Abstract
This review centers on updating the active research area of vascular calcification. This pathology underlies substantial cardiovascular morbidity and mortality, through adverse mechanical effects on vascular compliance, vasomotion, and, most likely, plaque stability. Biomineralization is a complex, regulated process occurring widely throughout nature. Decades ago, its presence in the vasculature was considered a mere curiosity and an unregulated, “dystrophic” process that does not involve biological mechanisms. While it remains controversial whether the process has any adaptive value or past evolutionary advantage, substantial advances have been made in understanding the biological mechanisms driving the process. Different types of calcific vasculopathy, such as inflammatory vs. metabolic, have parallel mechanisms in skeletal bone calcification, such as intramembranous and endochondral ossification. Recent work has identified important regulatory roles for inflammation, oxidized lipids, elastin, alkaline phosphatase, osteoprogenitor cells, matrix gamma-carboxyglutamic acid protein (MGP), transglutaminase, osteoclastic regulatory factors, phosphate regulatory hormones and receptors, apoptosis, prelamin A, autophagy, and microvesicles or microparticles similar to the matrix vesicles of skeletal bone. Recent work has uncovered fascinating interactions between MGP, vitamin K, warfarin and transport proteins. And, lastly, recent breakthroughs in inherited forms of calcific vasculopathy, have identified the genes responsible as well as an unexpected overlap of phenotypes.
Until recently, vascular calcification was considered a purely degenerative, unregulated process. Since then, investigative groups around the world have identified a wide range of etiological mechanisms and regulatory pathways, and some of the recent developments are highlighted in this review.
Clinical importance
The vasculature requires distensibility and elasticity to maintain normal hemodynamics, autoregulatory function, and vasomotion. Thus, rigid deposits of calcium mineral in the artery wall have important biomechanical consequences on circulatory function. Direct, in vivo stress-strain measurements in hyperlipidemic rabbits suggest that calcification increases stiffness and fragility,1 as expected from theoretical mechanical considerations. Increased stiffness also impairs vasomotion. Plaque rupture is a serious and devastating complication of atherosclerosis. Biomechanical analyses indicate that rigid inclusions in distensible materials, such as calcium deposits in arteries, introduce compliance mismatch and failure stress at the surfaces facing the principal direction of stress, and thereby increase the risk of rupture.2 Others have also used finite element analysis to show that the risk of rupture is further increased when two such deposits are in close proximity.3 When cardiac valves calcify, hemodynamic effects may be severe, as in calcific aortic stenosis, which blocks outflow from the left ventricle. Overall, vascular calcification promotes significant adverse clinical effects including systolic hypertension, left ventricular hypertrophy, coronary ischemia, congestive heart failure, and possibly plaque rupture, thrombosis, and myocardial infarction.
Proposed classification scheme
A variety of mechanisms have been proposed for vascular calcification. Rather than being mutually exclusive, they may correctly reflect multiple etiologies. At risk of gross oversimplification, artery wall calcification may be loosely categorized into three broad types as shown in Table I. For simplicity, certain known forms, such as those associated with irradiation, pharmacologic agents, toxins, and hypercalcemia of cancer, are not included here. The three different types listed may coexist or overlap in the same subject at the same time, and even at the same arterial site. Many patients and experimental models with diabetes have both hyperlipidemia and chronic kidney disease, resulting in both intimal atherosclerotic calcification and medial calcification in the same arterial segment. In addition, even though osteochondrogenic differentiation factors and ossification are primarily associated with intimal atherosclerotic calcification, evidence shows that medial calcification is also associated osteoblastic differentiation markers and, occasionally, even ossification.4 Many of the genetic disorders also have their effects through downstream metabolic mediators. Although osteogenic and chondrogenic differentiation are assumed to precede mineralization in the vasculature, as in bone, the reverse order may also occur, as described later.
Table I.
