Background: The large-conductance Ca2+-activated K+ channel (BKCa) is an important regulator of membrane excitability.
Results: N-terminal isoforms of the BKCa channel are differentially modulated by the regulatory β1-subunit.
Conclusion: Expression of different N-terminal isoforms is a novel mechanism of BKCa channel regulation.
Significance: Elucidating the modulation of BKCa activity by β1 provides a new understanding of ion channel physiology.
Keywords: Electrophysiology, Ion Channels, Patch Clamp Electrophysiology, Potassium Channels, Smooth Muscle, BKCa Channel, Auxiliary Subunits, Single-Channel Recordings
Abstract
The large-conductance Ca2+-activated K+ (BKCa) channel is essential for maintaining the membrane in a hyperpolarized state, thereby regulating neuronal excitability, smooth muscle contraction, and secretion. The BKCa α-subunit has three predicted initiation codons that generate proteins with N-terminal ends starting with the amino acid sequences MANG, MSSN, or MDAL. Because the N-terminal region and first transmembrane domain of the α-subunit are required for modulation by auxiliary β1-subunits, we examined whether β1 differentially modulates the N-terminal BKCa α-subunit isoforms. In the absence of β1, all isoforms had similar single-channel conductances and voltage-dependent activation. However, whereas β1 did not modulate the voltage-activation curve of MSSN, β1 induced a significant leftward shift of the voltage activation curves of both the MDAL and MANG isoforms. These shifts, of which the MDAL was larger, occurred at both 10 μm and 100 μm Ca2+. The β1-subunit increased the open dwell times of all three isoforms and decreased the closed dwell times of MANG and MDAL but increased the closed dwell times of MSSN. The distinct modulation of voltage activation by the β1-subunit may be due to the differential effect of β1 on burst duration and interburst intervals observed among these isoforms. Additionally, we observed that the related β2-subunit induced comparable leftward shifts in the voltage-activation curves of all three isoforms, indicating that the differential modulation of these isoforms was specific to β1. These findings suggest that the relative expression of the N-terminal isoforms can fine-tune BKCa channel activity in cells, highlighting a novel mechanism of BKCa channel regulation.
Introduction
The large-conductance voltage- and Ca2+-activated K+ channel (BKCa)2 is a key regulator of membrane excitability in a wide variety of cells such as neurons, smooth muscle, chromaffin cells, and immune cells (1–5). BKCa channel activation integrates both membrane depolarization and increases in intracellular Ca2+ to control membrane excitability (6). Although a single gene (KCNMA1) encodes the BKCa channel α-subunit, alternative splicing produces an array of BKCa isoforms that respond to a variety of modulators in tissue-specific manners (4, 7–9). Despite the high degree of splicing, all BKCa α-subunits are composed of seven conserved transmembrane domains (S0 through S6) and an extracellular N terminus. The N terminus and first transmembrane domain (S0) of the α-subunit are required for association with the auxiliary β1-subunit (10, 11), which is an important source of variable regulation for BKCa channel function (12–14).
Three possible translation initiation sites have been identified in the BKCa α-subunit N-terminal sequence (15, 16). Heterologous expression studies have used the third initiation site (methionine at position 66), starting with amino acid sequence MDAL, as the canonical human BKCa channel α-subunit to investigate β-subunit modulation (10, 14, 17). Other studies of the mouse (13, 18) or rat (9) BKCa channel have also used N-terminal truncated isoforms that start at the second or third translation initiation sites (starting with MSSN or MDAL, respectively). The full-length transcript, which can encode an isoform that starts with MANG, has been isolated from human smooth muscle (15), but whether the β1-subunit modulates this isoform of the human BKCa α-subunit has not been extensively investigated. Because the N-terminal region and first transmembrane domain (S0) are necessary for β1-subunit modulation (10, 11), we hypothesized that these three N-terminal isoforms of the BKCa α-subunit may be differentially modulated by the accessory β1-subunit. Here, we report that the β1-subunit differentially modulates the activation of the MANG, MSSN, and MDAL isoforms. We show that β1 modulates the voltage activation of the N-terminally extended isoforms less than that of the MDAL isoform. This effect may reflect differential modulation of channel kinetics of the three isoforms by β1. By contrast, the β2-subunit modulated voltage activation of MDAL, MSSN, and MANG isoforms to similar extents. These results suggest a new mechanism of fine-tuning the BKCa channel activity that depends on the expression of the N-terminal isoforms and their modulation by the β1-subunit.
EXPERIMENTAL PROCEDURES
Tissue Collection and Cloning of BKCa Isoforms
Non-pregnant human uteri were obtained from the Cooperative Human Tissue Network (Midwestern Division, Columbus, OH). Total RNA from human myometrium was reverse transcribed by using the First-strand RT-PCR kit (Stratagene, La Jolla, CA). The MANG (GenBankTM accession no. BC137137.1) and MSSN isoforms of the BKCa α-subunit were isolated from human myometrium by using sense primers 5′-ATGGCAAATGGTGGCGGC-3′ and 5′-ATGAGTAGCAATATCCAC-3′, respectively, with the antisense primer 5′-CCCAGTAGAGTCGTACTT-3′. The MDAL isoform (GenBankTM accession no. U11058.2) was subcloned by PCR using the MANG isoform as a template.
cDNA Constructs
All BKCa α-subunit N-terminal isoforms were inserted into the pCMV site of a pBudCE4.1 plasmid vector (Invitrogen) containing an optimal Kozak sequence (GACCACC) upstream of the start codon and including the mCherry reporter in the EF1-α site. The cDNA encoding the human β1-subunit (GenBankTM accession no. U25138.1) was cloned into the EF1-α site of pBudCE4.1; eGFP was cloned by PCR into the pCMV site as a reporter. The β2-subunit construct with its N terminus deleted (Δ2–20, β2ND) (19) was kindly provided by Dr. Jianmin Cui (Washington University in St. Louis) and was cloned into the EF1-α site of pBudCE4.1; eGFP was used as a reporter. Plasmid DNAs for transfection were isolated with a Plasmid Maxi kit (Qiagen, Hilden, Germany).
