproliferative.14
The use of small molecules to manipulate the functions of pp32 family members is of clinical interest; we were therefore intrigued when two members of the ANP32 family, ANP32A and ANP32B (April) were recently identified as novel molecules interacting with sphingolipid metabolites.16 We searched for similar compounds in the PubChem database (http://pubchem.ncbi.nlm.nih.gov/) and identified FTY720, a synthetic structural analog of sphingosine that is derived from the metabolite myriocin produced by the fungus Isaria sinclairii. FTY720 (Finguimod) was first described as an immunosuppressant and is used clinically in the prevention of kidney graft rejection17 and for the treatment of relapsing multiple sclerosis.18 More recently, FTY720 has also been shown to induce apoptosis in a variety of cancer cell lines19-26 and a number of different signaling pathways have been implicated in mediating its effects.
In this work we investigated the direct binding of pp32 proteins with FTY720 and its reduced apoptotic effects in cells transfected with pp32r1 or the pp32r1Y140H mutant.
Results
Interaction between pp32 proteins and the sphingosine analog FTY720
Affinity chromatography and proteomics screening recently identified pp32 (ANP32A) and April (ANP32B) as novel proteins interacting with sphingolipid metabolites.16 In this work pp32 was observed to interact with sphingosine or dimethyl sphingosine but not dihydrosphingosine or ceramide indicating that the presence of a double bond (present in sphingosine but absent in dihydrosphingosine) is essential for this interaction. (Fig. 1). The sphingosine analog FTY720 retains this pseudo double bond feature by virtue of the benzene ring within its structure (Fig. 1A).
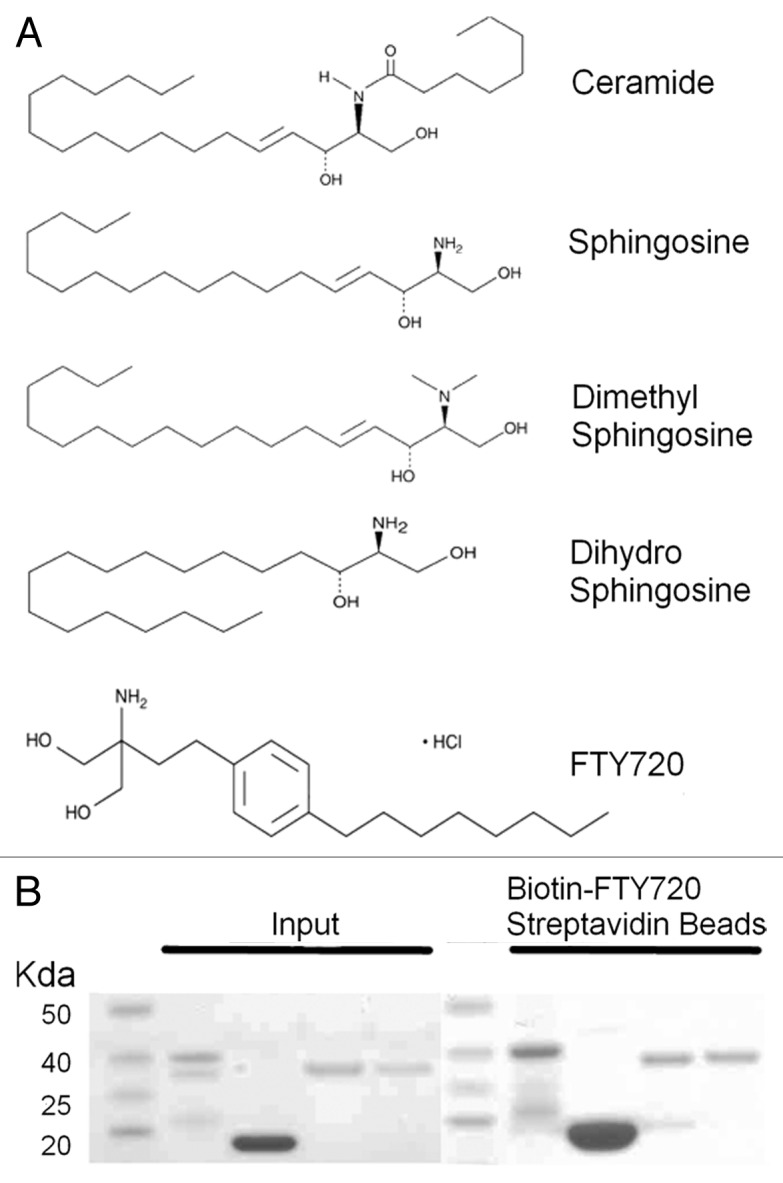
Figure 1. pp32 proteins and sphingosine metabolites. (A) 2D chemical structures of various sphingosine metabolites and the synthetic analog FTY720. (B) Molecular weight marker (lanes 1 and 6), pp32 full-length 1–249 (lanes 2 and 7), pp32ΔCT 1–149 (lanes 3 and 8), pp32r1 (lanes 4 and 9), pp32r1Y140H (lanes 5 and 10). Input protein is shown on the left and protein bound to biotin-FTY720 coupled streptavidin beads after washing is shown on the right.
Pp32 full-length (1–249), pp32ΔCT (1–149), pp32r1 (1–248), and pp32r1Y140H proteins were expressed in E. coli and purified as previously described (Fig. 1B). In our pull-down assay using biotin-FTY720 coupled to streptavidin beads pp32, pp32ΔCT, pp32r1, and pp32r1Y140H recombinant proteins were all bound to the beads after stringent washing.