Proposed categorization of common disease-associated pathological calcification (partial list)
| Category I | Category II | Category III | |
|---|---|---|---|
| Mediators | Inflammatory | Metabolic | Genetic |
| Commonly associated diseases | Atherosclerosis | Chronic kidney disease; diabetes mellitus | Genetic disorders, e.g. PXE, GACI, ACDC, Marfan syndrome |
| Arterial site | Intima | Media | Usually media |
| Regulatory manner | Gain of activators; loss of inhibitors | Loss of inhibitors; gain of activators | Loss of inhibitors; gain of activators |
| Molecular aspects | Osteogenesis and/or chondrogenesis; matrix vesicle release | Nucleation in vesicles or elastin; osteogenesis | Defects in various genes and miRs, e.g. ENPP1, ABCC6, NT5E, SLC20A2, MGP, OPG, Ank |
| Circulating factors | Lipids; cytokines; fetuin | Phosphate; uremic toxins; glucose; soluble RAGE; fetuin | Various, e.g. ATP, pyrophosphate, adenosine, inorganic phosphate |
| Example local factors | Oxidized lipids; cytokines; matrix metalloproteinases | Elastin; proteases; AGEs; RAGE; transglutaminase; MGP | Elastin; fibrillin; pyrophosphate |
| Functional effect | Vascular stiffness; plaque vulnerability | Vascular stiffness | Vascular stiffness |
| Possible analogous ossification | Endochondral ossification | Intramembranous ossification | Variable |
PXE = pseudoxanthoma elasticum; GACI = generalized arterial calcification of infancy; ACDC= arterial calcifications due to deficiency in CD73; miRs = micro RNAs; ENPP1 = ectonucleotidase pyrophosphate phosphodiesterase; ABCC6 = ATP-binding cassette sub-family C member 6; NT5E = 5’-nucleotidase, ecto (CD73); SLC20A2 = solute carrier family 20 (phosphate transporter), member 2; AGEs = advanced glycation endproducts; RAGE = receptor for AGEs; MGP = matrix gamma-carboxyglutamic acid protein; OPG = osteoprotegerin; Ank = ankylosis protein.
Comparable processes in skeletal bone formation
Although the majority of vascular calcium deposits appear to be amorphous calcification, about 15% have advanced to fully formed, structured bone tissue – true ossification -- including osteoid, trabeculae, osteocytes, osteoclast–like cells, and marrow. The use of more than one route to mineralization is also a feature of skeletal bone formation. These include intramembranous bone formation, by which the skull and clavicles arise; endochondral bone formation, by which long bones form; and callus formation, by which fractures repair. Intramembranous ossification occurs by direct mineralization of matrix produced by mesenchymal cells that undergo osteogenic differentiation.5 Endochondral ossification, on the other hand, occurs with cartilage as an intermediate step.5 For example, in the growth plate of long bone, chondrocytes occupy sites of future bone, creating a gradient of chondrocytes in stages of maturation: proliferation, prehypertrophy, hypertrophy and apoptosis. The apoptotic bodies serve as nucleation sites for calcium–phosphate crystals, producing calcified cartilage.6 This calcified cartilage matrix serves as the scaffold, into which microvessels invade, bringing endothelial cells and pericytes. Monocyte–derived chondroclasts dissolve the calcified cartilage and that the microvascular pericytes, on exposure to this calcified matrix and, possibly transglutaminase, undergo osteogenic differentiation and produce bone matrix, osteon, matrix vesicles, and new hydroxyapatite mineral to replace the cartilage.
As cells mature into osteoblasts or chondrocytes, they express osteochondrogenic genes and release a type of membrane–invested microparticle, known as “matrix vesicles,” into the extracellular milieu. These matrix vesicles contain a variety of enzymes and factors, including alkaline phosphatase and annexins. They concentrate calcium and initiate hydroxyapatite mineral crystallization. Over time, it appears that hydroxyapatite crystals nucleate within these matrix vesicles and propagate until they breach the vesicle membrane and, in some manner, merge with existing bone mineral. Matrix vesicles, themselves, arise from cells by more than one mechanism. A pioneer in this field, H. Clarke Anderson, noted that matrix vesicles may form by budding from the plasma membrane and/or from cell degeneration; more than one mechanism may function in the same tissue.7 The term “matrix vesicle” primarily refers to the microparticle found in the matrix of skeletal bone and cultured bone cells. Similar structures are found in other tissues, such as vascular tissue, and other cultured cells, including endothelial, dendritic, and smooth muscle cells. These have been termed extracellular vesicles, apoptotic bodies, and microvesicles.
Parallels between vascular and skeletal mineralization
As hypothesized by Anderson, the matrix vesicles in the vasculature appear to be analogous to the matrix vesicles of skeletal tissues. For example, Kapustin and colleagues8 and Chen et al9 showed that vascular smooth muscle cells (VSMC) produce matrix vesicles that, like bone matrix vesicles, contain alkaline phosphatase as well as annexins and that nucleate calcium mineral. Both groups further showed that the alkaline phosphatase activity in the VSMC matrix vesicles is regulated by exogenous calcium and beta–glycerophosphate. VSMC may also, like osteoblasts, produce nucleating vesicles as a result of primary osteogenic differentiation or as a result of degenerative processes. In novel work from the Aikawa group, evidence suggests that microvesicles released by inflammatory cells, such as macrophages, are capable of mineralization.10 And, as it does for pericytes, exposure to hydroxyapatite mineral may induce osteoblastic differentiation of VSMC.11
The concentrations of calcium and phosphate in extracellular fluid are near those required for spontaneous precipitation of calcium phosphate crystals. This is held in check at baseline by inhibitory factors. Extraosseous biomineralization may be driven not only by upregulation of activating factors but also by downregulation of such inhibiting factors. It is likely that some forms of vascular calcification are driven solely by gain of activators, others, solely by loss of inhibitors, and some, by a combination.