Cell Culture and Transfection
Human embryonic kidney (HEK) 293T cells were grown to 60–80% confluency in DMEM/F12 supplemented with 10% fetal bovine serum and 50 μg/ml gentamicin (all from Invitrogen). Cells were transiently transfected with constructs expressing the human BKCa channel N-terminal isoforms. Another set of cells was co-transfected with the BKCa α-subunit isoforms and the β1-subunit (in a 1:1 or 1:10 molar ratio) or the β2ND-subunit. All transfections were performed using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's instructions. Cells were used in subsequent experiments 24–48 h post-transfection.
Electrophysiology
Single-channel recordings in the inside-out configuration were performed at room temperature in a bath solution containing the following: 140 mm KCl, 20 mm KOH, 10 mm HEPES, 5 mm (H)EDTA, and 10 μm or 100 μm free-Ca2+ (pH 7.2 with HCl). Free Ca2+ concentration was measured by using a Ca2+-sensitive electrode (Thermo Fisher Scientific, Waltham, MA). Pipette solution contained the following: 140 mm KCl, 20 mm KOH, 2 mm MgCl2, and 10 mm HEPES (pH 7.4). Single-channel currents were recorded at a sampling rate of 100 kHz and filtered at 5 kHz by using an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA). Currents were evoked with 10 mV voltage steps (1000-ms duration) from −160 to +120 mV, from a holding potential of 0 mV by using pCLAMP software (version 10, Molecular Devices). This protocol was repeated at least three times on each patch with 10 μm or 100 μm Ca2+ in the bath. For analysis purposes, recordings on the same patch were concatenated, attaining at least a 3-s length recording from each voltage pulse. Mean open probability (Po) and unitary current (i) values were calculated by using pCLAMP software. Patches containing three or fewer channels were used for Po analysis; only patches containing one channel were used for i measurements. Po was plotted against membrane potential (V) and fitted to a Boltzmann function,
to determine half-maximal activation voltage (V0.5) and effective charge (z) values for each experiment by using Graph Pad software (San Diego, CA). Unitary conductance (γ) values were obtained from plotting the i-V relation (from −60 to +60 mV) and fitting to a linear regression; the slope of that regression was calculated as γ for each experiment. For analysis of open and closed dwell times of the channel (MANG, MSSN, or MDAL) in the presence or absence of β1-subunit, inside-out patches containing only one channel were held at −20 mV for 3 min with 10 μm Ca2+ in the bath. Open and closed dwell times histograms were plotted in log-bin time scales and fitted with double exponential functions to obtain time constants (τ) and relative distribution (P) of the data under the curve by using pCLAMP software. Burst analyses were performed on the same recordings as the dwell time analyses; bursts correspond to openings separated by a minimal closed interval defined as critical closed τ (τcrit). τcrit was estimated as the second or third component of a five component exponential function fitting within the closed dwell time histogram, as described previously (20). To ensure a clear estimation of τcrit, only recordings with a Po value less than 0.8 were included in the burst analysis.
Statistical Analysis
Data obtained were subjected to non-parametric Mann-Whitney U test by using Graph Pad software. A p value < 0.05 was considered significant. All data are presented as mean ± S.E.
RESULTS
BKCa N-terminal Isoforms Have Distinct Modes of Regulation by the Auxiliary β1-subunit
Previous studies identified three possible translation initiation sites in the BKCa α-subunit N-terminal sequence (15, 16). We cloned the longer N-terminal BKCa isoform, with amino acid sequence starting at MANG and the isoform starting at MSSN from human myometrium samples. Then, we used PCR to subclone the shorter N-terminal form starting at MDAL (Fig. 1); all constructs contained an optimal Kozak sequence at the 5′ end to optimize expression.
FIGURE 1.
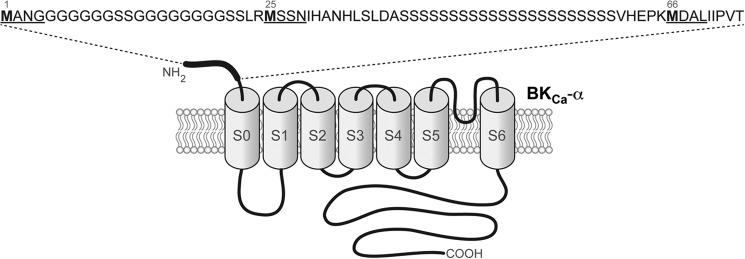
BKCa channel α-subunit structure and N-terminal sequence. A schematic representation of the human BKCa α-subunit is shown. The N-terminal peptide sequence of the α-subunit is shown in detail. Letters in boldface type show putative initiation methionines (Met-1, Met-25, and Met-66). Underlined stretches of four residues are names used in this study for each isoform. S0–S6 denote transmembrane domains.
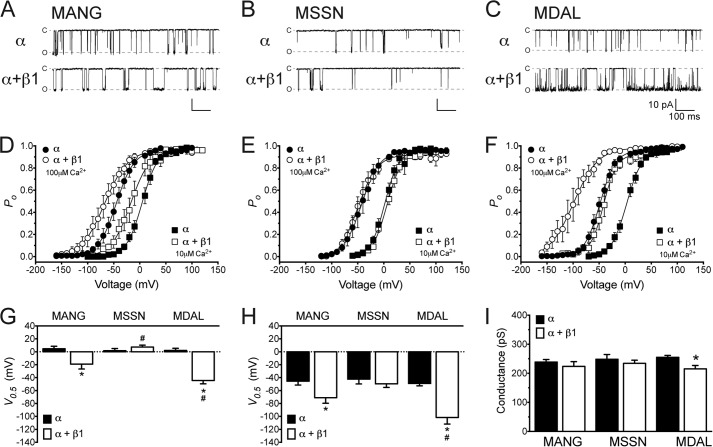
To test the functional properties of the N-terminal isoforms of the BKCa α-subunit, we expressed each construct individually in HEK293T cells and evaluated their voltage- and Ca2+-dependent currents. Inside-out patch-clamp recordings showed a similar voltage- and Ca2+-dependence of MANG, MSSN, and MDAL isoforms; all three channels showed a voltage-induced increase in Po (Fig. 2). Changes in Ca2+ concentration in the bath from 10 μm to 100 μm induced similar leftward shifts in the voltage-activation curves of MANG, MSSN, and MDAL (∼50 mV, ∼44 mV, and ∼51 mV, respectively, Fig. 2 and Table 1). In addition to Po, unitary conductances were comparable between these isoforms (Fig. 2I). Co-expression of the β1-subunit elicited a significant leftward shift in the voltage-activation curve of MDAL (∼47 and ∼53 mV at 10 and 100 μm Ca2+, respectively, Fig. 2 and Table 1). Although the β1-subunit also shifted voltage-activation curves of the MANG isoform to the left, the shifts were significantly smaller than those of MDAL (∼24 and ∼25 mV at 10 and 100 μm Ca2+, respectively, Fig. 2 and Table 1). In contrast, co-expression of β1 did not significantly shift the voltage-activation of the MSSN isoform (Fig. 2 and Table 1). The β1-subunit reduced unitary conductance only in the MDAL isoform (Fig. 2I). Moreover, the β1-subunit did not change the effective charge (z, given by the slope of voltage-activation curves) of any isoform at 10 μm or 100 μm Ca2+ (Table 1).