Generation of cell lines overexpressing pp32r1 or pp32r1Y140H
The renal carcinoma cell line ACHN and cervical carcinoma cell line HeLa were lentivirally transduced with constructs encoding pp32r1 and the pp32r1Y140H functional mutant as previously described14 and used to determine the effects of treatment with FTY720.
Cells overexpressing pp32r1 or pp32r1Y140H demonstrate increased viability when treated with FTY720
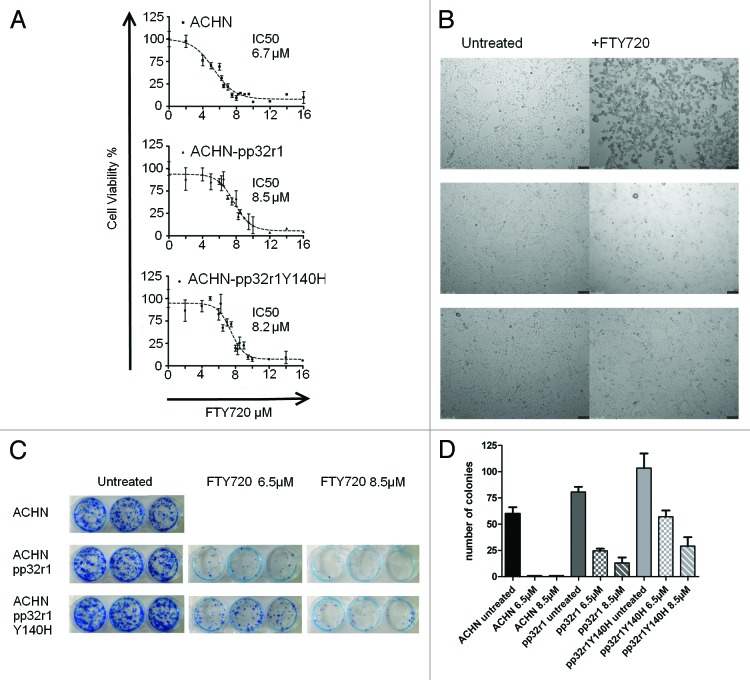
Untransduced and transduced cells were plated in 96-well plates and treated with 2–16 µM FTY720. The dose dependent effect of FTY720 on the cell viability was then measured using MTT and colony formation assays. The data for ACHN cells are shown in Figure 2 and for HeLa cells in Figure S1. The IC50 values for ACHN, ACHN-pp32r1, and ACHN-pp32r1Y140H cell lines were determined to be 6.7, 8.5, and 8.2 µM respectively estimated from nonlinear regression of the MTT assay data shown in Figure 2A. As can be seen in Figure 2B almost all ACHN cells treated with 6.5 µM FTY720 illustrated typical signs of apoptosis: detachment, shrinkage, and rounding, while in those cells overexpressing pp32r1 or pp32r1 only a small percentage showed any sign of apoptosis. Colony assays were performed at two concentrations (6.5 and 8.5 µM FTY720) for the three ACHN cell lines and are shown in Figure 2C and D. At these concentrations no colonies were observed in untransduced ACHN cells while pp32r1 and pp32r1Y140H cells retained their colony forming capacity.
Figure 2. Increased viability of pp32r1 and pp32r1Y140H cell lines treated with FTY720. (A) MTT assay performed on ACHN, ACHN-pp32r1, and ACHN-pp32r1Y140H cell lines treated with 2–16 µM FTY720 and compared with untreated cells. (B) Cells were treated with 6.5 µM FTY720 and visualized by light microscopy. ACHN cells show significant onset of apoptosis while transduced cell lines illustrate only a few cells with apoptotic features. (C) Colony formation assay of untransduced and transduced cell lines treated with 6.5 and 8.5 µM FTY720. (D) A graphical representation of the data shown in (C).
The enhanced resistance to FTY720 treatment seen in ACHN cell lines was similarly observed in HeLa cells transduced with pp32r1 and pp32r1Y140H (Fig. S1), with IC50 values calculated as 9.7, 10.5, and 12.1 µM, respectively. Again pp32r1 or pp32r1Y140H transduced cells showed increased colony forming capacity compared with wild-type untransduced cells.
FTY720 induces differential apoptosis in ACHN cell lines transduced with pp32r1 or the Y140H mutant
To further demonstrate that the loss of cell viability in the three ACHN cell lines was through FTY720 mediated apoptosis we treated cells with 6.5 µM FTY720 (just below the IC50 concentration for untransduced ACHN cells) and measured the amount of apoptosis by flow cytometry after staining with fluorescein-conjugated annexin V and propidium iodide. These results are illustrated in Figure 3A. While ACHN cells showed a high level of apoptosis (35.0%) the pp32r1 and pp32r1Y140H cell lines demonstrated a pronounced resistance showing only 8.7% and 17.0% respectively. Figure 3B illustrates these results graphically.
Figure 3. Reduced FTY720 mediated apoptosis in cells overexpressing pp32r1 or pp32r1Y140H. (A) Cells were either untreated, treated with DMSO control, or treated with 6.5 µM FTY720 and stained using an annexinV-FITC/ PI kit. Viable cells (unstained, bottom left quadrant Q4), early apoptotic cells with annexin V-FITC staining (bottom right quadrant Q3), late apoptotic cells with annexin V-FITC/PI staining (top right quadrant Q2), dead cells with PI staining (top left quadrant Q1). (B) The results from (A) for cells treated with 6.5 µM FTY720 are shown graphically as a percentage of viable Q4, apoptotic (early and late Q2/Q3) and dead cells Q1.