Teleology
Vascular calcification may often be a result of an adaptive mechanism gone awry. Evolutionary pressure from numerous parasitic and bacterial infections may have driven an emphasis on immunological and inflammatory responses. Soft tissue abscesses, ulcers, and other lesions of chronic inflammation often produce ectopic ossification, which may form a wall of bone entrapping the noxious site, an ultimate immune defense against objects resistant to ordinary immune defenses.
Relationship to osteoporosis
Some epidemiological studies have found that the correlation between vascular calcification and osteoporosis is independent of age.13, 14 The mechanisms for such an age–independent association are not clear. One possibility is that the same underlying trigger driving mineralization of vascular tissue also underlies demineralization of bone tissue, even though the outcomes are opposite in the two tissues. For example, it is well known that chronic inflammation has opposite effects on soft vs. hard tissue, promoting mineralization in the former and demineralization in the latter. Clinical examples are calcific tendonitis and osteomyelitis.5 Thus, as hyperlipidemia and diabetes promote systemic and local inflammation by glycoxidative modification of lipids in bone and artery wall tissue, simultaneous vascular calcification and osteoporosis may result. Lipid deposition and oxidation has been shown in skeletal bone.15–17 With respect to metabolic vascular calcification, the associated chronic kidney disease promotes hyperparathyroidism, which promotes bone loss, now known as the “chronic kidney disease-mineral and bone disorder.”
A common question is whether the calcium lost from bone in osteoporosis is transferred to the vascular tree. In normal individuals, this would be unlikely given that circulating levels of calcium are tightly regulated by parathyroid hormone and renal excretion and also because of abundant mineralization inhibitors in the circulation, such as fetuin.18 In contrast, patients with chronic kidney disease (CKD) often have dysregulated calcium-phosphate metabolism, due to hyperparathyroidism and/or treatment regimens, possibly resulting in hyperphosphatemic calcification. Another potential mechanism linking osteoporosis and vascular calcification is release into the circulation, during resorption of skeletal bone, of unidentified regulatory factors that trigger mineralization in the arteries.
Inflammation, oxidized lipids, hyperlipidemia, and fish oil
Chronic inflammation appears to be a central factor in aberrant soft tissue calcification in general, and sites of chronic inflammation in the vasculature have been shown to become sites of atherosclerotic calcification in mice.19 This phenomenon has now been confirmed at the level of human imaging studies. Abdelbaky and colleagues evaluated imaging data from 137 patients over 1 to 5 years and showed that sites of focal aortic inflammation, detected by 18F-deoxyglucose PET scanning, are significantly associated with subsequent calcification detected by CT scanning.20 These findings support the view that atherosclerotic calcification in humans arises from chronic inflammation. The most common source of chronic vascular inflammation is atherosclerosis, and its underlying contributing factor is accumulation of oxidized lipids. Earlier studies showed that fish oils, such as eicosapentaenoic acid (EPA), which inhibit lipid oxidation, also inhibit vascular calcification.21 Addressing the mechanism of this effect, Kageyama and colleagues recently showed that osteoblastic differentiation and mineralization in VSMC induced by the free-fatty acid, palmitic acid, is blocked by EPA through a mechanism requiring long-chain acyl-CoA synthetase-3 and nuclear factor-κB.22 These findings suggest that pro-oxidant lipids positively regulate, and anti-oxidant lipids negatively regulate, vascular calcification.
The importance of osteogenic differentiation in hyperlipidemic vascular calcification was shown in vivo by Sun et al., who generated mice with vascular-specific deficiency of Runx2, the master-regulatory factor for osteogenic differentiation.23 This defect markedly inhibited vascular calcification induced by a high-fat diet. Interestingly, this group at University of Alabama also observed a reduction in infiltration of macrophages and their differentiation into osteoclastic cells,23 which may have important implications as we develop a better understanding of the coupling between osteoblastic and osteoclastic cells in the artery wall, similar to the functionally important coupling between these cell types in bone.