FIGURE 2.
MANG, MSSN, and MDAL isoforms of the BKCa channel are differentially modulated by the β1-subunit. Representative excised patch recordings from HEK293T cells transfected with constructs expressing either the MANG (A), MSSN (B), or MDAL (C) α-subunit without (upper traces) or with (lower traces) β1-subunit, at −100 mV with 100 μm Ca2+ in the bath. Dashed lines indicate open (O) or closed (C) states of the channels. Voltage dependence of BKCa activation of MANG (D), MSSN (E), or MDAL (F) with (open symbols) or without (closed symbols) β1-subunit, expressed as open probability of the channel (Po), in the presence of 10 μm (squares) or 100 μm (circles) Ca2+ (n = 6–14). Half-maximal activation voltage (V0.5) obtained from voltage-activation curves for MANG, MSSN, or MDAL isoforms in 10 μm (G) or 100 μm Ca2+ (H) with (white bars) or without (black bars) β1-subunit. Single channel conductances of the different N-terminal isoforms (I) in the absence (black bars) or presence (white bars) of β1-subunit obtained at 10 μm Ca2+ (n = 5–10). *, p < 0.05 compared with α-subunit alone; #, p < 0.05 compared with MANG+β1 and MSSN+β1.
TABLE 1.
Effect of β1-subunit on the voltage activation of different BKCa channel α-subunit N-terminal isoforms
| Isoform | Half-maximal activation voltage (V0.5) mV, mean ± S.E. (n) |
Effective charge (z) mean ± S.E. |
||
|---|---|---|---|---|
| α | α + β1 | α | α + β1 | |
| MANG | ||||
| 10 μm Ca2+ | 4.7 ± 3.9 (10) | −19.1 ± 7.6 (7)a | −2.26 ± 0.15 | −2.15 ± 0.40 |
| 100 μm Ca2+ | −45.6 ± 5.9 (10) | −71.0 ± 8.7 (11)a | −2.00 ± 0.12 | −1.84 ± 0.14 |
| MSSN | ||||
| 10 μm Ca2+ | 1.7 ± 3.4 (10) | 7.3 ± 3.0 (8)b | −2.46 ± 0.17 | −2.87 ± 0.93 |
| 100 μm Ca2+ | −42.4 ± 7.3 (6) | −49.6 ± 5.6 (6) | −1.94 ± 0.12 | −1.62 ± 0.26 |
| MDAL | ||||
| 10 μm Ca2+ | 2.1 ± 3.3 (14) | −44.5 ± 5.2 (7)c,d,e | −2.36 ± 0.29 | −3.14 ± 0.81 |
| 100 μm Ca2+ | −49.0 ± 3.6 (9) | −101.8 ± 10.0 (6)b,c,e | −2.09 ± 0.15 | −1.73 ± 0.18 |
a p < 0.05 compared with α-subunit.
b p < 0.05 compared with MANG.
c p < 0.01 compared to α-subunit.
d p < 0.01 compared to MANG.
e p < 0.01 compared to MSSN.
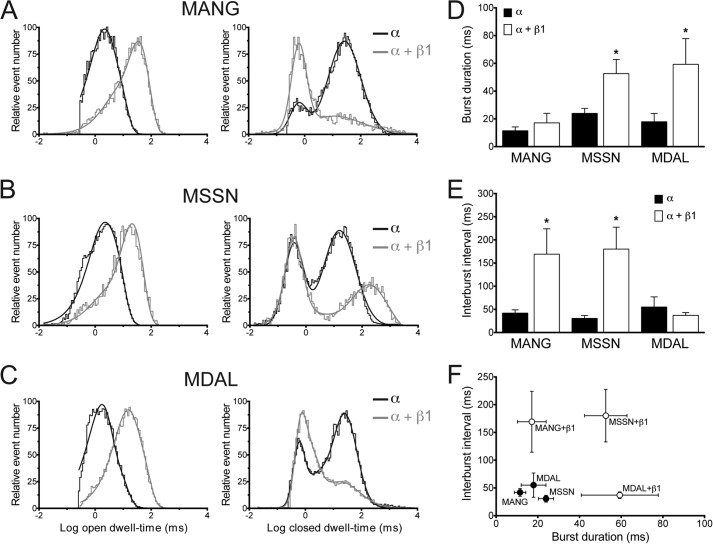
We further analyzed the effect of the β1-subunit on the MANG, MSSN, and MDAL channel kinetics by recording single channel currents at −20 mV for 3 min with 10 μm Ca2+ in the bath. In the presence of the β1-subunit, the open time constants (τ) were increased in MANG and MDAL, whereas only the larger τ (τ2) was increased in MSSN (Fig. 3 and Table 2). Additionally, β1 decreased the τ of the closed state of both MANG and MDAL isoforms, but increased the τ of the closed state on MSSN (Fig. 3 and Table 2). Co-expression of β1 significantly increased the burst duration of both MDAL (from 18.0 ± 6.0 ms to 59.3 ± 18.5 ms, p < 0.05, Fig. 3D) and MSSN (from 23.9 ± 3.6 ms to 52.6 ± 10.2, p < 0.05, Fig. 3D), but not of MANG (from 11.5 ± 2.8 ms to 17.1 ± 6.9 ms, Fig. 3D). Interestingly, the interburst interval was increased on MANG and MSSN isoforms in the presence of the β1 subunit, but not in MDAL (Fig. 3E), indicating that the β1-induced stabilization of the burst state was different between these isoforms (Fig. 3F). Together, these observations suggest that the extended N-terminal sequence in the MANG isoform reduces, and in the case of MSSN abolishes, the β1-induced changes in voltage dependence of channel activation, which might be due, in part, to a differential β1-dependent modulation of channel kinetics.
