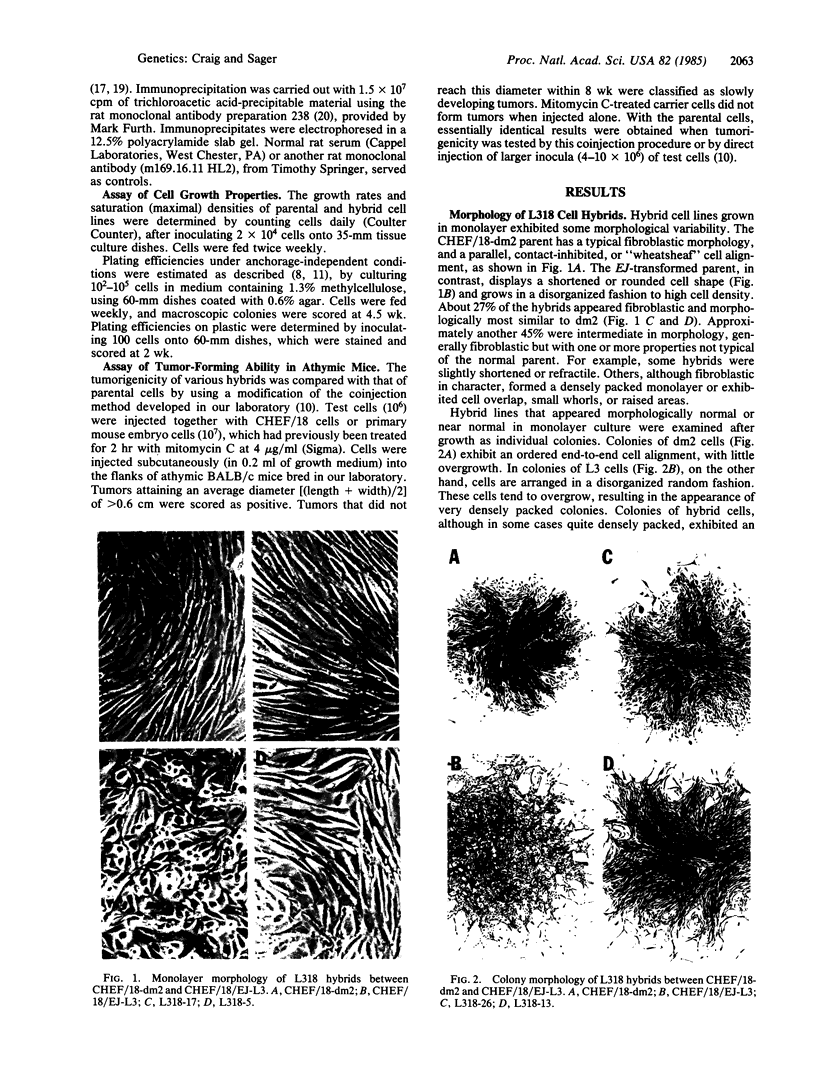
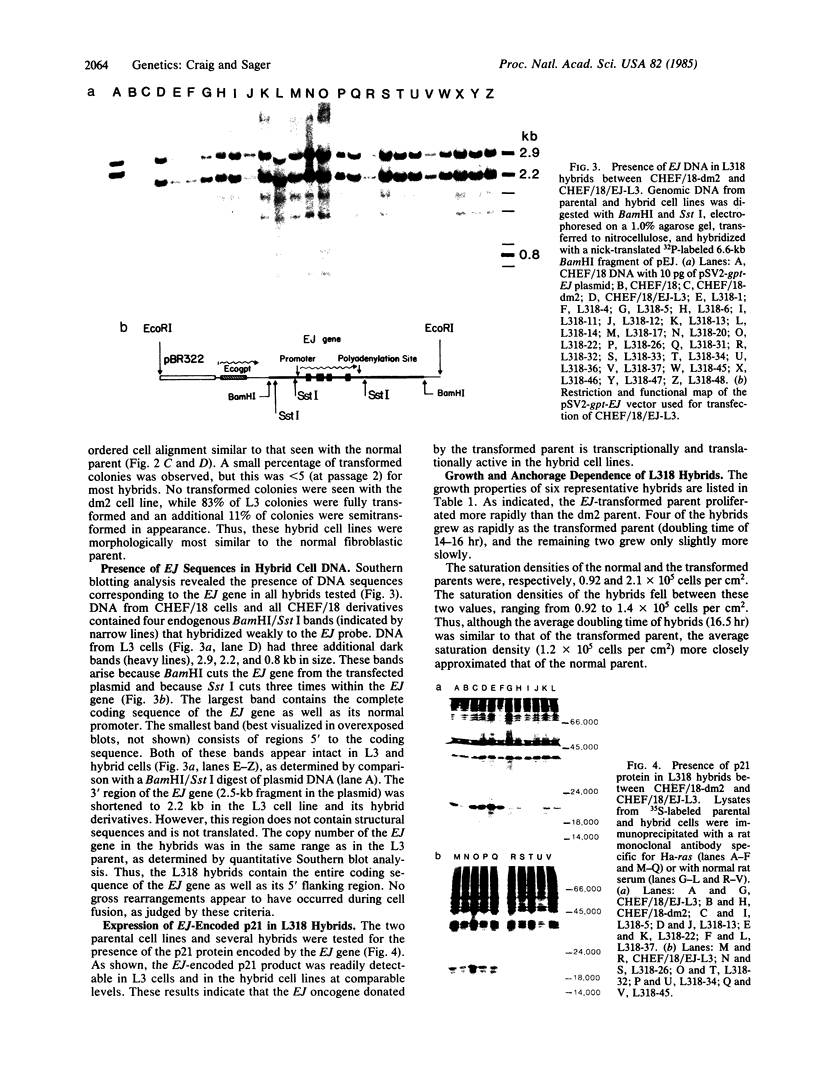
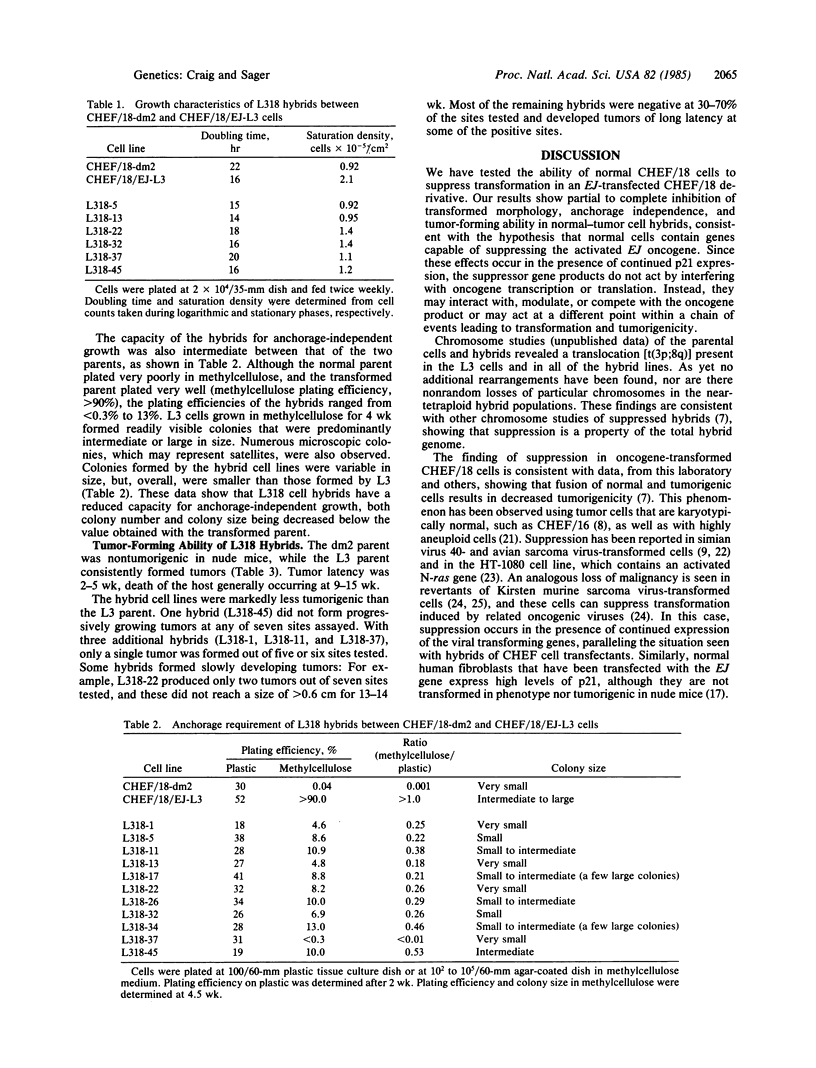
Abstract
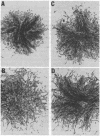
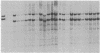
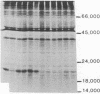
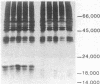
Somatic cell hybridization experiments were carried out to determine whether normal cells have the ability to suppress the transforming effects of a defined oncogene. A nontransformed Chinese hamster embryo fibroblast cell line (CHEF/18-dm2) was used as the normal parent, and a CHEF/18 transfectant carrying the human mutant c-Ha-ras (EJ) oncogene was used as the tumorigenic parent. Selected hybrids (L318 cell lines) were assayed for the presence of EJ DNA, for the p21 product of the c-Ha-ras gene, and for various indices of cell transformation. These hybrids exhibited a fibroblastic morphology similar to the normal parent, although they contained the EJ gene and expressed its p21 protein product at levels comparable with the transformed parent. They had a reduced capacity for anchorage-independent growth (plating efficiency in methylcellulose of less than 0.3-13%, as compared with greater than 90% for the transformed parent) and decreased tumor-forming ability in athymic mice. These findings show that normal CHEF/18 cells contain suppressor genes capable of inhibiting expression of the transformed phenotype, and tumor-forming ability, in the presence of an activated EJ oncogene.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benedict W. F., Weissman B. E., Mark C., Stanbridge E. J. Tumorigenicity of human HT1080 fibrosarcoma X normal fibroblast hybrids: chromosome dosage dependency. Cancer Res. 1984 Aug;44(8):3471–3479. [PubMed] [Google Scholar]