Overexpression of pp32r1 or pp32r1Y140H does not regulate FTY720 resistance through inhibition of PP2A activity
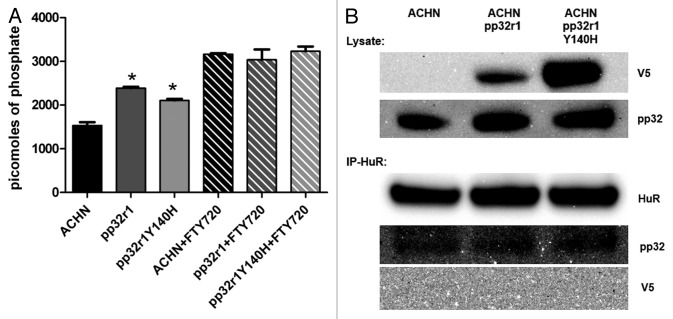
PP2A activity has been linked to FTY720 activity by virtue of it binding to the PP2A inhibitors pp32 and SET causing them to dissociate and alleviating inhibition. We therefore measured the levels of PP2A activity in untransduced and transduced cell lysates using a PP2A immunoprecipitation assay (Millipore) by calculating the phosphate release during dephosphorylation of the threonine phosphopeptide (K-R-pT-I-R-R) compared with a phosphate standard curve. Assays performed using the ACHN, pp32r1, and pp32r1Y140H cell lysates showed averages of 1529, 2383 (~56% increased activity), and 2103 (~38% increased activity) picomoles of phosphate released, with both transduced cell line lysates showing increased PP2A activity (Fig. 4A). Assays performed in parallel using lysates spiked with 20 µM FTY720 showed significantly higher PP2A activity with ~3000 picomoles of phosphate released in each lysate and only minor differences were observed between lysates from untransduced and transduced cells (Fig. 4A). These data suggest that overexpression of pp32r1 or pp32r1Y140H in ACHN cells does not inhibit PP2A activity and therefore is unlikely to be a contributing factor in pp32r1 mediated FTY720 resistance.
Figure 4. Investigation into the mechanism of pp32r1-mediated FTY720 resistance. (A) Protein phosphatise 2A assay performed on ACHN cell lysates in the absence and presence of FTY720, *P < 0.05. (B) Transduced cell lysates were shown to contain overexpressed pp32r1/pp32r1Y140H (anti-V5) while endogenous pp32 protein is observed in all three lysates. Co-immunoprecipitation,with anti- HuR antibody is only able to co-immunoprecipitate endogenous pp32.
pp32r1 and pp32r1Y140H do not interact with HuR
The pp32 family members pp32 and April are both known to interact with Hu-antigen R (HuR) a protein that regulates the mRNA export, stability of many cytokines and cell cycle regulators through binding to the AU-rich elements in their mRNAs.27 We were unaware of any current data demonstrating an interaction of pp32r1 with HuR and therefore performed immunoprecipitations to determine if this interaction could stabilize specific mRNAs that could be critical in mediating FTY720 resistance, as has been observed in pp32-mediated gemcitabine resistance.27,28 Despite high levels of both HuR and recombinant pp32r1 proteins in ACHN cell lysates (Fig. 4B), immunoprecipitation with an anti-HuR antibody and blotting with anti-V5 to detect the recombinant pp32r1 or pp32r1Y140H proteins demonstrated no interaction between pp32r1 or pp32r1Y140H and HuR. In contrast control experiments blotting with anti-pp32 clearly showed that endogenous pp32 and HuR in cell lysates could be co-immunoprecipitated (Fig. 4B). These results suggest that at least in the context of these cells pp32r1 is unable to associate with HuR and cannot contribute to the stabilization of specific mRNA’s responsible for FTY720 resistance. The immunoprecipitation experiment was identically performed using HeLa untransduced and transduced cell lysates with similar outcome (data not shown). It may however be possible that pp32r1 might be able to stabilize specific HuR-mRNA complexes in other cell types, thus a pp32r1 interaction with HuR cannot be totally ruled out.
Molecular docking
To determine the possible binding site of FTY720 on the surface of the pp32 LRR domain we performed molecular docking studies using the program Autodock.29 A grid surrounding the entire pp32 crystal structure was used and the ensemble of docking conformations was then ranked based upon the binding energy score as output by Autodock.
The majority of docked conformers surrounded a binding site where the aromatic ring of FTY720 formed a pi-stacking interaction with residue F136 of the pp32 LRR structure, the best energy conformer (−4.0 kcal/mol) is shown in Figure 5.
