Abstract
The detection of nutrients, both in food and within the body, is crucial for the regulation of feeding behavior, growth, and metabolism. While the molecular basis for sensing food chemicals by the taste system has been firmly linked to specific taste receptors, relatively little is known about the molecular nature of the sensors that monitor nutrients internally. Recent reports of taste receptors expressed in other organ systems, foremost in the gastrointestinal tract of mammals and insects, has led to the proposition that some taste receptors may also be used as sensors of internal nutrients. Indeed, we provided direct evidence that the Drosophila gustatory receptor 43a (Gr43a) plays a critical role in sensing internal fructose levels in the fly brain. In addition to the brain and the taste system, Gr43a is also expressed in neurons of the proventricular ganglion and the uterus. Here, we discuss the multiple potential roles of Gr43a in the fly. We also provide evidence that its activation in the brain is likely mediated by the neuropeptide Corazonin. Finally, we posit that Gr43a may represent only a precedent for other taste receptors that sense internal nutrients, not only in flies but, quite possibly, in other animals, including mammals.
Keywords: brain, corazonin, fructose, nutrient sensor, proventriculus, receptor, taste, uterus, valence
Omnivores consume a wide selection of nutrients such as carbohydrates, amino acids, fatty acids and numerous salts to meet their needs for energy expenditure, growth, and development. Absence of a single group of nutrients can result in stunted growth, morbidity, metabolic dysfunction, and premature death. The sense of taste plays a central role for evaluating the palatability of potential food sources, and recent progress in uncovering the molecular and cellular principle that underlie taste perception have led to a broad understanding of how mammals and insects identify and discriminate among different food chemicals and avoid the many non-nutritious, toxic chemicals which often taste bitter.1 Intriguingly, it has been demonstrated recently in both vertebrates and insects that at least some of these nutrients can be sensed not only by the taste systems, but also by internal sensors present in the gastrointestinal system and the brain. For example, mammals appear to sense glucose (and other sugars) in the gut using the T1R2/T1R3 taste receptors,2,3 and the glucose transporter GLUT2 mediates glucose uptake in the pancreas and probably also in selected hypothalamic and other neurons in the brain.4 These glucose-sensing processes are essential for the regulation of nutrient metabolisms and behaviors via the secretion of insulin, glucagon, and numerous neuropeptides.5 In insects, the G-protein coupled receptor BOSS was proposed to function as a glucose sensor in the fat body to regulate insulin signaling.6 Gastrointestinal systems have also been implicated in sensing bitter substances, since gut endothelial cells of mammals and insects express T2R and Gr bitter taste receptors, respectively.7,8 Sodium is probably sensed by the mechanosensory channel TRPV1 and the atypical sodium channel NaX in the brain,9,10 while PKD2L1, a sour taste sensor, is expressed in the neurons surrounding the central canal of the spinal cord.11 Finally, both mammals and insects can sense internal levels of amino acids, which is used to modulate their feeding behavior; specifically, uncharged tRNAs are suggested to be mediators of amino acid sensing in brain neurons of mammals.12
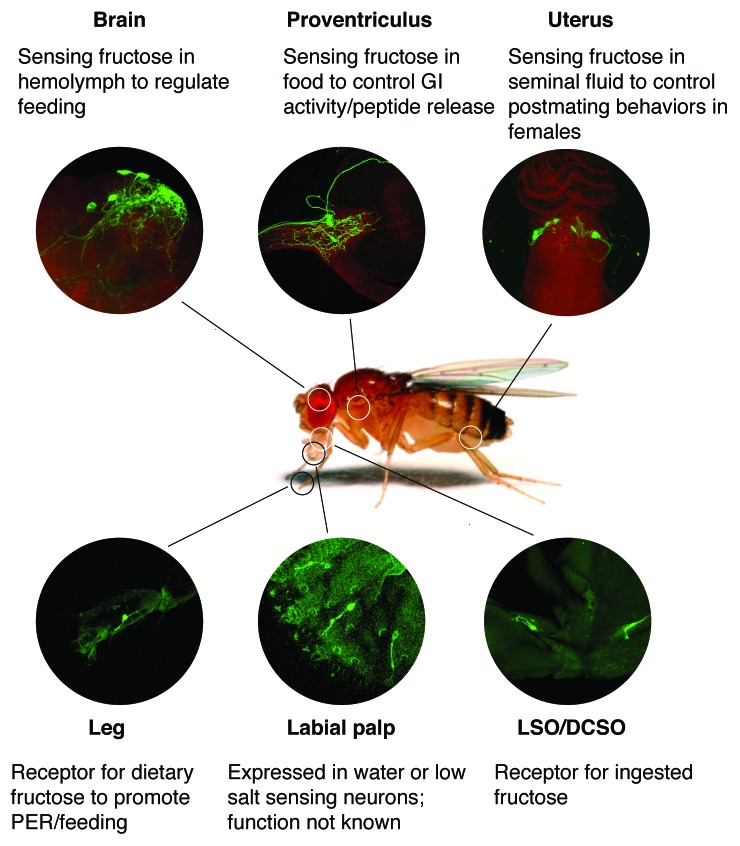
We recently discovered that the Drosophila gustatory receptor 43a (Gr43a), one of the most conserved insect taste receptors, is expressed not only in taste neurons, but also in neurons associated with internal organs such as the brain, the proventricular ganglion, and the uterus.13 Expression in these organs was established with a GAL4 knock-in allele (Gr43aGAL4), in which the Gr43a coding sequence was replaced with that of the GAL4 gene. Using Ca2+ imaging, we found that Gr43a functions as a narrowly tuned receptor for fructose. These observations raised the intriguing possibility that fructose is not only sensed as a dietary component by the taste system, but also serves as a carbohydrate component in the hemolymph to reflect the internal nutrient status. Here, we discuss potential functions of Gr43a expressing neurons in each of the organ system where its expression has been established (Fig. 1). We also show for the first time that the Gr43a expressing uterus neurons respond to fructose in a manner similar to the brain neurons. Finally, we provide evidence that Gr43a expressing neurons use distinct modes of neurotransmission in different organs to propagate stimulation by fructose. Specifically, the Gr43a expressing brain neurons co-express Corazonin, a highly conserved insect neuropeptide, suggesting that downstream neurons, which mediate Gr43a activity, express the Corazonin receptor.