Alkaline phosphatase and PHOSPHO1
Tissue nonspecific alkaline phosphatase (TNAP) is a phosphatase that breaks down the mineralization inhibitor, pyrophosphate (PPi).24 Recently, Millan’s group identified an additional phosphatase, PHOSPHO1, in matrix vesicles.25 They demonstrated that mice deficient in PHOSPHO1 have abnormalities in skeleton, including osteomalacia and reduced plasma levels of TNAP and PPi.25 Interestingly, mutant mice deficient in PHOSPHO1 but overexpressing TNAP do not have corrected mineralization phenotype in skeleton, despite corrected PPi levels, while mutant mice deficient in both PHOSPHO1 and TNAP have complete absence of skeletal mineralization.25 These findings led the authors to conclude that PHOSPHO1 has a non-redundant functional role as an initiator of mineralization.25 Most recently, Kiffer-Moreira showed that inhibition of PHOSPHO1 in vascular SMC also inhibits matrix calcification in vitro.26
Osteoprogenitor cells
The osteoprogenitor cells in the artery wall may arise from several potential sources, and there is evidence for most of them. These include, among others: resident progenitor cells; mesenchymal stem cells from the marrow stroma via the circulation; subendothelial pericyte-like cells; calcifying vascular cells, also known as vascular mesenchymal cells; adventitial myofibroblasts; transdifferentiated smooth muscle cells; previously mature SMC that have dedifferentiated and redifferentiated; and endothelial cells that have undergone epithelial-mesenchymal transition/transformation. Whether mature smooth muscle cells have the plasticity to de- and redifferentiate is an area of controversy.27–29 Many of these cells undergo mineralization in vitro. In pericytes and calcifying vascular cells, the mineral forms within 3–dimensional cellular aggregates known as nodules, which self–organize in periodic patterns through a mechanism involving reaction–diffusion, polarized cell division,30 and left–right chirality.31
In an exciting new development from Towler’s group, Cheng et al. showed that vascular endothelial cells, previously considered relatively innocent bystanders in vascular calcification, underwent endothelial–mesenchymal transition and osteogenic differentiation in response to overexpression of the Wnt7 inhibitor, Dkk1.32 Furthermore, Yao et al. showed that cells with endothelial markers colocalize with osteogenic markers in Mgp−/− mice and Ins2Akita/+, a diabetes mouse model, and that overexpression of MGP in human umbilical vein endothelial cells attenuates osteogenic differentiation.33 An adventitial source of multipotent vascular cells is evidenced by the findings from Yang et al.34 Altogether, such findings begin to paint an image similar to the transitional stages of differentiation and renewal found in intestinal epithelium and skin.
Matrix gamma-carboxyglutamic acid protein (MGP) and transglutaminase 2
This small protein, MGP, is well established as a key inhibitor of vascular calcification, and its function is affected by inflammatory state 35. MGP deficient mice are known to develop rapid and extensive vascular calcification.36 Recent studies suggest that MGP can bind and inhibit BMP37 as well as calcium mineral itself,38 suggesting that the aortic calcification in the deficient mice may be due to unopposed BMP activity or unopposed mineral growth. Both mechanisms may have a role, given that the mice develop aortic calcification within 9 days of age, and express osteochondrogenic genes 5 days later.39 Thus, osteoblastic differentiation may result either from unopposed BMP activity, or it may proceed as a consequence of mineral formation perhaps due to endocytosed calcium nanocrystal effects on gene induction.11
An interesting feature of MGP is that it requires post-translational modification, vitamin K-dependent gamma-glutamyl carboxylation.40 Some epidemiological evidence supports a role for vitamin K in vascular calcification.41,42 Rats with vitamin K deficiency and CKD have increased vascular calcification, which is attenuated by vitamin K repletion.43 Warfarin, which blocks vitamin K action by blocking the reductase step of vitamin K cycling44, interferes with MGP gamma-glutamyl carboxylation, and induces vascular calcification in rats and in DBA/2J mice, which is reversed by vitamin K treatment.45 In catalyzing gamma-carboxylation, vitamin K is converted to vitamin K oxide, then recycled to active vitamin K through a reductase.44 Recently, Schurgers et al., extended this experimental model and superimposed it on the hyperlipidemic model of atherosclerotic calcification by showing that warfarin significantly increases vascular calcification in hyperlipidemic mice.40 Clinical studies are underway to assess the role of warfarin in human vascular and valvular calcification. Together, these findings support the concept that vitamin K deficiency promotes vascular calcification through insufficient post-translational modification of MGP. This may have important clinical applications.
The mechanism of warfarin-induced vascular calcification may also involve transglutaminase 2 (TG2), which is known to crosslink matrix proteins. It is key in intermediate stages of endochondral ossification in the appendicular bones, where it is released by hypertrophic chondrocytes during production of calcified cartilage matrix.46 Crosslinking appears to render the matrix more vulnerable to mineral deposition, possibly by increasing or enhancing nucleation sites. This is consistent with the increase in calcification of bioprosthetic valves treated with another cross-linker, glutaraldehyde.47
Using cultured vascular SMC and organ culture of aortic rings from Tg2−/− deficient mice, Johnson and colleagues showed a substantial inhibition of phosphate-induced calcium deposition, indicating an essential role for TG2 in this form of vascular calcification.48 Beazley and colleagues went on to show that TG2 inhibitors block vascular calcification in vitro and in vivo in warfarin-treated rats, possibly independently of effects on MGP.49, 50 Chen and colleagues demonstrated elevated tissue levels of TG2 in rats with chronic kidney disease and inhibition of ex vivo calcification in rat aortic rings by TG2 inhibition.51 Together, these findings support a role for TG2 in two metabolic forms of vascular calcification driven by warfarin and CKD.