FIGURE 3.
BKCa channel kinetics of the N-terminal α-subunit isoforms are differentially regulated by the β1-subunit. Open and closed dwell time distributions of single channels in HEK293T cells expressing MANG (A), MSSN (B), or MDAL (C) isoforms of the BKCa channel in the absence (black lines) or presence (gray lines) of β1-subunit (n = 3–7). Currents were evoked by holding the membrane potential at −20 mV for 3 min in the presence of 10 μm Ca2+ in the bath. Histograms were plotted in log-bin time scales and fitted with double exponential functions (solid line). Burst duration (D) and interburst (E) analyses of same recordings as in A–C. *, p < 0.05 compared with α-subunit alone. F, burst duration was plotted against interburst interval for each construct in the presence (open symbols) and absence (closed symbols) of β1-subunit.
TABLE 2.
Effect of β1-subunit on the open and closed dwell-times of different BKCa α-subunit N-terminal isoforms
Time constants (τ1 and τ2) are expressed in milliseconds. P1 and P2 are relative distributions of data under curves used to fit the results shown in Fig. 3.
| Isoform | Open, mean ± S.E. |
Closed, mean ± S.E. |
||
|---|---|---|---|---|
| α | α + β1 | α | α + β1 | |
| MANG | ||||
| τ1 | 1.46 ± 0.09 | 2.82 ± 0.11a | 1.2 ± 0.12 | 1.02 ± 0.07 |
| P1 | 0.49 ± 0.06 | 0.13 ± 0.01a | 0.13 ± 0.01 | 0.69 ± 0.03a |
| τ2 | 4.71 ± 0.08 | 34.15 ± 0.02a | 35.55 ± 0.04 | 33.2 ± 0.23a |
| P2 | 0.51 ± 0.06 | 0.87 ± 0.01a | 0.87 ± 0.02 | 0.31 ± 0.03a |
| MSSN | ||||
| τ1 | 1.06 ± 0.22 | 1.10 ± 0.18 | 0.61 ± 0.08 | 0.54 ± 0.05 |
| P1 | 0.32 ± 0.13 | 0.11 ± 0.01 | 0.36 ± 0.02 | 0.54 ± 0.02a |
| τ2 | 3.60 ± 0.92 | 20.28 ± 0.02a | 29.02 ± 0.04 | 40.34 ± 0.26a |
| P2 | 0.78 ± 1.50 | 0.89 ± 0.01 | 0.64 ± 0.02 | 0.46 ± 0.03a |
| MDAL | ||||
| τ1 | 1.45 ± 0.07 | 9.84 ± 0.05a | 1.22 ± 0.09 | 1.54 ± 0.08b |
| P1 | 0.68 ± 0.06 | 0.48 ± 0.03a | 0.27 ± 0.02 | 0.66 ± 0.04a |
| τ2 | 5.42 ± 0.15 | 32.13 ± 0.05a | 35.35 ± 0.04 | 23.98 ± 0.28a |
| P2 | 0.32 ± 0.06 | 0.52 ± 0.03a | 0.73 ± 0.02 | 0.34 ± 0.05a |
a p < 0.01 compared to α-subunit.
b p < 0.05 compared with α-subunit.
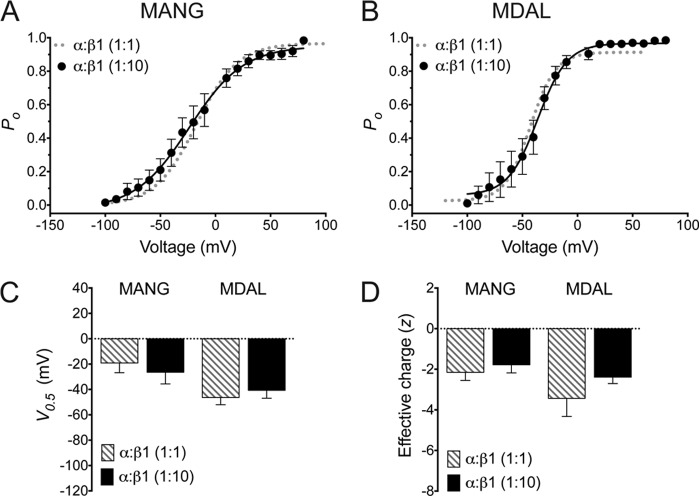
A typical BKCa channel is thought to have a stoichiometry of 4α:4β1-subunits (21). However, the relative expression of each of these proteins has been reported to influence the overall effect of the β1-subunit on BKCa currents; binding of each β1 molecule can sequentially shift the V0.5 of the channel (22). Therefore, it is feasible that the intermediate effect of β1 observed in the MANG isoform was due to fewer β1-subunit molecules binding to the α-subunit when expressed in 1:1 ratio. To address this possibility, we used an α:β1 cDNA ratio of 1:10 to express the MANG and MDAL isoforms in the presence of saturating concentrations of β1. The resulting voltage activation curves for both MANG and MDAL were similar to those observed when a 1:1 cDNA ratio was used (Fig. 4). Thus, the reduced β1-induced leftward shift of MANG voltage-activation curves was not due to fewer β1-subunits binding to this isoform.
FIGURE 4.