Figure 1. Overview of Gr43a expression and putative function in a female fly. The 6 sites of Gr43a expression in an adult female fly are depicted and actual expression sites are shown in individual images. Established (brain, forelegs), proposed (VSO/DCSO, proventriculus and uterus) or unknown (labial palps) are indicated.
The Adult Taste Sensory System
Fly taste neurons, referred to as gustatory receptor neurons (GRNs), are organized in taste sensilla and taste pegs, located in various appendages.14 The 2 labial palps harbor close to 80 taste sensilla and taste pegs, and there are ~30 to 40 taste sensilla on each leg and about 20 on each anterior wing margin; while the function of labial and tarsal taste sensilla in mediating feeding responses is well established, the contribution to taste of sensilla on the wing margin are largely unknown.
Most taste sensilla contain 4 GRNs, each thought to detect distinct groups of food and other chemicals: the sweet neuron senses various sugars, the bitter/high salt neuron responds to various non-nutritious and often harmful organic chemicals, as well as high concentration of salt (> 400 mM), a third neuron responds to low salt solutions and the last neuron responds to water.15 In addition, the fly harbors internal taste neurons, located in 3 pharyngeal structures, the labral, dorsal and ventral cibarial sense organs (LSO, 18 neurons; DCSO, 6 and VCSO, 8).16
Function of Gr43a as a Taste Receptor
Tarsal taste sensilla
The most prominent expression of Gr43a in the taste system is observed in the legs: Gr43aGAL4 is expressed in a single GRN of 2 taste sensilla located on the 5th tarsal segment of each leg (Fig. 1).13 These GRNs also express several members of the sugar gustatory receptor (sugar Gr) subfamily consisting of Gr5a, Gr61a, and Gr64a-f and are broadly tuned to and activated by most sugars, as determined by Ca2+ imaging.13 Lack of Gr43a reduces the response specifically to fructose, while absence of the sugar Gr genes abolishes the response to all sugars except fructose and sucrose (disaccharide of fructose and glucose). Finally, lack of all sugar Gr genes and Gr43a completely abolishes fructose and sucrose response, and transgene expression of Gr43a alone is sufficient to restore both responses. Thus, Gr43a functions as a secondary tarsal fructose receptor. We note that the Gr43a expressing neuron housed in the 5V1 sensillum is significantly more sensitive to sugars—especially to fructose and sucrose—than other sweet sensing GRNs that do not express Gr43a.17 A possible explanation for the large contribution of Gr43a to fructose sensing in this sensillum is its high level of expression: analysis from tarsal tissue shows that Gr43a transcripts are represented approximately 10 times more than transcripts of any of the classical sugar receptor genes (data not shown), even though the former is expressed in fewer cells than the latter.13,17,18 Behavioral relevance for the high sensitivity of Gr43a expressing neurons has yet to be established, as the standard behavioral proboscis extension reflex (PER) response is not significantly affected for any sugars in Gr43a mutant flies when compared with wild type flies.13
Labial palp neurons
Gr43a is expressed in ~8 GRNs in the labial palp (Fig. 1).13 Surprisingly, Gr64f, a marker expressed in virtually all sugar neurons, is not co-expressed with Gr43a in these neurons, and Gr66a, a receptor for caffeine and a marker for bitter sensing GRNs, is not co-expressed with Gr43a either.13 Thus, by default, these Gr43aGAL4 expressing neurons appear to correspond to water or low salt sensing neurons. Single neuron Ca2+ imaging has not yet been possible on sensilla located in the palps and, hence, the response properties of these Gr43a expressing neurons are not known. Compared with other taste organs (i.e., tarsal neurons or pharyngeal neurons; see below), the expression level of Gr43aGAL4 in the labial palp is much lower,13 and it is therefore also possible that Gr43a has no obvious function in these neurons.