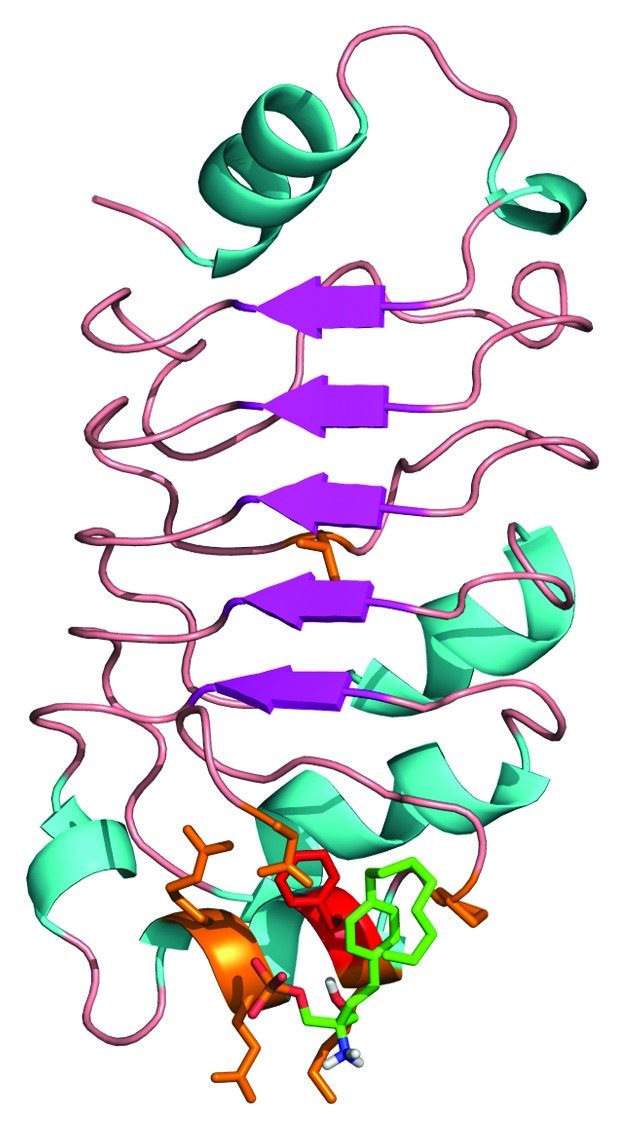
Figure 5. Docking of FTY720 to the crystal structure of the pp32 LRR domain. The structure of the pp322 LRR domain is illustrated as ribbons and colored according to secondary structure. The proposed FTY720 (green) binding site identified by docking studies is illustrated as sticks with residue F136 highlighted in red.
Discussion
Increased pp32r1 expression has been observed in both tumor cells7,8 and also in activated stem cells;15 however the functional relevance of increased levels of pp32r1 in different cell types is still unclear. The pp32 family represents an intriguing balance between proteins that share very high sequence identity and yet appear to illustrate opposing functions, although this can be attributed in part to their alternate cellular localization and as is seen between pp32 (ANP32A) and pp32r1 (ANP32C).14 In this work we confirmed our hypothesis that pp32 proteins are able to bind to the sphingosine analog FTY720 (Finguimod) using biotin-FTY720 coupled streptavidin beads to pull-down recombinant pp32 proteins. Interestingly pp32, pp32r1, and the pp32r1Y140H mutant were all able to be pulled down indicating that sphingosine or FTY720 binding might be a general feature of pp32 family proteins. More importantly the pp32ΔCT protein comprising the residues 1–149 also interacted with FTY720, highlighting that the N-terminal LRR domain is required for this interaction.
Our molecular docking studies identified a possible FTY720 binding site surrounding a conserved residue (F136) on the face of the LRR domain (Fig. 5). Despite our attempts at soaking existing pp32 crystals with FTY720 we have been hampered by the solubility of this compound in our current crystallization conditions. The data collected on soaked crystals however does show weak density existing around residue F136 which we have attributed to FTY720 binding at low occupancy (data not shown) and supports our conclusion that F136 is involved in FTY720 binding. The mutation of residue F136 however appears to influence the solubility of recombinant protein making supportive pull-down experiments difficult to perform. Further biophysical characterization of this interaction using mutagenesis and X-ray crystallographic analysis is currently in progress and will reveal more about the molecular details of the pp32:FTY720 interaction site.
Despite its initial application as an immunomodulating drug to treat multiple sclerosis FTY720 has now been shown to induce apoptosis in a variety of cancer cell lines leading to a number of clinical trials to treat among others ovarian cancer, lung cancer, and chronic myelogenous leukemia (CML).30 We overexpressed pp32r1 and pp32r1Y140H proteins in both ACHN and Hela cell lines and investigated cell viability after treatment with different FTY720 concentrations using MTT and colony formation assays (Fig. 2; Fig. S1). Our observations show that cells overexpressing pp32r1 or pp32r1Y140H were more resistant to the effects of FTY720 illustrating higher viability and reduced levels of apoptosis.
The detailed molecular mechanism of FTY720 action is very complex and a number of pathways have been implicated allowing FTY720 to induce apoptosis31 or caspase-independent cell death.32 Very recent work has however implicated the protein SET, a known pp32 binding partner that is found in both the INHAT and SET complexes,33,34 as an FTY720 binding protein.35 Like SET, pp32 proteins are also known to be potent inhibitors of protein phosphatise 2A (PP2A),36 a tumor suppressor protein that is responsible for the regulation of a number of oncoproteins including c-Myc.37 The targeting of SET by FTY720 was demonstrated to suppress tumor growth through apoptosis-independent programmed cell death (necroptosis) following PP2A-dependent RIPK1 activation.35 Given the close connection between SET and pp32 it seemed likely that this mechanism of FTY720 action could be a common feature. We therefore performed PP2A assays on the ACHN and pp32r1 transduced cell lystates in the absence and presence of FTY720 (Fig. 4A). Interestingly PP2A activity was slightly higher in pp32r1 or pp32r1Y140H expressing cell lines, suggesting that unlike pp32 the overexpressed pp32r1 protein is unable to act as an inhibitor of PP2A, although the reason for significantly increased activity compared with control cells remains unclear. Incubation of the cell lysates with FTY720 increased the observed PP2A activity significantly and to a similar level in all three lysates, presumably due to FTY720 interaction with endogenous pp32 and SET proteins thereby alleviating the basal cellular level of PP2A inhibition as has been previously demonstrated.35 These combined results suggest however that pp32r1 mediated resistance to FTY720 does not occur through increased inhibition of PP2A by pp32r1 or pp32r1Y140H overexpression and that another pathway must be involved.