Elastin, pseudoxanthoma elasticum (PXE), generalized arterial calcification of infancy (GACI), ABCC6, and ENPP1
Pathologists have long observed that early stages of human vascular calcification occur along the edges and frayed ends of elastin fibers.52 As noted earlier, elastin haploinsufficiency reduces the vascular calcification associated with MGP deficiency in mice.39 The authors interpreted this finding to mean that MGP either protects mineralization initiation sites on elastin or that MGP alters extracellular matrix. These findings suggest that, in the context of MGP insufficiency, elastin calcification is metabolic in origin, and that metabolic mineralization may lead to osteochondrogenic differentiation. Results showed a reduction in calcification and an increase in life span.39 The potential importance of elastin in other forms of vascular calcification is supported by its role as the target of calcification in the hereditary human disease, pseudoxanthoma elasticum, described below.
Clues about the role of elastin in metabolic vascular calcification come from the hereditary human disorder, PXE, which features fragmentation and calcification of elastin in arteries and connective tissue, as well as changes in the skin. Its autosomal recessive inheritance was traced to mutations in the gene ABCC6, resulting in absence or nonfunction of the multidrug resistance protein, MRP6, a transmembrane organic anion transporter found primarily in kidney and liver. However, it is not clear whether ABCC6 is located on the basal membrane of hepatocytes,53 or on the mitochondrial membrane,54 and, more importantly, the organic anion whose transport is disrupted in this disease remains to be identified.
Several lines of evidence suggest that PXE is a systemic, rather than local, disease. ABCC6 is not found at the sites of disease, and disease expression requires absence of ABCC6 in the liver, suggesting that the mechanism is related to transport of some unknown factor from the liver to the periphery. In recent work, making use of the observation that mice with PXE develop whisker calcification, Jiang et al. transplanted snout tissue, including whiskers, between Abcc6 null and wild type mice.55 The whiskers from null mice calcified when grafted to Abcc6null mice, but did not when grafted to wild-type mice. Conversely, whiskers from wild-type mice, grafted to null mice, did calcify, indicating that the calcification depended on the recipient (systemic) condition rather than donor (local) condition.55
This, together with the evidence for vitamin K effects described above, led investigators in Amsterdam to the exciting theory that vascular calcification in PXE is due to inadequate delivery of vitamin K to the periphery, by absence of a factor needed to deliver it.56 This raised the possibility of vitamin K treatment for PXE. Interestingly, vitamin K, being a fat-soluble vitamin, must be carried to the periphery on lipoproteins, which are produced by the liver, and levels of lipoproteins are influenced by ABCC6 deficiency.57 Other investigators in Amsterdam proceeded to test the possibility of vitamin K therapy and found benefit in some types of vascular calcification, but, surprisingly, not that in PXE mice.58, 59
In an unexpected twist, mutations of ABCC6 were recently found to underlie some cases of a different, equally mysterious, inherited disorder of vascular calcification, now known as generalized arterial calcification of infancy (GACI),52 which was previously believed to arise from mutations of the gene, ENPP1, which encodes a pyrophosphate-generating enzyme. Apparently, the two disorders, GACI and PXE, overlap substantially in phenotype, having a final common pathway of diffuse vascular calcification, with variable degree of severity. Even more remarkably, the converse was shown full features of each disease may arise from mutations in either gene: some cases of GACI are due to ABCC6 mutations, and some cases of PXE are due to ENPP1 mutations.60
Receptor activator of nuclear factor-kappaB ligand (RANKL) and osteoprotegerin (OPG)
Oxidized lipids and oxidant stress also act on the key driver of osteoclastic bone resorptive activity, RANKL. Byon and colleagues demonstrated that oxidant stress induces RANKL in mouse vascular SMC via the osteogenic differentiation factor, Runx2.61 Similarly, Maziere and colleagues showed that oxidized lipids upregulate RANKL in a dose-dependent manner in human vascular cells, possibly through the associated increase in reactive oxygen species.62 Earlier work showed that mice deficient in a decoy receptor for RANKL, OPG, develop extensive vascular calcification63 and that OPG treatment reduced vascular calcification in hyperlipidemic mice.64 This effect of OPG treatment may occur through downregulation of Notch1-RBP-Jkappa pathway and downstream mediators, Msx2 and alkaline phosphatase.65 To determine whether the vascular calcification in Opg−/− mice results from “leaking” of mineral from skeletal bone where excessive osteoclastic differentiation promotes resorption, Callegari et al., showed that vascular calcification in Opg−/− mice was reduced by transplantation of Opg+/+ marrow.66 Interestingly, angiotensin II infusion increases both RANKL expression and calcification in hyperlipidemic mice.67
CKD, FGF23, and Klotho