LSO and VCSO neurons
Gr43a is expressed in 2 neurons located in the LSO and the VCSO13(Fig. 1). A putative sugar receptor gene, Gr64f is also co-expressed in the 2 of the Gr43aGAL4 positive LSO neurons, but not in the VCSO neurons.13 The only established role for pharyngeal taste neurons has been reported for the VCSO, where bitter sensing (Gr66a-expressing) nuerons contribute to egg laying preference on lobeline containing food substrates.19 This is interesting, because bitter chemicals sensed by labial or tarsal neurons suppress feeding responses in proboscis extension reflex (PER) assays.20 Regardless, we observed no co-expression between Gr43a and Gr66a in any pharyngeal taste neurons. Based on partial co-expression with Gr64f, the role of Gr43a is likely to be related to sensing sugars while food is ingested, but new behavioral paradigms will have to be developed to assess their specific role in feeding.
Functions of Gr43a as an Internal Nutrient Sensor
In addition to gustatory neurons, Gr43a is also expressed in defined sets of neurons of the proventricular ganglion, the brain and the uterus13 (Fig. 1). Ca2+ imaging experiments using ex-vivo preparations of brains and uterus confirmed that Gr43a also functions as a fructose receptor in these organs13(Fig. 3).
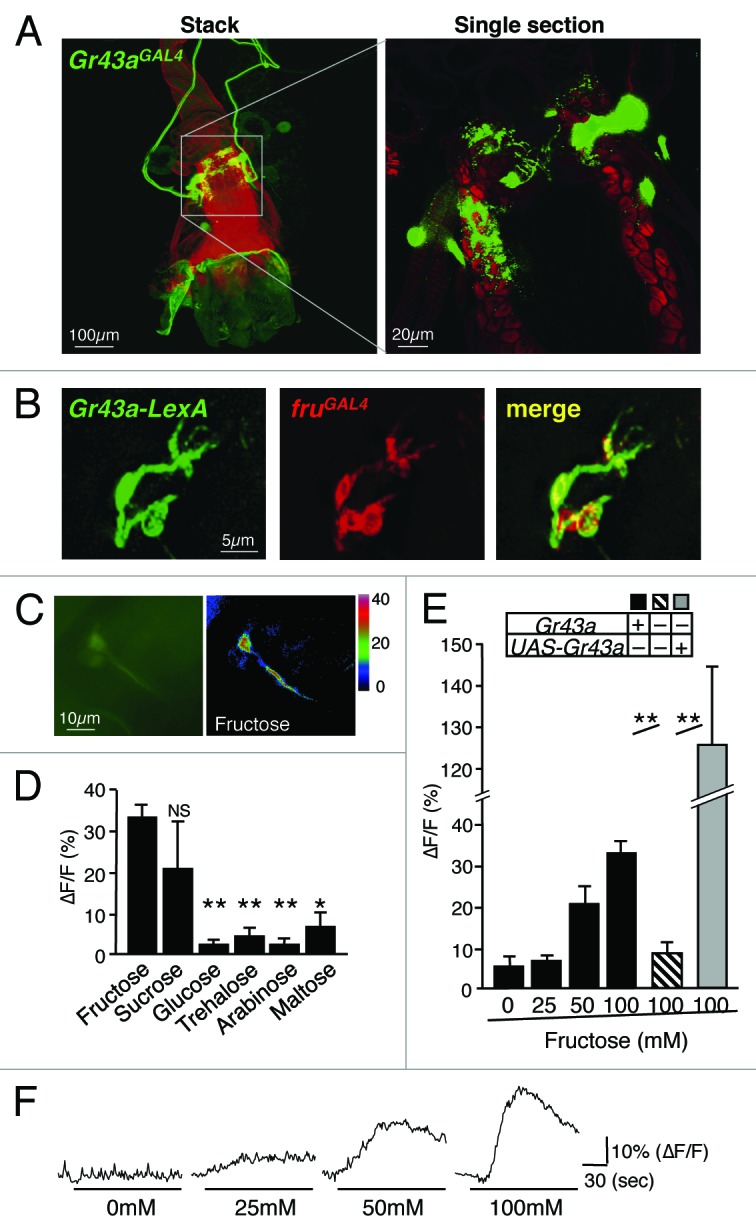
Figure 3.Gr43a expressing neurons in the uterus. (A) Gr43aGAL4 drives UAS-mCD8GFP expression in 4–6 neurons. Cell bodies are located at the middle of the uterus, sending the axons to the abdominal ganglion. The dendrites innervate the lumen of the uterus, clearly shown by the single section view. (B) Gr43a-LexA and SPR are co-expressed in the same neurons. Gr43a-LexA drives lexAop-rCD2GFP, fruGAL4 drives UAS-mCD8RFP. Note that fruGAL4 expression serves as a proxy for SPR, which are co-expressed in the uterus.35,36 (C) Uterus neurons expressing G-CaMP3.0 under control of Gr43aGAL4. ∆F pseudocolor fluorescence image was taken 85 s after application of 100mM fructose (right). (D) Gr43aGAL4 neurons specifically respond to fructose. Max ∆F/F within 135 s of application is shown. All sugars are 100mM. Flies contained 2 genomic copies of Gr43a. **P < 0.0001; ANOVA. Error bars represent standard error. 5 ≤ n ≤ 8. (E) Response of Gr43aGAL4 neurons to fructose is Gr43a dependent. **P < 0.0001; ANOVA. Error bars represent standard error. 5 ≤ n ≤ 9. (F) Time-course of G-CaMP3.0 fluorescence changes in Gr43aGAL4 neurons stimulated with different concentrations of fructose. Note that ∆F/F in Gr43aGal4/Gr43aGAL4;UAS-Gr43a rescue flies is higher than in Gr43a+ controls, likely due to higher expression levels of Gr43a.