Increasing α:β1 cDNA ratio does not affect the differential modulation by β1 of the MANG or MDAL isoforms. Voltage dependence of BKCa activation of MANG (A) or MDAL (B) in the presence of the β1-subunit in HEK293T cells transfected at an α:β1 cDNA ratio of 1:1 (dotted gray lines; same data as shown in Fig. 2D, α+β1, 10 μm Ca2+ (n = 6–7)) or 1:10 (closed circles (n = 8–10)), expressed as open probability of the channel (Po). The bath solution contained 10 μm Ca2+. Half-maximal activation voltage (V0.5, C) and effective charge (z, D) were obtained from voltage-activation curves for each isoform at 1:1 (striped bars) and 1:10 (black bars) α:β1 cDNA ratio.
All Three N-terminal Isoforms Are Similarly Modulated by the β2-subunit
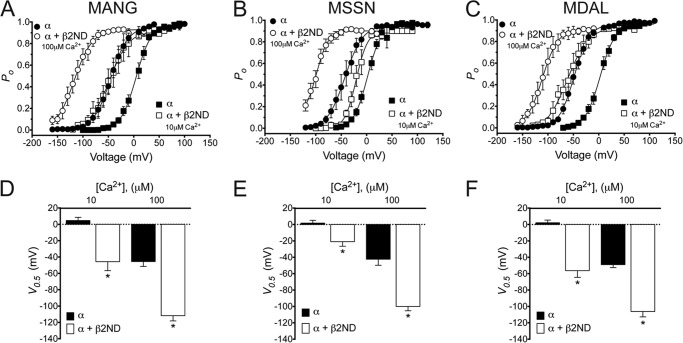
Similar to β1, the related β2-subunit increases BKCa channel Ca2+ sensitivity (23), although it is likely that modulation by these subunits occurs by different mechanisms (19, 24, 25). To assess whether the significantly attenuated β1-induced leftward shift of the voltage-activation curve observed in the MANG isoform, and the lack of effect on the MSSN isoform, were specific for the β1-subunit, we tested the modulation of MANG, MSSN, and MDAL by β2. Because β2 inactivates the BKCa α-subunit channel currents by N-type inactivation, we used a truncated form of the β2-subunit (β2ND) that does not inactivate the channel (19, 23). We observed a significant leftward shift in the voltage-activation curves at two Ca2+ concentrations in cells co-expressing any of the α-subunit isoforms with β2ND as compared with those expressing α-subunit alone (curves were shifted to the left by ∼50 mV and ∼66 mV for MANG, by ∼23 mV and ∼58 mV for MSSN, and by ∼59 mV and ∼57 mV for MDAL, in the presence of 10 and 100 μm Ca2+, respectively, Fig. 5). Hence, the decreased shift in channel activation by the extended N terminus of the MANG and MSSN isoforms is specific for the β1-subunit.
FIGURE 5.
The MANG, MSSN, and MDAL α-subunit isoforms are similarly modulated by the β2-subunit. Shown is the voltage dependence of BKCa activation of MANG (A), MSSN (B), and MDAL (C) constructs in the presence (open symbols) or absence (closed symbols) of a β2-subunit construct with its N terminus deleted (β2ND), expressed as open probability of the channel (Po) (n = 6–12). The bath solution contained 10 μm (squares) or 100 μm (circles) Ca2+. Half-maximal activation voltage (V0.5) obtained from voltage-activation curves for MANG (D), MSSN (E), or MDAL (F) isoforms for each Ca2+ concentration with (open columns) or without (closed columns) β2ND-subunit. *, p < 0.01 compared with α-subunit alone.
DISCUSSION
The BKCa channel plays an important role in regulating the membrane potential of excitable cells. Association of the pore-forming α-subunit of the BKCa channel with distinct auxiliary subunits (β1–β4 or the recently described γ1–γ4) is a significant form of channel modulation (17, 26). Accordingly, interaction of the α-subunit with the β1-subunit, which predominates in smooth muscle, confers increased Ca2+ sensitivity and decreased voltage-dependence to the BKCa channel (12, 13). Both the N-terminal region and the S0 transmembrane domain of the BKCa α-subunit are essential for both its interaction with the auxiliary β1-subunit (10, 11) and for β2-subunit modulation (19). In this work, we studied the properties of three different N-terminal isoforms (MANG, MSSN, and MDAL) and how they are modulated by the auxiliary β1- and β2-subunits. We observed that whereas the voltage-activation curve of MDAL was shifted to the left by ∼50 mV, that of MANG was only shifted by ∼25 mV, and that of the MSSN isoform was not affected by the β1-subunit.
The diminished β1-induced shift in voltage-activation curves observed in the MANG isoform, and the lack of modulation in MSSN, were reflected in the modulation of channel kinetics. We observed that the β1-subunit modulated channel kinetics of all three BKCa α-subunit N-terminal isoforms, as evidenced by increases in open dwell times. We also observed that the closed dwell times of MANG and MDAL decreased, whereas the closed dwell times of MSSN increased; these data are consistent with other reports showing that the β1-subunit modifies the kinetics of the channel by stabilizing its open state (14, 18). The β1-subunit also increases the apparent Ca2+ sensitivity of the channel by increasing burst duration (20). Analysis of single-channel kinetics revealed that both MDAL and MSSN isoforms were maintained in the bursting state by β1, as indicated by an increased burst duration, but the burst duration of the MANG isoform was not affected by the β1-subunit. Furthermore, β1 increased the interval between bursts of MANG and MSSN isoforms but did not change the interval of the MDAL isoform. These observations (summarized in Table 3) support a model of allosteric modulation of BKCa channel kinetics by the β1-subunit in which the MANG-extended N-terminal sequence reduces the β1-dependent stabilization of the bursting state, but not the change in overall open and closed dwell times. In the MSSN isoform, the bursting state is stabilized by β1, but the time between bursts is longer, which might explain the net null effect of β1 on the voltage-activation of the MSSN isoform. Differential modifications by β1 in the burst duration and open/closed time constants of these channels could lead to changes in Po and apparent Ca2+ sensitivity (20), altering membrane excitability. Thus, β1-dependent increase in burst duration of the MDAL isoform will result in an increase of the overall time the channel remains in the open state, a greater Po, and a subsequent leftward shift in voltage activation.