The interaction between pp32 and HuR has previously been shown to stabilize specific mRNAs leading to the enhanced resistance of cells to the chemotherapeutic gemcitabine.28 In our current studies we observed interaction between endogenous pp32 and HuR proteins in cell lysates, but were unable to detect any interaction between HuR and overexpressed pp32r1 or pp32r1Y140H proteins (Fig. 4B). These data suggest that the various non-overlapping functions of pp32 family proteins are likely to contribute to FTY720 resistance and the mechanism of pp32r1 mediated FTY720 resistance still remains unclear.
It is difficult to determine the clinical implications of our observations as details regarding the frequency of pp32r1 overexpressing tumors are not available. However pp32r1 appears to be frequently overexpressed in a high percentage of prostate, breast, and pancreatic tumors,38 which implies that treatment with FTY720 in these cases may be less effective at normal dosages.
The levels of pp32r1 expression are known to be increased in poorly differentiated tumors38,39 while in cell lines mRNA expression is highest in LNCaP cells with moderate levels in PC-3 and low levels in DU145 cells.15 In prostate cancer cell line DU145 cells have been shown to be more sensitive to FTY720 than normal human prostate stromal cells.40 The chemotherapeutic sensitivity of DU145 cells has however been attributed to Sphingosine Kinase 1 activity in these cells.41 However, it is possible that the known decrease in pp32r1 levels across LNCaP, PC-3, and DU145 contributes to the relative greater sensitivity of DU145 to FTY720. We hope that future studies aimed at silencing native pp32r1 expression in cancer cell lines will confirm the role of pp32r1 in FTY720 resistance. Future structural and biochemical analysis are also still required to fully determine how FTY720 might target individual pp32 proteins, its relevance in tumor cells where pp32r1 is overexpressed, or even in neural cells where other pp32 family members like ANP32E show higher levels of expression.1
Materials and Methods
Expression and purification of pp32 and pp32r1 proteins
The pp32 (Anp32a) full-length gene (residues 1–249) and pp32ΔCT fragment (residues 1–149) was amplified by PCR from a full-length clone (RZPD). The pp32r1 (ANP32C) full-length gene (residues 1–249) was amplified via nested PCR using HeLa cell genomic DNA as a template. The fragments were then cloned into the pProEX HTb expression vector in frame with an N-terminal (His)6 tobacco etch virus (TEV) cleavable tag. The Y140H mutant construct was generated using the Quickchange mutagenesis kit (Stratagene) according to the manufacturers protocol. Expression and purification of pp32 proteins in E. coli was essentially as described for the pp32ΔCT fragment.11
FTY720 pull-down experiments
Streptavidin beads (Pierce) were equilibrated in PBS, loaded with 20 µM biotinylated FTY720 (Cayman Chemicals) and washed with PBS to remove unbound ligand. Purified pp32 proteins ~100 µg/mL were then incubated with beads for 30 min before washing 4 times with PBS. The beads were then resuspended in SDS sample buffer and proteins resolved by SDS-PAGE on a 4–12% gradient gel (Invitrogen) before staining with coomassie blue.
Cell culture and letiviral transduction
ACHN cells (DSMZ) were grown at 37 °C in a 5% CO2-humidified atmosphere in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 50 U/mL penicillin, 50 μg/mL streptomycin, 2 mM l-glutamine, 1% NEAA, 1 mM sodium pyruvate, and 10% fetal bovine serum (FBS). Lentivirus was produced in HEK293T cells using standard protocols and ACHN or Hela cells were transduced as previously described.14
Cell viability
The MTS assay (Promega, Cell Titer 96 AQueous non-radioactive cell proliferation assay) was performed in triplicates as per manufactures protocol. Cells were first seeded at 4 × 103 cells per well in 96-well plates and incubated for 24 h. Cells were then treated for an additional 24 h with FTY720 at various concentrations (2–16 µM) while control cells were left untreated. To each well MTS (0.5 mg/mL), was added, incubated for 3 h and the absorbance measured at 490 nm using a plate reader (BioTek, Synergy 2).
Colony formation assays
Cell lines were treated as above for MTS assays in 96-well plates. After 24 h incubation with FTY720 at 6.5 and 8.5 µM, 1000 viable cells (as judged from trypan blue staining and counting) were plated into 6-well plates and left to grow for 10–14 d with regular media exchange. Colonies (>30 cells) were fixed and stained using methylene blue before counting, each experiment was performed in triplicate.
Apoptosis
The percentage of apoptotic cells was evaluated using an annexinV-FITC/PI kit (Beckman Coulter) according to the manufacturer’s protocol. All flow cytometry measurements were made using a Becton-Dickinson FacsCanto. Briefly, 1 × 106 cells washed with PBS before being stained with annexin V-FITC and 5 mg/mL propidium iodide in binding buffer (10 mM HEPES/NaOH [pH 7.4], 140 mM NaOH, and 2.5 mM CaCl2) for 10 min at room temperature in the dark. The samples were then analyzed by flow cytometry within 1 h to determine the percentage of cells displaying annexin V (early apoptosis) or annexin V/propidium iodide staining (late apoptosis). Multiple independent experiments were performed for each cell line, a representative of which is shown.