- Campisi J., Gray H. E., Pardee A. B., Dean M., Sonenshein G. E. Cell-cycle control of c-myc but not c-ras expression is lost following chemical transformation. Cell. 1984 Feb;36(2):241–247. doi: 10.1016/0092-8674(84)90217-4. [DOI] [PubMed] [Google Scholar]
- Chang E. H., Furth M. E., Scolnick E. M., Lowy D. R. Tumorigenic transformation of mammalian cells induced by a normal human gene homologous to the oncogene of Harvey murine sarcoma virus. Nature. 1982 Jun 10;297(5866):479–483. doi: 10.1038/297479a0. [DOI] [PubMed] [Google Scholar]
- Der C. J., Cooper G. M. Altered gene products are associated with activation of cellular rasK genes in human lung and colon carcinomas. Cell. 1983 Jan;32(1):201–208. doi: 10.1016/0092-8674(83)90510-x. [DOI] [PubMed] [Google Scholar]
- Dyson P. J., Quade K., Wyke J. A. Expression of the ASV src gene in hybrids between normal and virally transformed cells: specific suppression occurs in some hybrids but not others. Cell. 1982 Sep;30(2):491–498. doi: 10.1016/0092-8674(82)90246-x. [DOI] [PubMed] [Google Scholar]
- Furth M. E., Davis L. J., Fleurdelys B., Scolnick E. M. Monoclonal antibodies to the p21 products of the transforming gene of Harvey murine sarcoma virus and of the cellular ras gene family. J Virol. 1982 Jul;43(1):294–304. doi: 10.1128/jvi.43.1.294-304.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyette M., Petropoulos C. J., Shank P. R., Fausto N. Regulated transcription of c-Ki-ras and c-myc during compensatory growth of rat liver. Mol Cell Biol. 1984 Aug;4(8):1493–1498. doi: 10.1128/mcb.4.8.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell N., Sager R. Noncoordinate expression of SV40-induced transformation and tumorigenicity in mouse cell hybrids. Somatic Cell Genet. 