TABLE 3.
Summary of the β1-subunit effect on the different properties of BKCa channel α-subunit N-terminal isoforms
| Isoform | Voltage dependence | Conductance | Open dwell time constants | Close dwell time constants | Burst duration | Interburst interval |
|---|---|---|---|---|---|---|
| MANG | Leftward shift | No change | Both increased | Decreased | No change | Increased |
| MSSN | No shift | No change | Only τ2 increased | Increased | Increased | Increased |
| MDAL | Larger leftward shift | Decreased | Both increased | Decreased | Increased | No change |
The association between BKCa α- and its β1-subunit plays an important role in multiple tissues, most notably in regulating smooth muscle contractility (12, 27–31). For example, the β1-subunit controls arterial tone in resistance arteries (29) and contractility in the urinary bladder (31). Because the interaction between BKCa α and β1 seems to be important for human myometrial contractility (32, 33), and both subunits are abundantly expressed in this tissue (34), we explored the presence of the different N-terminal isoforms in smooth muscle tissue derived from human myometrium obtained from non-laboring pregnant women at term. However, detection of peptides corresponding to the various N termini by using mass spectrometry was unsuccessful (data not shown), likely because the low complexity of the sequence between amino acids Met-1 and Met-66 (polyglycine and polyserine stretches, Fig. 1) complicates the detection of these peptides by mass spectrometry. Further studies, such as N-terminal protein sequencing or development of specific antibodies targeted to the N-terminal region of the BKCa α-subunit, will be necessary to determine the endogenous expression of these isoforms.
Our data suggest that the additional N-terminal sequence in the MANG isoform might dampen the effects of the β1-subunit on channel activation by a specific allosteric mechanism rather than by hampering protein-protein interaction. The case of MSSN seems to be more complex; extending the N-terminal sequence by 41 amino acids completely abolished the modulation by β1 of the voltage-dependent activation observed with MDAL, but adding 24 more amino acids partially restored this modulation, as observed with the MANG isoform. The secondary structure of this N-terminal sequence is not clear because of its low complexity poly-glycine and poly-serine stretches (Fig. 1). Further studies aimed at dissecting the length of residues necessary to prevent and restore β1 modulation might elucidate the structural determinants of this effect.
Several studies have reported that the β1-subunit increases the Ca2+ and voltage sensitivity of recombinant BKCa channels (9–11, 13–15, 17, 18, 35–37). However, most reports used an α-subunit isoform starting at the third initiation site, the MDAL isoform (10, 14, 15, 17). Although three predicted initiation codons are encoded in the DNA sequence of the α-subunit (15, 16), neither the first nor second initiation sites (corresponding to MANG and MSSN isoforms, respectively) generate functional channels when expressed in Xenopus laevis oocytes (15). One study demonstrated that expression of functional BKCa channels might depend on the taxonomic class of the cell line used for heterologous expression. Erxleben et al. (9) described a BKCa channel in a rat pituitary cell line, starting at MSSN, whose kinetics are slower in the presence of β1, but lack the typical change in Ca2+ sensitivity at 1–10 μm Ca2+. Interestingly, a truncated form starting at MDAL restores modulation by the β1-subunit. These authors propose that the poly-serine stretch between Met-25 and Met-66 is important in blocking Ca2+ sensitivity modulation by β1. We found comparable results in that the human MSSN isoform lacked, and the MDAL isoform retained, voltage-dependent activation modulation by the β1-subunit. In our study, using a mammalian heterologous expression system, both MANG and MSSN N-terminal isoforms formed functional channels with intrinsic properties indistinguishable from those observed in the MDAL isoform. In addition, expression of the N-terminal isoforms in a mouse fibroblast cell line also generated functional channels, comparable to those expressed in HEK293T cells (data not shown). Thus, expression systems may account for the differences in BKCa channel N-terminal α-subunit function observed between our experimental conditions and those in other reports; however, the underlying mechanism for tissue specific expression of these N-terminal isoforms is still unclear.
The functional complexity of the N-terminal BKCa isoforms, and how their expression is regulated, has not been fully explored. Alternative translation initiation has been described to modulate the expression and function of N-terminal truncated forms of certain potassium channels: the voltage-gated potassium channel Kv3.3 (38) and the two P-domain potassium channels K2P2.1 and K2P10.1 (39, 40). Alternative translation initiation is a mechanism to regulate protein diversity whereby proteins with different N termini are produced from a single mRNA (41). Alternative translation initiation occurs during translation when the ribosome binds to an initiation codon (AUG) that is not the first cap-proximal in the mRNA coding region, thereby generating N-terminally truncated protein isoforms. Generally, AUG sequences are flanked by Kozak consensus sequences, which facilitate binding of the ribosome to the AUG sequence and thus translation initiation (42). The extent of this facilitation is determined by the relative strength of the ribosome binding given by the Kozak sequence; thus, some sequences will promote binding of the ribosome to a certain initiation site over others (43). In examining the mRNA sequence of the BKCa channel α-subunit, we found that the third initiation codon (Met-66) is flanked by a Kozak sequence stronger than those found in the first or second initiation codon (Met-1 and Met-25, respectively); this might lead to leaky scanning by ribosomes to initiate translation at MDAL. In our study, our N-terminal constructs contained an optimal Kozak consensus sequence (GACCACC) before their respective initiation codons to ensure their optimal heterologous expression. Nonetheless, it is possible that the expression of the MDAL isoform was facilitated by alternative translation initiation, at the expense of expression of MANG or MSSN isoforms; therefore, additional studies are necessary to clarify the molecular mechanisms of this regulation.
Our data suggest that the relative expression of N-terminal isoforms can act as a novel mechanism of regulation of BKCa channel activity by the auxiliary β1-subunit. Thus, our results showing the differential activity of BKCa channel N-terminal isoforms might be relevant to several tissues and pathological processes in which BKCa channels, and their β1-subunits, are involved and could lead to development of specific therapeutic strategies to regulate channel activity.