Hyperphosphatemia, a metabolic disorder that is almost universal in CKD, stimulates release of the hormone fibroblast growth factor 23 (FGF23) from skeletal osteocytes as part of a negative feedback loop. It appears that FGF23 and Klotho have a role in vascular calcification, however, whether it is direct or indirect remains under investigation. Together with a coreceptor, Klotho, FGF23 acts on FGF receptors in the kidney to decrease renal phosphate resorption and downregulate vitamin D activation, both effects serving to reduce serum phosphate. Klotho deficiency is associated with premature aging syndromes.68 As evidence for a role for Klotho in CKD-related vascular calcification, Hu et al. showed that mice overexpressing Klotho have reduced vulnerability to CKD-induced vascular calcification and, conversely, that mice with Klotho insufficiency have greater vulnerability to CKD-induced vascular calcification.69 It has been suggested that CKD-associated vascular calcification represents a state of klotho-deficiency. Lim et al. showed that Klotho knockdown in vascular SMC potentiated development of cell calcification via Runx2 and myocardin serum response factor-dependent pathways.70 Some investigators have shown endogenous Klotho expression in human vascular SMC and arteries as well as responsiveness to FGF23;70 whereas others have found neither expression of Klotho nor FGF23 responsiveness in human or mouse vascular SMC or arteries.71
In clinical studies, a cross-sectional analysis of over 2000 patients with atherosclerosis showed a positive and independent association between FGF23 and coronary calcification.72 However, in a study that analyzed over 1500 patients with CKD, the independent correlation between FGF23 levels and coronary calcification severity lost significance after adjustment for cardiovascular risk factors.71 These divergent findings may be explained, in part, by the different patient populations, atherosclerosis vs. CKD. Another consideration is that the statistical adjustment included several factors that may lie on a causal pathway between FGF23 and calcification, such as prior cardiovascular disease, diabetes, hypertension, smoking history. In this manner, a causal relationship between FGF23 and coronary calcification, if present, could be masked by the statistical adjustment.
Diabetes
Though not as dramatic as in patients with chronic kidney disease, there is a strong predilection for vascular calcification in subjects with diabetes,73 resulting in a major clinical problem, given the large and growing numbers of such patients. Several mechanisms may be involved. One is the formation of advanced glycation endproducts. The receptor for these advanced glycation end products (RAGE) has a role in atherosclerosis, and, in calcific human carotid artery specimens, RAGE co-localizes with inflammatory cells in unstable regions with microcalcifications.74 In mice with diet-induced diabetes, RAGE is upregulated and co-localizes with vascular smooth muscle cells undergoing osteochondrogenic differentiation.75 Ligands for RAGE are quenched by the soluble form of the receptor (sRAGE), and serum levels of this decoy receptor in hemodialysis patients are inversely associated with vascular calcification,76 suggesting a role for RAGE. Moreover, hyperlipidemic mice overexpressing extracellular RAGE-binding protein, S100A12, in VSMC have accelerated vascular calcification, and this is mediated, in part by oxidative stress.77 Another mechanism by which diabetes may affect vascular calcification is through promoting release of osteoprogenitor cells from the marrow into the circulation. Two recent studies found that the proportion of circulating progenitor cells with osteogenic markers is significantly increased in patients with diabetes.78, 79 An additional mechanistic consideration, based on a streptozotocin-treated rat model, is that diabetes may promote vascular calcification by reducing the vitamin K-dependent activation of the inhibitor of calcification, MGP.80 A combination of diabetic factors may synergize to promote vascular calcification. For example, the combination of hyperglycemia and elastin degradation products, coupled with TGF-β1 (commonly elevated in diabetes) increases osteogenic markers, such as alkaline phosphatase, osteocalcin, and Runx2 in vascular cells.81 These and other mechanisms may explain a predilection for initiation of vascular calcification in diabetic subjects. There may also be features of diabetes that facilitate progression of existing vascular calcification. One study of about 200 veterans with diabetes suggests that coronary vascular calcification progresses more rapidly with the use of cholesterol-lowering statins.82 Although statins are expected to reduce inflammation, since they also promote bone growth,83 the counter-intuitive findings from the study of veterans raises the interesting possibility that, once vascular calcium deposits achieve a certain level of maturity in osteogenic differentiation, then statins may enhance progression.
Apoptosis, DNA damage, prelamin A, autophagy, and matrix vesicles
The chronic inflammatory milieu generally includes cell injury and death, including DNA damage, autophagy, and apoptosis. During apoptosis, VSMC release both matrix vesicles and other microparticles, such as apoptotic bodies, that are larger than matrix vesicles, but share many functions, including the ability to concentrate calcium and nucleate calcium-phosphate crystals.6 In further studies related to cell injury, Liu et al. have shown that in vitro prelamin A overexpression, which blocks DNA damage repair, induces vascular cell osteoblastic differentiation and mineralization.84 This finding suggests that DNA damage signaling induces pro-osteogenic expression. Another recent important finding is that an autophagic response, transiently observed in chondrocyte maturation, which reduces matrix vesicle release from cells exposed to excess phosphate, prevents in vitro vascular calcification, supporting the concept that matrix vesicle release is critical to the process of hyperphosphatemic vascular calcification.85
Conclusion
As with many clinical disorders, there are multiple etiologies for calcific vasculopathy, including inflammatory, metabolic, genetic, and epigenetic mechanisms, and these have substantial mechanistic overlap, suggesting that soft tissue calcification may be viewed as a spectrum. Lessons learned from this research in vascular calcification may also contribute to the understanding of a broad range of inflammatory diseases that result in soft tissue calcification.