1979 Jan;5(1):129–143. doi: 10.1007/BF01538791. [DOI] [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Krontiris T. G., Cooper G. M. Transforming activity of human tumor DNAs. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1181–1184. doi: 10.1073/pnas.78.2.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Marshall C. J., Kitchin R. M., Sager R. Genetic analysis of tumorigenesis: XII. Genetic control of the anchorage requirement in CHEF cells. Somatic Cell Genet. 1982 Nov;8(6):709–721. doi: 10.1007/BF01543013. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M., Selinger Z., Scolnick E. M., Bassin R. H. Flat revertants isolated from Kirsten sarcoma virus-transformed cells are resistant to the action of specific oncogenes. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5602–5606. doi: 10.1073/pnas.80.18.5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton J. D., Cook F., Roberts P. C., Clewley J. P., Avery R. J. Expression of Kirsten murine sarcoma virus in transformed nonproducer and revertant NIH/3T3 cells: evidence for cell-mediated resistance to a viral oncogene in phenotypic reversion. J Virol. 1984 May;50(2):439–444. doi: 10.1128/jvi.50.2.439-444.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager R., Anisowicz A., Howell N. Genomic rearrangements in a mouse cell line containing integrated SV40 DNA. Cell. 1981 Jan;23(1):41–50. doi: 10.1016/0092-8674(81)90268-3. [DOI] [PubMed] [Google Scholar]
- Sager R., Kovac P. E. Genetic analysis of tumorigenesis: I. Expression of tumor-forming ability in hamster hybrid cell lines. Somatic Cell Genet. 1978 May;4(3):375–392. doi: 10.1007/BF01542849. [DOI] [PubMed] [Google Scholar]