Proventricular ganglion
The taste organs examine food chemicals before they enter the digestive system, but it is now well documented that both nutrients and potential toxins are also re-evaluated when they are in the gastrointestinal tract. For example, in mammals, sugar taste receptors expressed in the gastrointestinal tract stimulate glucagon-like peptide 1 secretion in response to sugar ingestions,21 and bitter taste receptors are also expressed in gut epithelial cells of both mammals and insects.7,8 In the mouse, activation of T2Rs in the gut leads to secretion of cholecystokinin from enteroendocrine cells, which limits absorption of dietary toxins. In addition, cholecystokinin signaling also increases expression of the ABCB1 efflux transporter, thereby actively limiting absorption of bitter-tasting toxins.22,23 In Drosophila, ingested food passes through the pharynx and the foregut and is initially deposited in the crop. The stored food is then moved into the midgut through the proventriculus, a muscular organ that separates foregut and midgut. The proventricular ganglion is located on the dorsal side of the foregut.24 This ganglion contains about 30–40 neurons, 6 of which express Gr43a (Fig. 2); they send dendritic terminals into the lumen of the foregut, but not into the crop duct. One group of neurons sends axonal projections to the SOG along the esophagus through the brain, forming a nerve bundle with axons of GRNs located in the LSO and the VCSO. The other group of neurons extends axons posteriorly, where they innervate cells in the midgut.13 The anatomy and location of processes of these neurons suggests that fructose content of food may be monitored immediately before entering the crop and the midgut. While the neurons projecting to the SOG may serve similar roles as taste neurons (PER, food intake), the neurons projecting to the midgut may regulate food transport and/or secretion of neuropeptides/hormones in response to sugar consumption. Expression of Gr43a in the gastrointestinal system appears to be conserved across different insect species; the Gr43a orthologs in the silkworm Bombyx mori (BmGr-9) and in the cotton bollworm Helicoverpa armigera (HaGR9) are also expressed in their digestive systems.25,26
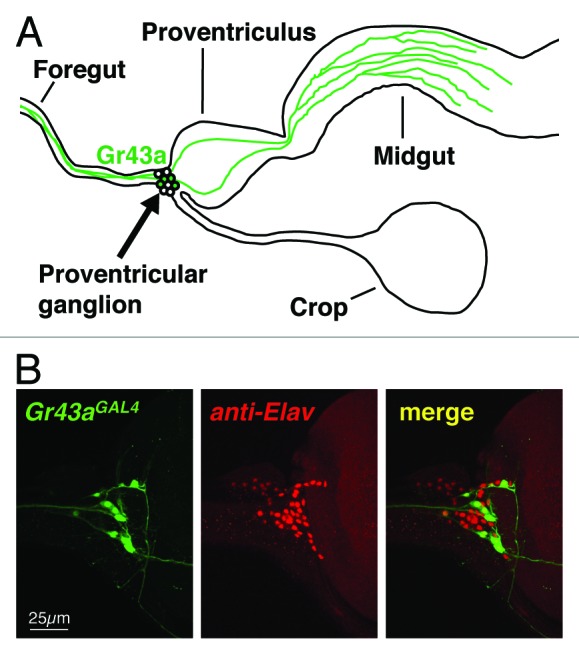
Figure 2.Gr43a expressing neurons in the proventricular ganglion. (A) Schematic diagram of Gr43a expressing neurons. All cell bodies are in the proventricular ganglion. One group sends axons along the foregut to the SOG, the other projects axons to the midgut. The dendrites innervate the lumen of the foregut. (B) The proventricular ganglion consists of 30–40 neurons (pan-neuronal marker, Elav), Gr43aGAL4 drives UAS-mCD8GFP expression in 4–8 of them (Gr43aGAL4).
Brain
Gr43a is expressed in 2–4 neurons located in the posterior superior lateral protocerebrum of each brain hemisphere.13 Two neurons are easily identifiable using live GFP imaging, but 2 additional neurons are characterized by lower level of expression and are only detected using antibody staining of dissected brains. We showed that the Gr43a expressing neurons respond to fructose, using Ca2+ imaging of ex vivo brain preparations at levels as low as 5 mM. Indeed, hemolymph sugar measurements have revealed that fructose levels increase to at least ~5 mM after flies feed on various nutritious carbohydrates, suggesting that these neurons are activated after ingestion of a carbohydrate rich meal.13 The steep increase of hemolymph fructose can be observed regardless of the type of sugar present in the meal, as long as it is metabolized, whereas non-nutritious carbohydrates (sucralose, xylose, arabinose) fail to increase hemolymph fructose. This observation suggests that a fraction of dietary, nutritious sugar is converted into fructose after ingestion, probably via the polyol pathway,27 and that this conversion is used to signal to the brain that carbohydrates are consumed. We think that this signaling event is integrated with the feeding status (hungry vs. satiated), thereby establishing positive or negative valence (see below).