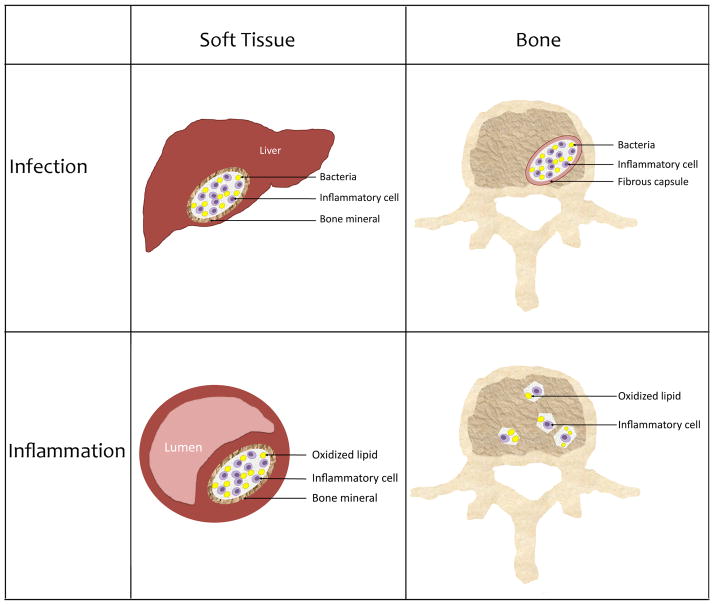
Figure. Schematic depicting relationships between soft tissue mineralization vs. bone mineralization in chronic infection vs. chronic inflammation.
Soft tissue calcification associated with bacterial infection and abscess in liver; vascular calcification associated with chronic inflammation; bone tissue decalcification with bacterial infection in osteomyelitis; and bone tissue calcification with chronic inflammation in osteoporosis.
Table 2.
Types of physiological bone formation12
| Intramembranous | Endochondral | Callus formation12 | |
|---|---|---|---|
| Skeletal site Primary events | Cranial and clavicular | Long bones | Fracture sites |
| Primary osteogenic differentiation of resident osteoprogenitors | Chondrocyte hypertrophy; release of transglutaminase; autophagy; apoptosis; release of nucleating microvesicles; amorphous calcification of cartilage matrix | Hematoma containing marrow mesenchymal stem cells; chondrogenic differentiation of marrow stem cells into “soft callus” | |
| Secondary events | Production of osteon; release of nucleating microvesicles; bone formation | Microvascular invasion; resorption of calcified matrix; osteoblastic differentiation of pericytes; production of osteon; release of nucleating microvesicles; bone formation | Microvascular invasion; resorption; osteoblastic differentiation of pericytes; osteon formation; release of nucleating microvesicles; formation of bone as “hard callus” |
Significance.
Calcific vasculopathy is widespread, affecting approximately 60% of individuals over age 60, and 80% of those over age 80. Its biomechanical effects on the artery wall promote hypertension, left ventricular hypertrophy, coronary insufficiency, congestive heart failure, and, most likely, plaque rupture. When present in tibial arteries, it portends amputation risk. When present in coronary arteries, it is predictive of “subclinical” atherosclerosis. Detection of vascular calcification tends to enhance or outperform traditional risk factors. The molecular mechanisms that control the initiation and progression of vascular mineralization are being elucidated.
Acknowledgments
The authors are grateful to Lizeth Fabiola Gomez for preparation of the artwork.
Sources of Funding
This work was supported in part by funding from National Institute of Health (DK081346, HL 109628, and HL114709).