Acknowledgments
We thank Dr. Deborah J. Frank, Dr. Vivian Gonzalez-Perez, Prof. Christopher J. Lingle, and Prof. Jianmin Cui for critical reading of the manuscript. We also thank Prof. Jianmin Cui for kindly providing the β2-subunit construct.
This work was supported by National Institutes of Health Grant R01 HD037831 and American Heart Association Grant 12GRNT10990002 (to S. K. E.), American Heart Association Postdoctoral Fellowship 12POST10660000 (to R. A. L.), and National Center for Research Resources, Clinical and Translational Science Award M01-RR-00059 for human tissue obtainment.
- BKCa
- large-conductance voltage- and Ca2+-activated K+ channel.
REFERENCES
- 1. Scott R. S., Bustillo D., Olivos-Oré L. A., Cuchillo-Ibañez I., Barahona M. V., Carbone E., Artalejo A. R. (2011) Contribution of BK channels to action potential repolarisation at minimal cytosolic Ca2+ concentration in chromaffin cells. Pflugers Arch. 462, 545–557 [DOI] [PubMed] [Google Scholar]
- 2. Yazejian B., Sun X. P., Grinnell A. D. (2000) Tracking presynaptic Ca2+ dynamics during neurotransmitter release with Ca2+-activated K+ channels. Nat. Neurosci. 3, 566–571 [DOI] [PubMed] [Google Scholar]
- 3. Essin K., Gollasch M., Rolle S., Weissgerber P., Sausbier M., Bohn E., Autenrieth I. B., Ruth P., Luft F. C., Nauseef W. M., Kettritz R. (2009) BK channels in innate immune functions of neutrophils and macrophages. Blood 113, 1326–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Korovkina V. P., Brainard A. M., England S. K. (2006) Translocation of an endoproteolytically cleaved maxi-K channel isoform: mechanisms to induce human myometrial cell repolarization. J. Physiol. 573, 329–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brayden J. E., Nelson M. T. (1992) Regulation of arterial tone by activation of calcium-dependent potassium channels. Science 256, 532–535 [DOI] [PubMed] [Google Scholar]
- 6. Cui J., Cox D. H., Aldrich R. W. (1997) Intrinsic voltage dependence and Ca2+ regulation of mslo large conductance Ca-activated K+ channels. J. Gen. Physiol. 109, 647–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tian L., Duncan R. R., Hammond M. S., Coghill L. S., Wen H., Rusinova R., Clark A. G., Levitan I. B., Shipston M. J. (2001) Alternative splicing switches potassium channel sensitivity to protein phosphorylation. J. Biol. Chem. 276, 7717–7720 [DOI] [PubMed] [Google Scholar]
- 8. Chen L., Tian L., MacDonald S. H., McClafferty H., Hammond M. S., Huibant J. M., Ruth P., Knaus H. G., Shipston M. J. (2005) Functionally diverse complement of large conductance calcium- and voltage-activated potassium channel (BK) α-subunits generated from a single site of splicing. J. Biol. Chem. 280, 33599–33609 [DOI] [PubMed] [Google Scholar]
- 9. Erxleben C., Everhart A. L., Romeo C., Florance H., Bauer M. B., Alcorta D. A., Rossie S., Shipston M. J., Armstrong D. L. (2002) Interacting effects of N-terminal variation and strex exon splicing on slo potassium channel regulation by calcium, phosphorylation, and oxidation. J. Biol. Chem. 277, 27045–27052 [DOI] [PubMed] [Google Scholar]
- 10. Wallner M., Meera P., Toro L. (1996) Determinant for beta-subunit regulation in high-conductance voltage-activated and Ca2+-sensitive K+ channels: an additional transmembrane region at the N terminus. Proc. Natl. Acad. Sci. U.S.A. 93, 14922–14927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morrow J. P., Zakharov S. I., Liu G., Yang L., Sok A. J., Marx S. O. (2006) Defining the BK channel domains required for β1-subunit modulation. Proc. Natl. Acad. Sci. U.S.A. 103, 5096–5101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tanaka Y., Meera P., Song M., Knaus H. G., Toro L. (1997) Molecular constituents of maxi KCa channels in human coronary smooth muscle: predominant α + β subunit complexes. J. Physiol. 502, 545–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McManus O. B., Helms L. M., Pallanck L., Ganetzky B., Swanson R., Leonard R. J. (1995) Functional role of the β subunit of high conductance calcium-activated potassium channels. Neuron 14, 645–650 [DOI] [PubMed] [Google Scholar]
- 14. Orio P., Torres Y., Rojas P., Carvacho I., Garcia M. L., Toro L., Valverde M. A., Latorre R. (2006) Structural determinants for functional coupling between the β and α subunits in the Ca2+-activated K+ (BK) channel. J. Gen. Physiol. 127, 191–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wallner M., Meera P., Ottolia M., Kaczorowski G. J., Latorre R., Garcia M. L., Stefani E., Toro L. (1995) Characterization of and modulation by a β-subunit of a human maxi KCa channel cloned from myometrium. Recept Channels 3, 185–199 [PubMed] [Google Scholar]
- 16. McCobb D. P., Fowler N. L., Featherstone T., Lingle C. J., Saito M., Krause J. E., Salkoff L. (1995) A human calcium-activated potassium channel gene expressed in vascular smooth muscle. Am. J. Physiol. 269, H767–777 [DOI] [PubMed] [Google Scholar]
- 17. Contreras G. F., Neely A., Alvarez O., Gonzalez C., Latorre R. (2012) Modulation of BK channel voltage gating by different auxiliary β subunits. Proc. Natl. Acad. Sci. U.S.A. 109, 18991–18996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nimigean C. M., Magleby K. L. (2000) Functional coupling of the β(1) subunit to the large conductance Ca2+-activated K+ channel in the absence of Ca2+. Increased Ca2+ sensitivity from a Ca2+-independent mechanism. J. Gen. Physiol. 115, 719–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee U. S., Shi J., Cui J. (2010) Modulation of BK channel gating by the β2 subunit involves both membrane-spanning and cytoplasmic domains of Slo1. J. Neurosci. 30, 16170–16179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nimigean C. M., Magleby K. L. (1999) The β subunit increases the Ca2+ sensitivity of large conductance Ca2+-activated potassium channels by retaining the gating in the bursting states. J. Gen. Physiol. 113, 425–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Knaus H. G., Garcia-Calvo M., Kaczorowski G. J., Garcia M. L. (1994) Subunit composition of the high conductance calcium-activated potassium channel from smooth muscle, a representative of the mSlo and slowpoke family of potassium channels. J. Biol. Chem. 269, 3921–3924 [PubMed] [Google Scholar]