Animals can sense the nutritional value of food and regulate their food intake through non-taste mechanisms.28-31 Feeding experiments of wild type flies and flies in which Gr43a expression was restricted to specific cells revealed that the brain neurons function as a nutrient sensor: in hungry flies, the Gr43a brain neurons are necessary and sufficient to promote feeding, while in satiated flies, they function to suppress feeding.13 Based on these observations, we proposed that ingestion of nutritious carbohydrates rapidly increases circulating fructose, resulting in activation of Gr43a-expressing brain neurons. This activation is perceived positively in hungry flies and reinforces feeding, but it is perceived negatively in satiated flies, leading to termination/suppression of feeding. Evoking opposite perceptions by a single group of neurons is unusual, but not unprecedented. In mice, a small set of neurons in the piriform cortex, a higher order olfactory integration center, mediates opposite valence (attraction vs. avoidance), depending on the nature of stimuli during conditioning.32 The mechanisms by which piriform neurons in the mouse or Gr43a brain neurons in the fly accomplish such binary valence are unknown. In the fly, it is possible that the Gr43a brain neurons communicate with 2, functionally distinct, group of target neurons, a notion that is supported by distinct projection tracts of subsets of these neurons.13
Uterus
One role of the insect uterus is to provide a receptacle for sperm and seminal fluid during copulation. In the fly, the seminal fluid not only contains sperm to fertilize the egg, but it serves also as a source of signals that induce numerous changes in the female’s behavior. In addition, the uterus also serves as a storage space for the egg prior deposition, and as a contractible muscle for expunging the mature egg.24
The uterus harbors 3 neuronal clusters, and Gr43a is expressed in approximately 4 out of 10 neurons in 1 of them (Fig. 3A). These neurons send dendritic and axonal projections to the uterus lumen and the abdominal ganglion, respectively. To assess and confirm ligand specificity of Gr43a expressing uterus neurons, we established an ex vivo preparation and performed Ca2+ imaging studies (Fig. 3C–F). These experiments demonstrate that fructose specifically activates these neurons, though the sensitivity and magnitude is lower than that of leg or brain neurons.
Activation of the sex peptide receptor (SPR) in neurons of the uterus is essential for females to undergo various post-mating changes, such as increase in egg production, reduction in mating activity, and a switch from a carbohydrate-rich diet to one containing more protein.33,34 SPR was shown to be activated by sex peptide (SP), 1 of several small proteins present in seminal fluid, which is transferred to the female reproductive tract during mating.34 While SPR is broadly expressed throughout the nervous system, expression in the uterus neurons alone is sufficient to induce changes in post-mating behaviors.35,36 We note that fructose is abundantly present in the seminal fluids of mammals and some insects,37,38 albeit we currently do not know whether it is found in Drosophila seminal fluid.
Interestingly, we found that Gr43a and SPR are co-expressed in the uterus neurons (Fig. 3B), suggesting that these neurons may sense several cues present in male seminal fluid. Interaction of SP with SPR leads to silencing of the neuron,35,36 and hence, simultaneous binding of SPR and Gr43a to their ligand might have counteracting (inhibitory and excitatory) effects on these cells, leading to modulation of one system by the other. However, it is also possible that hemolymph fructose is sensed by these neurons, which would imply that feeding on carbohydrate can modulate post-mating responses through counteracting SPR mediated silencing. Future studies will be necessary to elucidate the physiological role of Gr43a in uterus neurons for post-mating behavior.
Corazonin is the Likely Neurotransmitter in Gr43a Brain Neurons
Fructose and its receptor play roles in Drosophila nutrient sensing in multiple organ systems. A well-defined function is currently only evident in the brain, where it provides distinct valence to the experience of food intake. To dissect the mechanism of satiation-dependent valence setting, it will be crucial to define the neural circuit that is governed by Gr43a. A first and important step toward this goal is to identify the neurotransmitter that is released in response to Gr43a neural activation. We noticed a striking similarity of brain expression patterns between Gr43aGAL4 and Corazonin (Crz).39,40 We therefore examined potential co-expression of these 2 genes, and we indeed observed that all Gr43aGAL4 - positive neurons also express this peptide (Fig. 4). Crz, a short neuropeptide/hormone, and its receptor (crzR) are orthologs of the mammalian gonadotropin releasing hormone and its receptor, respectively. In Drosophila, a role for Crz and crzR has been reported in resistance to alcohol sedation41 and the regulation of sperm and seminal fluid transfer during mating.42 Interestingly, the latter study provided evidence that Crz acts as a neurotransmitter, rather than a hormone. Thus, our next goal is to identify the brain neurons that express the crzR gene, which will provide critical information about the downstream targets in this neural circuit. We note that Crz is not expressed in other internal Gr43aGAL4 expressing neurons (uterus, proventriculus) or in taste neurons (data not shown) and, therefore, another neurotransmitter must be used to mediate Gr43a activity in these organ systems.
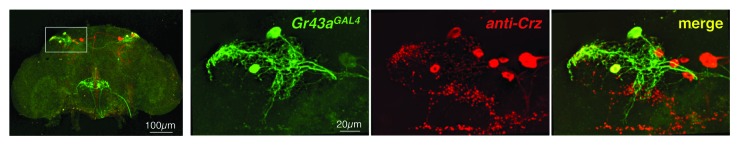
Figure 4.Gr43a is expressed in a subpopulation of Crz expressing neurons in the brain. Whole brain preparation of Gr43aGAL4;UAS-mCD8GFP fly stained with anti GFP and anti Crz antibody.40 Crz (red) is expressed in all of the Gr43aGAL4 positive neurons (green); note the presence of 4 to 6 additional Crz expressing cells in each hemisphere that do not express the fructose receptor.