Protein phosphatise 2A (PP2A) assay
The protein phosphatise 2A (PP2A) immunoprecipitation assay kit (Millipore) was used to determine the PP2A activity in cell lysates, where indicated, 20 µM FTY720 was added to the lysate before immunoprecipitation. All experiments were performed in duplicate and are representative of multiple experiments.
Immunoprecipitation and western blotting
Cell lysates were prepared on ice using NP40 lysis buffer (50 mM Tris, 150 mM NaCl, 1% NP40 protease inhibitor cocktail) and protein quantitation was performed using a BCA assay (Uptima). Lysates were precleared with protein A agarose (GE Healthcare) before A total of 50 µg protein was immunoprecipitated using a monoclonal mouse anti-HuR antibody (Santa Cruz, sc5261), proteins were then resolved on 4–20% SDS-PAGE gels (Invitrogen). After transfer to polyvinylidene difluoride (PVDF) the membrane was blocked for 1 h in 5% low fat milk powder in PBST. The membrane was then incubated overnight at 4 °C with 1:1000 dilution of mouse anti-V5 tag (Serotech, MCA1360), mouse anti-ANP32A (Sigma clone, 2G11-4A5 WH0008125M1), mouse anti-ANP32C (Abcam, ab65037) in blocking solution. The blot was then washed 3 times with PBST followed by incubation with 1:2500 dilution of goat-anti-mouse-HRP (DAKO) or goat-anti-rabbit-HRP (Bio-Rad) conjugated secondary antibody for 1 h at room temperature. Following 3 washes with PBST detection was performed by incubating the blot directly with Roti-Lumin substrate (Roth) and measured on a MultiImage II chemiluminescence imaging system (Alpha Innotech).
Molecular docking
Autodock29 was used to dock FTY720 to the structure of the pp32 LRR domain (PDB 2JE0), using a grid spanning the entire molecule. The generated ensemble of docked conformations was then ranked according to the corresponding binding energy scores as output by Autodock.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
S.B. is a graduate student of the REBIRTH “Regenerative Sciences” PhD program, Hannover Medical School. This work was supported in part by grants from the Erich und Gertrud Roggenbuck-Stiftung (THU).
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/27307
References
- 1.Matilla A, Radrizzani M. The Anp32 family of proteins containing leucine-rich repeats. Cerebellum. 2005;4:7–18. doi: 10.1080/14734220410019020. [DOI] [PubMed] [Google Scholar]
- 2.Malek SN, Katumuluwa AI, Pasternack GR. Identification and preliminary characterization of two related proliferation-associated nuclear phosphoproteins. J Biol Chem. 1990;265:13400–9. [PubMed] [Google Scholar]
- 3.Matilla A, Koshy BT, Cummings CJ, Isobe T, Orr HT, Zoghbi HY. The cerebellar leucine-rich acidic nuclear protein interacts with ataxin-1. Nature. 1997;389:974–8. doi: 10.1038/40159. [DOI] [PubMed] [Google Scholar]
- 4.Opal P, Garcia JJ, McCall AE, Xu B, Weeber EJ, Sweatt JD, Orr HT, Zoghbi HY. Generation and characterization of LANP/pp32 null mice. Mol Cell Biol. 2004;24:3140–9. doi: 10.1128/MCB.24.8.3140-3149.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reilly PT, Afzal S, Gorrini C, Lui K, Bukhman YV, Wakeham A, Haight J, Ling TW, Cheung CC, Elia AJ, et al. Acidic nuclear phosphoprotein 32kDa (ANP32)B-deficient mouse reveals a hierarchy of ANP32 importance in mammalian development. Proc Natl Acad Sci U S A. 2011;108:10243–8. doi: 10.1073/pnas.1106211108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reilly PT, Afzal S, Wakeham A, Haight J, You-Ten A, Zaugg K, Dembowy J, Young A, Mak TW. Generation and characterization of the Anp32e-deficient mouse. PLoS One. 2010;5:e13597. doi: 10.1371/journal.pone.0013597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brody JR, Kadkol SS, Mahmoud MA, Rebel JM, Pasternack GR. Identification of sequences required for inhibition of oncogene-mediated transformation by pp32. J Biol Chem. 1999;274:20053–5. doi: 10.1074/jbc.274.29.20053. [DOI] [PubMed] [Google Scholar]
- 8.Kadkol SS, Brody JR, Pevsner J, Bai J, Pasternack GR. Correction to “Modulation of oncogenic potential by alternative gene use in human prostate cancer”. Nat Med. 1999;5:1087. doi: 10.1038/12530. [DOI] [PubMed] [Google Scholar]
- 9.Adegbola O, Pasternack GR. Phosphorylated retinoblastoma protein complexes with pp32 and inhibits pp32-mediated apoptosis. J Biol Chem. 2005;280:15497–502. doi: 10.1074/jbc.M411382200. [DOI] [PubMed] [Google Scholar]