- 22. Wang Y. W., Ding J. P., Xia X. M., Lingle C. J. (2002) Consequences of the stoichiometry of Slo1 α and auxiliary β subunits on functional properties of large-conductance Ca2+-activated K+ channels. J. Neurosci. 22, 1550–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wallner M., Meera P., Toro L. (1999) Molecular basis of fast inactivation in voltage and Ca2+-activated K+ channels: a transmembrane β-subunit homolog. Proc. Natl. Acad. Sci. U.S.A. 96, 4137–4142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Orio P., Latorre R. (2005) Differential effects of β1 and β2 subunits on BK channel activity. J. Gen. Physiol. 125, 395–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang H., Zhang G., Shi J., Lee U. S., Delaloye K., Cui J. (2008) Subunit-specific effect of the voltage sensor domain on Ca2+ sensitivity of BK channels. Biophys. J. 94, 4678–4687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yan J., Aldrich R. W. (2012) BK potassium channel modulation by leucine-rich repeat-containing proteins. Proc. Natl. Acad. Sci. U.S.A. 109, 7917–7922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Behrens R., Nolting A., Reimann F., Schwarz M., Waldschütz R., Pongs O. (2000) hKCNMB3 and hKCNMB4, cloning and characterization of two members of the large-conductance calcium-activated potassium channel β subunit family. FEBS Lett. 474, 99–106 [DOI] [PubMed] [Google Scholar]
- 28. Knaus H. G., Folander K., Garcia-Calvo M., Garcia M. L., Kaczorowski G. J., Smith M., Swanson R. (1994) Primary sequence and immunological characterization of β-subunit of high conductance Ca2+-activated K+ channel from smooth muscle. J. Biol. Chem. 269, 17274–17278 [PubMed] [Google Scholar]
- 29. Brenner R., Peréz G. J., Bonev A. D., Eckman D. M., Kosek J. C., Wiler S. W., Patterson A. J., Nelson M. T., Aldrich R. W. (2000) Vasoregulation by the β1 subunit of the calcium-activated potassium channel. Nature 407, 870–876 [DOI] [PubMed] [Google Scholar]
- 30. Plüger S., Faulhaber J., Fürstenau M., Löhn M., Waldschütz R., Gollasch M., Haller H., Luft F. C., Ehmke H., Pongs O. (2000) Mice with disrupted BK channel beta1 subunit gene feature abnormal Ca2+ spark/STOC coupling and elevated blood pressure. Circ. Res. 87, E53–60 [DOI] [PubMed] [Google Scholar]
- 31. Petkov G. V., Bonev A. D., Heppner T. J., Brenner R., Aldrich R. W., Nelson M. T. (2001) β1-subunit of the Ca2+-activated K+ channel regulates contractile activity of mouse urinary bladder smooth muscle. J. Physiol. 537, 443–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Matharoo-Ball B., Ashford M. L., Arulkumaran S., Khan R. N. (2003) Down-regulation of the α- and β-subunits of the calcium-activated potassium channel in human myometrium with parturition. Biol. Reprod. 68, 2135–2141 [DOI] [PubMed] [Google Scholar]
- 33. Brainard A. M., Korovkina V. P., England S. K. (2007) Potassium channels and uterine function. Semin. Cell Dev. Biol. 18, 332–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chan Y. W., van den Berg H. A., Moore J. D., Quenby S., Blanks A. M. (2014) Assessment of myometrial transcriptome changes associated with spontaneous human labour by high throughput RNA-seq. Exp. Physiol. 99, 510–524 [DOI] [PubMed] [Google Scholar]
- 35. Cox D. H., Aldrich R. W. (2000) Role of the β1 subunit in large-conductance Ca2+-activated K+ channel gating energetics. Mechanisms of enhanced Ca2+ sensitivity. J. Gen. Physiol. 116, 411–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bao L., Cox D. H. (2005) Gating and ionic currents reveal how the BKCa channel's Ca2+ sensitivity is enhanced by its β1 subunit. J. Gen. Physiol. 126, 393–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sun X., Shi J., Delaloye K., Yang X., Yang H., Zhang G., Cui J. (2013) The interface between membrane-spanning and cytosolic domains in Ca2+-dependent K+ channels is involved in β subunit modulation of gating. J. Neurosci. 33, 11253–11261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fernandez F. R., Morales E., Rashid A. J., Dunn R. J., Turner R. W. (2003) Inactivation of Kv3.3 potassium channels in heterologous expression systems. J. Biol. Chem. 278, 40890–40898 [DOI] [PubMed] [Google Scholar]
- 39. Simkin D., Cavanaugh E. J., Kim D. (2008) Control of the single channel conductance of K2P10.1 (TREK-2) by the amino-terminus: role of alternative translation initiation. J. Physiol. 586, 5651–5663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thomas D., Plant L. D., Wilkens C. M., McCrossan Z. A., Goldstein S. A. (2008) Alternative translation initiation in rat brain yields K2P2.1 potassium channels permeable to sodium. Neuron 58, 859–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cai J., Huang Y., Li F., Li Y. (2006) Alteration of protein subcellular location and domain formation by alternative translational initiation. Proteins 62, 793–799 [DOI] [PubMed] [Google Scholar]
- 42. Kozak M. (1991) Structural features in eukaryotic mRNAs that modulate the initiation of translation. J. Biol. Chem. 266, 19867–19870 [PubMed] [Google Scholar]
- 43. Kozak M. (1997) Recognition of AUG and alternative initiator codons is augmented by G in position +4 but is not generally affected by the nucleotides in positions +5 and +6. EMBO J. 16, 2482–2492 [DOI] [PMC free article] [PubMed] [Google Scholar]