Outlook
The role of fructose as a nutrient signal is not well understood. We think that the identification of an internal fructose sensor and the observation that fructose is a regulated component of Drosophila hemolymph, features likely to be conserved in many other insect species, will stimulate at least 3 avenues of future studies. First, other Gr proteins known to function as taste receptors are expressed in internal organs, including the brain, and are therefore likely employed to sense internal signaling molecules (other sugars, especially glucose and trehalose, as well as amino acids). In this regard, it is noteworthy that numerous members of the Gr28 gene family are expressed in many neuronal and non-neuronal cell populations43 throughout development and in the adult, and several putative bitter and sugar receptors were found to be expressed in the gastrointestinal tract of larvae.8 Thus, identification of their ligands and their specific function in feeding and other behaviors will be of great interest. Second, the Gr43a brain neurons represent a highly tractable and relatively simple case of a brain structure that mediates opposite valence, and they therefore provide an ideal case to dissect the mechanism of choice behavior encoded in the insect brain. And third, we think that the emergence of hemolymph fructose in insects warrants efforts to explore the potential role of this sugar as a nutrient ligand in other organisms, including mammals. In humans, the increase of fructose consumption is strongly associated with a steady increase in obesity, insulin resistance and other metabolic syndromes.44,45 In addition, several studies in humans and mice suggest that fructose and glucose, the most common dietary sugars, are absorbed in distinct regions of the gastrointestinal tract and metabolized differently,46,47 and that fructose and glucose affect brain activity and feeding behavior in a disparate manner.48,49 Thus, it will be interesting to see whether this sugar—and a specific receptor for it—plays also a role in nutrient sensing in mammals.
Methods
Immunostaining
Immunostaining of whole-mount tissues was performed as described previously.50 anti-Crz was kindly provided by Dr Park (University of Tennessee). anti-Crz and anti-ELAV were used at 1:100 dilution.
Calcium imaging
The uterus was dissected in a sugar-free ringer solution (5 mM HEPES pH 7.2, 130 mM NaCl, 5 mM KCl, 2 mM CaCl2, 2mM MgCl2) and placed under agarose mounted in a glass bottom dish (MatTek corp.) such that the region containing the Gr43a expressing cells were viewable under the microscope. The preparation was covered with 100 ml of ringer, and 100 ml test solutions was administered through a pipette. Images for data analysis were acquired from the cell body for 30 s before and 120 s after application (1 frame/1 s). Adjacent regions were used to determine autofluorescence background. Imaging was performed with a Nikon eclipse Ti inverted microscope with 20 × water objective. The light source was a Lumen 200 lamp (Prior Scientific Inc.). Samples were excited at 488 nm (metal halide lamp), and emitted light was collected through a 515–555 nm filter. Data acquisition was performed with NIS-Elements software (Nikon). Average of 5 frames taken immediately before the application were used to define base fluorescence.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgment
The fly image was taken with permission from the website of the Obbard lab, University of Edinburgh, GB. This work was supported by grants from the National Institute of Health (RO1-DC009014 and RO1-DC005606) to H.A.
Footnotes
Previously published online: www.landesbioscience.com/journals/fly/article/27241
References
- 1.Scott K. Taste recognition: food for thought. Neuron. 2005;48:455–64. doi: 10.1016/j.neuron.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 2.Dyer J, Salmon KS, Zibrik L, Shirazi-Beechey SP. Expression of sweet taste receptors of the T1R family in the intestinal tract and enteroendocrine cells. Biochem Soc Trans. 2005;33:302–5. doi: 10.1042/BST0330302. [DOI] [PubMed] [Google Scholar]
- 3.Margolskee RF, Dyer J, Kokrashvili Z, Salmon KS, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B, Shirazi-Beechey SP. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci U S A. 2007;104:15075–80. doi: 10.1073/pnas.0706678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leturque A, Brot-Laroche E, Le Gall M. GLUT2 mutations, translocation, and receptor function in diet sugar managing. Am J Physiol Endocrinol Metab. 2009;296:E985–92. doi: 10.1152/ajpendo.00004.2009. [DOI] [PubMed] [Google Scholar]