- 10.Kobe B, Kajava AV. The leucine-rich repeat as a protein recognition motif. Curr Opin Struct Biol. 2001;11:725–32. doi: 10.1016/S0959-440X(01)00266-4. [DOI] [PubMed] [Google Scholar]
- 11.Huyton T, Wolberger C. The crystal structure of the tumor suppressor protein pp32 (Anp32a): structural insights into Anp32 family of proteins. Protein Sci. 2007;16:1308–15. doi: 10.1110/ps.072803507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen TH, Brody JR, Romantsev FE, Yu JG, Kayler AE, Voneiff E, Kuhajda FP, Pasternack GR. Structure of pp32, an acidic nuclear protein which inhibits oncogene-induced formation of transformed foci. Mol Biol Cell. 1996;7:2045–56. doi: 10.1091/mbc.7.12.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brennan CM, Gallouzi IE, Steitz JA. Protein ligands to HuR modulate its interaction with target mRNAs in vivo. J Cell Biol. 2000;151:1–14. doi: 10.1083/jcb.151.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buddaseth S, Göttmann W, Blasczyk R, Huyton T. Dysregulation of cell cycle control caused by overexpression of the oncogene pp32r1 (ANP32C) and the Tyr>His mutant pp32r1Y140H. Biochim Biophys Acta. 2013;1833:1212–21. doi: 10.1016/j.bbamcr.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Kochevar GJ, Brody JR, Kadkol SS, Murphy KM, Pasternack GR. Identification of a functional mutation in pp32r1 (ANP32C) Hum Mutat. 2004;23:546–51. doi: 10.1002/humu.20030. [DOI] [PubMed] [Google Scholar]
- 16.Habrukowich C, Han DK, Le A, Rezaul K, Pan W, Ghosh M, Li Z, Dodge-Kafka K, Jiang X, Bittman R, et al. Sphingosine interaction with acidic leucine-rich nuclear phosphoprotein-32A (ANP32A) regulates PP2A activity and cyclooxygenase (COX)-2 expression in human endothelial cells. J Biol Chem. 2010;285:26825–31. doi: 10.1074/jbc.M110.147058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tedesco-Silva H, Mourad G, Kahan BD, Boira JG, Weimar W, Mulgaonkar S, Nashan B, Madsen S, Charpentier B, Pellet P, et al. FTY720, a novel immunomodulator: efficacy and safety results from the first phase 2A study in de novo renal transplantation. Transplantation. 2004;77:1826–33. [PubMed] [Google Scholar]
- 18.Kappos L, Antel J, Comi G, Montalban X, O’Connor P, Polman CH, Haas T, Korn AA, Karlsson G, Radue EW, FTY720 D2201 Study Group Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med. 2006;355:1124–40. doi: 10.1056/NEJMoa052643. [DOI] [PubMed] [Google Scholar]
- 19.Shen Y, Cai M, Xia W, Liu J, Zhang Q, Xie H, Wang C, Wang X, Zheng S. FTY720, a synthetic compound from Isaria sinclairii, inhibits proliferation and induces apoptosis in pancreatic cancer cells. Cancer Lett. 2007;254:288–97. doi: 10.1016/j.canlet.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 20.Azuma H, Takahara S, Horie S, Muto S, Otsuki Y, Katsuoka Y. Induction of apoptosis in human bladder cancer cells in vitro and in vivo caused by FTY720 treatment. J Urol. 2003;169:2372–7. doi: 10.1097/01.ju.0000064938.32318.91. [DOI] [PubMed] [Google Scholar]
- 21.Liu Q, Zhao X, Frissora F, Ma Y, Santhanam R, Jarjoura D, Lehman A, Perrotti D, Chen CS, Dalton JT, et al. FTY720 demonstrates promising preclinical activity for chronic lymphocytic leukemia and lymphoblastic leukemia/lymphoma. Blood. 2008;111:275–84. doi: 10.1182/blood-2006-10-053884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liao A, Broeg K, Fox T, Tan SF, Watters R, Shah MV, Zhang LQ, Li Y, Ryland L, Yang J, et al. Therapeutic efficacy of FTY720 in a rat model of NK-cell leukemia. Blood. 2011;118:2793–800. doi: 10.1182/blood-2011-01-331447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chua CW, Lee DT, Ling MT, Zhou C, Man K, Ho J, Chan FL, Wang X, Wong YC. FTY720, a fungus metabolite, inhibits in vivo growth of androgen-independent prostate cancer. Int J Cancer. 2005;117:1039–48. doi: 10.1002/ijc.21243. [DOI] [PubMed] [Google Scholar]
- 24.Ho JW, Man K, Sun CK, Lee TK, Poon RT, Fan ST. Effects of a novel immunomodulating agent, FTY720, on tumor growth and angiogenesis in hepatocellular carcinoma. Mol Cancer Ther. 2005;4:1430–8. doi: 10.1158/1535-7163.MCT-05-0021. [DOI] [PubMed] [Google Scholar]
- 25.Lee TK, Man K, Ho JW, Sun CK, Ng KT, Wang XH, Wong YC, Ng IO, Xu R, Fan ST. FTY720 induces apoptosis of human hepatoma cell lines through PI3-K-mediated Akt dephosphorylation. Carcinogenesis. 2004;25:2397–405. doi: 10.1093/carcin/bgh250. [DOI] [PubMed] [Google Scholar]