- Sager R., Kovac P. E. Genetic analysis of tumorigenesis: IV. Chromosome reduction and marker segregation in progeny clones from Chinese hamster cell hybrids. Somatic Cell Genet. 1979 Jul;5(4):491–502. doi: 10.1007/BF01538883. [DOI] [PubMed] [Google Scholar]
- Sager R., Tanaka K., Lau C. C., Ebina Y., Anisowicz A. Resistance of human cells to tumorigenesis induced by cloned transforming genes. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7601–7605. doi: 10.1073/pnas.80.24.7601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih C., Weinberg R. A. Isolation of a transforming sequence from a human bladder carcinoma cell line. Cell. 1982 May;29(1):161–169. doi: 10.1016/0092-8674(82)90100-3. [DOI] [PubMed] [Google Scholar]
- Slamon D. J., deKernion J. B., Verma I. M., Cline M. J. Expression of cellular oncogenes in human malignancies. Science. 1984 Apr 20;224(4646):256–262. doi: 10.1126/science.6538699. [DOI] [PubMed] [Google Scholar]
- Smith B. L., Anisowicz A., Chodosh L. A., Sager R. DNA transfer of focus- and tumor-forming ability into nontumorigenic CHEF cells. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1964–1968. doi: 10.1073/pnas.79.6.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. L., Sager R. Genetic analysis of tumorigenesis: XXI. Suppressor genes in CHEF cells. Somat Cell Mol Genet. 1985 Jan;11(1):25–34. doi: 10.1007/BF01534731. [DOI] [PubMed] [Google Scholar]
- Stacey D. W., Kung H. F. Transformation of NIH 3T3 cells by microinjection of Ha-ras p21 protein. Nature. 1984 Aug 9;310(5977):508–511. doi: 10.1038/310508a0. [DOI] [PubMed] [Google Scholar]
- Stanbridge E. J., Der C. J., Doersen C. J., Nishimi R. Y., Peehl D. M., Weissman B. E., Wilkinson J. E. Human cell hybrids: analysis of transformation and tumorigenicity. Science. 1982 Jan 15;215(4530):252–259. doi: 10.1126/science.7053574. [DOI] [PubMed] [Google Scholar]