- 5.Thorens B. Of fat, β cells, and diabetes. Cell Metab. 2011;14:439–40. doi: 10.1016/j.cmet.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Kohyama-Koganeya A, Kim YJ, Miura M, Hirabayashi Y. A Drosophila orphan G protein-coupled receptor BOSS functions as a glucose-responding receptor: loss of boss causes abnormal energy metabolism. Proc Natl Acad Sci U S A. 2008;105:15328–33. doi: 10.1073/pnas.0807833105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu SV, Rozengurt N, Yang M, Young SH, Sinnett-Smith J, Rozengurt E. Expression of bitter taste receptors of the T2R family in the gastrointestinal tract and enteroendocrine STC-1 cells. Proc Natl Acad Sci U S A. 2002;99:2392–7. doi: 10.1073/pnas.042617699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park JH, Kwon JY. Heterogeneous expression of Drosophila gustatory receptors in enteroendocrine cells. PLoS One. 2011;6:e29022. doi: 10.1371/journal.pone.0029022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hiyama TY, Watanabe E, Ono K, Inenaga K, Tamkun MM, Yoshida S, Noda M. Na(x) channel involved in CNS sodium-level sensing. Nat Neurosci. 2002;5:511–2. doi: 10.1038/nn0602-856. [DOI] [PubMed] [Google Scholar]
- 10.Liedtke W, Choe Y, Martí-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000;103:525–35. doi: 10.1016/S0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang AL, Chen X, Hoon MA, Chandrashekar J, Guo W, Tränkner D, Ryba NJ, Zuker CS. The cells and logic for mammalian sour taste detection. Nature. 2006;442:934–8. doi: 10.1038/nature05084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hao S, Sharp JW, Ross-Inta CM, McDaniel BJ, Anthony TG, Wek RC, Cavener DR, McGrath BC, Rudell JB, Koehnle TJ, et al. Uncharged tRNA and sensing of amino acid deficiency in mammalian piriform cortex. Science. 2005;307:1776–8. doi: 10.1126/science.1104882. [DOI] [PubMed] [Google Scholar]
- 13.Miyamoto T, Slone J, Song X, Amrein H. A fructose receptor functions as a nutrient sensor in the Drosophila brain. Cell. 2012;151:1113–25. doi: 10.1016/j.cell.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stocker RF. The organization of the chemosensory system in Drosophila melanogaster: a review. Cell Tissue Res. 1994;275:3–26. doi: 10.1007/BF00305372. [DOI] [PubMed] [Google Scholar]
- 15.Hallem EA, Dahanukar A, Carlson JR. Insect odor and taste receptors. Annu Rev Entomol. 2006;51:113–35. doi: 10.1146/annurev.ento.51.051705.113646. [DOI] [PubMed] [Google Scholar]
- 16.Gendre N, Lüer K, Friche S, Grillenzoni N, Ramaekers A, Technau GM, Stocker RF. Integration of complex larval chemosensory organs into the adult nervous system of Drosophila. Development. 2004;131:83–92. doi: 10.1242/dev.00879. [DOI] [PubMed] [Google Scholar]
- 17.Miyamoto T, Chen Y, Slone J, Amrein H. Identification of a Drosophila glucose receptor using Ca2+ imaging of single chemosensory neurons. PLoS One. 2013;8:e56304. doi: 10.1371/journal.pone.0056304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slone J, Daniels J, Amrein H. Sugar receptors in Drosophila. Curr Biol. 2007;17:1809–16. doi: 10.1016/j.cub.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joseph RM, Heberlein U. Tissue-specific activation of a single gustatory receptor produces opposing behavioral responses in Drosophila. Genetics. 2012;192:521–32. doi: 10.1534/genetics.112.142455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montell C. A taste of the Drosophila gustatory receptors. Curr Opin Neurobiol. 2009;19:345–53. doi: 10.1016/j.conb.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, Zhou J, Kim HH, Xu X, Chan SL, Juhaszova M, et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci U S A. 2007;104:15069–74. doi: 10.1073/pnas.0706890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeon TI, Seo YK, Osborne TF. Gut bitter taste receptor signalling induces ABCB1 through a mechanism involving CCK. Biochem J. 2011;438:33–7. doi: 10.1042/BJ20110009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeon TI, Zhu B, Larson JL, Osborne TF. SREBP-2 regulates gut peptide secretion through intestinal bitter taste receptor signaling in mice. J Clin Invest. 2008;118:3693–700. doi: 10.1172/JCI36461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Demerec M. The Biology of Drosophila melanogaster1965: Hafner Publishing Company Ltd. [Google Scholar]
- 25.Sato K, Tanaka K, Touhara K. Sugar-regulated cation channel formed by an insect gustatory receptor. Proc Natl Acad Sci U S A. 2011;108:11680–5. doi: 10.1073/pnas.1019622108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu W, Zhang HJ, Anderson A. A sugar gustatory receptor identified from the foregut of cotton bollworm Helicoverpa armigera. J Chem Ecol. 2012;38:1513–20. doi: 10.1007/s10886-012-0221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harvey RA, Ferrier DR, eds. Biochemistry (Lippincott's Illustrated Reviews) 5th edition ed., ed. R.A. Harvey2011, Lippincott, Williams and Wilkins: Philadelphia, PA. [Google Scholar]
- 28.Burke CJ, Waddell S. Remembering nutrient quality of sugar in Drosophila. Curr Biol. 2011;21:746–50. doi: 10.1016/j.cub.2011.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MA, Simon SA. Food reward in the absence of taste receptor signaling. Neuron. 2008;57:930–41. doi: 10.1016/j.neuron.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 30.Dus M, Min S, Keene AC, Lee GY, Suh GS. Taste-independent detection of the caloric content of sugar in Drosophila. Proc Natl Acad Sci U S A. 2011;108:11644–9. doi: 10.1073/pnas.1017096108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujita M, Tanimura T. Drosophila evaluates and learns the nutritional value of sugars. Curr Biol. 2011;21:751–5. doi: 10.1016/j.cub.2011.03.058. [DOI] [PubMed] [Google Scholar]