- 26.Permpongkosol S, Wang JD, Takahara S, Matsumiya K, Nonomura N, Nishimura K, Tsujimura A, Kongkanand A, Okuyama A. Anticarcinogenic effect of FTY720 in human prostate carcinoma DU145 cells: modulation of mitogenic signaling, FAK, cell-cycle entry and apoptosis. Int J Cancer. 2002;98:167–72. doi: 10.1002/ijc.10178. [DOI] [PubMed] [Google Scholar]
- 27.Costantino CL, Witkiewicz AK, Kuwano Y, Cozzitorto JA, Kennedy EP, Dasgupta A, Keen JC, Yeo CJ, Gorospe M, Brody JR. The role of HuR in gemcitabine efficacy in pancreatic cancer: HuR Up-regulates the expression of the gemcitabine metabolizing enzyme deoxycytidine kinase. Cancer Res. 2009;69:4567–72. doi: 10.1158/0008-5472.CAN-09-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams TK, Costantino CL, Bildzukewicz NA, Richards NG, Rittenhouse DW, Einstein L, Cozzitorto JA, Keen JC, Dasgupta A, Gorospe M, et al. pp32 (ANP32A) expression inhibits pancreatic cancer cell growth and induces gemcitabine resistance by disrupting HuR binding to mRNAs. PLoS One. 2010;5:e15455. doi: 10.1371/journal.pone.0015455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J Comput Chem. 2009;30:2785–91. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neviani P, Santhanam R, Oaks JJ, Eiring AM, Notari M, Blaser BW, Liu S, Trotta R, Muthusamy N, Gambacorti-Passerini C, et al. FTY720, a new alternative for treating blast crisis chronic myelogenous leukemia and Philadelphia chromosome-positive acute lymphocytic leukemia. J Clin Invest. 2007;117:2408–21. doi: 10.1172/JCI31095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Don AS, Martinez-Lamenca C, Webb WR, Proia RL, Roberts E, Rosen H. Essential requirement for sphingosine kinase 2 in a sphingolipid apoptosis pathway activated by FTY720 analogues. J Biol Chem. 2007;282:15833–42. doi: 10.1074/jbc.M609124200. [DOI] [PubMed] [Google Scholar]
- 32.Wallington-Beddoe CT, Hewson J, Bradstock KF, Bendall LJ. FTY720 produces caspase-independent cell death of acute lymphoblastic leukemia cells. Autophagy. 2011;7:707–15. doi: 10.4161/auto.7.7.15154. [DOI] [PubMed] [Google Scholar]
- 33.Seo SB, Macfarlan T, McNamara P, Hong R, Mukai Y, Heo S, Chakravarti D. Regulation of histone acetylation and transcription by nuclear protein pp32, a subunit of the INHAT complex. J Biol Chem. 2002;277:14005–10. doi: 10.1074/jbc.M112455200. [DOI] [PubMed] [Google Scholar]
- 34.Chakravarti D, Hong R. SET-ting the stage for life and death. Cell. 2003;112:589–91. doi: 10.1016/S0092-8674(03)00151-X. [DOI] [PubMed] [Google Scholar]
- 35.Saddoughi SA, Gencer S, Peterson YK, Ward KE, Mukhopadhyay A, Oaks J, Bielawski J, Szulc ZM, Thomas RJ, Selvam SP, et al. Sphingosine analogue drug FTY720 targets I2PP2A/SET and mediates lung tumour suppression via activation of PP2A-RIPK1-dependent necroptosis. EMBO Mol Med. 2013;5:105–21. doi: 10.1002/emmm.201201283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li M, Damuni Z. I1PP2A and I2PP2A. Two potent protein phosphatase 2A-specific inhibitor proteins. Methods Mol Biol. 1998;93:59–66. doi: 10.1385/0-89603-468-2:59. [DOI] [PubMed] [Google Scholar]
- 37.Yeh E, Cunningham M, Arnold H, Chasse D, Monteith T, Ivaldi G, Hahn WC, Stukenberg PT, Shenolikar S, Uchida T, et al. A signalling pathway controlling c-Myc degradation that impacts oncogenic transformation of human cells. Nat Cell Biol. 2004;6:308–18. doi: 10.1038/ncb1110. [DOI] [PubMed] [Google Scholar]
- 38.Kadkol SS, Brody JR, Pevsner J, Bai J, Pasternack GR. Modulation of oncogenic potential by alternative gene use in human prostate cancer. Nat Med. 1999;5:275–9. doi: 10.1038/12530. [DOI] [PubMed] [Google Scholar]
- 39.Kadkol SS, El Naga GA, Brody JR, Bai J, Gusev Y, Dooley WC, Pasternack GR. Expression of pp32 gene family members in breast cancer. Breast Cancer Res Treat. 2001;68:65–73. doi: 10.1023/A:1017919507109. [DOI] [PubMed] [Google Scholar]
- 40.Wang JD, Takahara S, Nonomura N, Ichimaru N, Toki K, Azuma H, Matsumiya K, Okuyama A, Suzuki S. Early induction of apoptosis in androgen-independent prostate cancer cell line by FTY720 requires caspase-3 activation. Prostate. 1999;40:50–5. doi: 10.1002/(SICI)1097-0045(19990615)40:1<50::AID-PROS6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 41.Pchejetski D, Bohler T, Brizuela L, Sauer L, Doumerc N, Golzio M, Salunkhe V, Teissié J, Malavaud B, Waxman J, et al. FTY720 (fingolimod) sensitizes prostate cancer cells to radiotherapy by inhibition of sphingosine kinase-1. Cancer Res. 2010;70:8651–61. doi: 10.1158/0008-5472.CAN-10-1388. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.