- 32.Choi GB, Stettler DD, Kallman BR, Bhaskar ST, Fleischmann A, Axel R. Driving opposing behaviors with ensembles of piriform neurons. Cell. 2011;146:1004–15. doi: 10.1016/j.cell.2011.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ribeiro C, Dickson BJ. Sex peptide receptor and neuronal TOR/S6K signaling modulate nutrient balancing in Drosophila. Curr Biol. 2010;20:1000–5. doi: 10.1016/j.cub.2010.03.061. [DOI] [PubMed] [Google Scholar]
- 34.Yapici N, Kim YJ, Ribeiro C, Dickson BJ. A receptor that mediates the post-mating switch in Drosophila reproductive behaviour. Nature. 2008;451:33–7. doi: 10.1038/nature06483. [DOI] [PubMed] [Google Scholar]
- 35.Häsemeyer M, Yapici N, Heberlein U, Dickson BJ. Sensory neurons in the Drosophila genital tract regulate female reproductive behavior. Neuron. 2009;61:511–8. doi: 10.1016/j.neuron.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 36.Yang CH, Rumpf S, Xiang Y, Gordon MD, Song W, Jan LY, Jan YN. Control of the postmating behavioral switch in Drosophila females by internal sensory neurons. Neuron. 2009;61:519–26. doi: 10.1016/j.neuron.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mann T. Secretory function of the prostate, seminal vesicle and other male accessory organs of reproduction. J Reprod Fertil. 1974;37:179–88. doi: 10.1530/jrf.0.0370179. [DOI] [PubMed] [Google Scholar]
- 38.King M, Eubel H, Millar AH, Baer B. Proteins within the seminal fluid are crucial to keep sperm viable in the honeybee Apis mellifera. J Insect Physiol. 2011;57:409–14. doi: 10.1016/j.jinsphys.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 39.Nässel DR, Winther AM. Drosophila neuropeptides in regulation of physiology and behavior. Prog Neurobiol. 2010;92:42–104. doi: 10.1016/j.pneurobio.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 40.Choi YJ, Lee G, Park JH. Programmed cell death mechanisms of identifiable peptidergic neurons in Drosophila melanogaster. Development. 2006;133:2223–32. doi: 10.1242/dev.02376. [DOI] [PubMed] [Google Scholar]
- 41.McClure KD, Heberlein U. A small group of neurosecretory cells expressing the transcriptional regulator apontic and the neuropeptide corazonin mediate ethanol sedation in Drosophila. J Neurosci. 2013;33:4044–54. doi: 10.1523/JNEUROSCI.3413-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tayler TD, Pacheco DA, Hergarden AC, Murthy M, Anderson DJ. A neuropeptide circuit that coordinates sperm transfer and copulation duration in Drosophila. Proc Natl Acad Sci U S A. 2012;109:20697–702. doi: 10.1073/pnas.1218246109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thorne N, Amrein H. Atypical expression of Drosophila gustatory receptor genes in sensory and central neurons. J Comp Neurol. 2008;506:548–68. doi: 10.1002/cne.21547. [DOI] [PubMed] [Google Scholar]
- 44.Pollock NK, Bundy V, Kanto W, Davis CL, Bernard PJ, Zhu H, Gutin B, Dong Y. Greater fructose consumption is associated with cardiometabolic risk markers and visceral adiposity in adolescents. J Nutr. 2012;142:251–7. doi: 10.3945/jn.111.150219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nomura K, Yamanouchi T. The role of fructose-enriched diets in mechanisms of nonalcoholic fatty liver disease. J Nutr Biochem. 2012;23:203–8. doi: 10.1016/j.jnutbio.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 46.Coate KC, Kraft G, Lautz M, Smith M, Neal DW, Cherrington AD. A high-fat, high-fructose diet accelerates nutrient absorption and impairs net hepatic glucose uptake in response to a mixed meal in partially pancreatectomized dogs. J Nutr. 2011;141:1643–51. doi: 10.3945/jn.111.145359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McGuinness OP, Cherrington AD. Effects of fructose on hepatic glucose metabolism. Curr Opin Clin Nutr Metab Care. 2003;6:441–8. doi: 10.1097/01.mco.0000078990.96795.cd. [DOI] [PubMed] [Google Scholar]
- 48.Page KA, Chan O, Arora J, Belfort-Deaguiar R, Dzuira J, Roehmholdt B, Cline GW, Naik S, Sinha R, Constable RT, et al. Effects of fructose vs glucose on regional cerebral blood flow in brain regions involved with appetite and reward pathways. JAMA. 2013;309:63–70. doi: 10.1001/jama.2012.116975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Purnell JQ, Klopfenstein BA, Stevens AA, Havel PJ, Adams SH, Dunn TN, Krisky C, Rooney WD. Brain functional magnetic resonance imaging response to glucose and fructose infusions in humans. Diabetes Obes Metab. 2011;13:229–34. doi: 10.1111/j.1463-1326.2010.01340.x. [DOI] [PubMed] [Google Scholar]
- 50.Miyamoto T, Amrein H. Courtship Suppression by a Drosophila pheromone recpetor. Nat Neurosci. 2008 doi: 10.1038/nn.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]