Footnotes
Disclosure
None
References
- 1.Demer LL. Effect of calcification on in vivo mechanical response of rabbit arteries to balloon dilation. Circulation. 1991;83:2083–2093. doi: 10.1161/01.cir.83.6.2083. [DOI] [PubMed] [Google Scholar]
- 2.Hoshino T, Chow LA, Hsu JJ, Perlowski AA, Abedin M, Tobis J, Tintut Y, Mal AK, Klug WS, Demer LL. Mechanical stress analysis of a rigid inclusion in distensible material: a model of atherosclerotic calcification and plaque vulnerability. Am J Physiol Heart Circ Physiol. 2009;297:H802–810. doi: 10.1152/ajpheart.00318.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelly-Arnold A, Maldonado N, Laudier D, Aikawa E, Cardoso L, Weinbaum S. Revised microcalcification hypothesis for fibrous cap rupture in human coronary arteries. Proc Natl Acad Sci U S A. 2013;110:10741–10746. doi: 10.1073/pnas.1308814110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qiao JH, Mertens RB, Fishbein MC, Geller SA. Cartilaginous metaplasia in calcified diabetic peripheral vascular disease: morphologic evidence of enchondral ossification. Hum Pathol. 2003;34:402–407. doi: 10.1053/hupa.2003.72. [DOI] [PubMed] [Google Scholar]
- 5.Schiller AL, Teitelbaum SL. Bones and joints. In: Rubin E, Farber, John L, editors. Pathology. Lippincott-Raven; 1999. pp. 1337–1347. [Google Scholar]
- 6.Proudfoot D, Skepper JN, Hegyi L, Bennett MR, Shanahan CM, Weissberg PL. Apoptosis regulates human vascular calcification in vitro: evidence for initiation of vascular calcification by apoptotic bodies. Circ Res. 2000;87:1055–1062. doi: 10.1161/01.res.87.11.1055. [DOI] [PubMed] [Google Scholar]
- 7.Anderson HC. Mineralization by matrix vesicles. Scan Electron Microsc. 1984:953–964. [PubMed] [Google Scholar]
- 8.Kapustin AN, Davies JD, Reynolds JL, McNair R, Jones GT, Sidibe A, Schurgers LJ, Skepper JN, Proudfoot D, Mayr M, Shanahan CM. Calcium regulates key components of vascular smooth muscle cell-derived matrix vesicles to enhance mineralization. Circ Res. 2011;109:e1–12. doi: 10.1161/CIRCRESAHA.110.238808. [DOI] [PubMed] [Google Scholar]
- 9.Chen NX, O'Neill KD, Chen X, Moe SM. Annexin-mediated matrix vesicle calcification in vascular smooth muscle cells. J Bone Miner Res. 2008;23:1798–1805. doi: 10.1359/JBMR.080604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.New SE, Goettsch C, Aikawa M, Marchini JF, Shibasaki M, Yabusaki K, Libby P, Shanahan CM, Croce K, Aikawa E. Macrophage-derived matrix vesicles: an alternative novel mechanism for microcalcification in atherosclerotic plaques. Circ Res. 2013;113:72–77. doi: 10.1161/CIRCRESAHA.113.301036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sage AP, Lu J, Tintut Y, Demer LL. Hyperphosphatemia-induced nanocrystals upregulate the expression of bone morphogenetic protein-2 and osteopontin genes in mouse smooth muscle cells in vitro. Kidney Int. 2011;74:414–422. doi: 10.1038/ki.2010.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ross MH, Pawlina W. Histology: A Text and Atlas with Correlated Cell and Molecular Biology. Wolters Kluwer, Lippincott Williams & Wilkins; 2011. pp. 218–252. [Google Scholar]
- 13.Farhat GN, Cauley JA, Matthews KA, Newman AB, Johnston J, Mackey R, Edmundowicz D, Sutton-Tyrrell K. Volumetric BMD and vascular calcification in middle-aged women: the Study of Women's Health Across the Nation. J Bone Miner Res. 2006;21:1839–1846. doi: 10.1359/jbmr.060903. [DOI] [PubMed] [Google Scholar]
- 14.Hak AE, Pols HA, van Hemert AM, Hofman A, Witteman JC. Progression of aortic calcification is associated with metacarpal bone loss during menopause: a population-based longitudinal study. Arterioscler Thromb Vasc Biol. 2000;20:1926–1931. doi: 10.1161/01.atv.20.8.1926. [DOI] [PubMed] [Google Scholar]
- 15.Kawai K, Maruno H, Watanabe Y, Hirohata K. Fat necrosis of osteocytes as a causative factor in idiopathic osteonecrosis in heritable hyperlipemic rabbits. Clin Orthop Relat Res. 1980:273–282. [PubMed] [Google Scholar]
- 16.Watanabe Y, Kawai K, Hirohata K. Histopathology of femoral head osteonecrosis in rheumatoid arthritis: the relationship between steroid therapy and lipid degeneration in the osteocyte. Rheumatol Int. 1989;9:25–31. doi: 10.1007/BF00270286. [DOI] [PubMed] [Google Scholar]
- 17.Tintut Y, Morony S, Demer LL. Hyperlipidemia promotes osteoclastic potential of bone marrow cells ex vivo. Arterioscler Thromb Vasc Biol. 2004;24:e6–10. doi: 10.1161/01.ATV.0000112023.62695.7f. [DOI] [PubMed] [Google Scholar]
- 18.Schafer C, Heiss A, Schwarz A, Westenfeld R, Ketteler M, Floege J, Muller-Esterl W, Schinke T, Jahnen-Dechent W. The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest. 2003;112:357–366. doi: 10.1172/JCI17202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aikawa E, Nahrendorf M, Figueiredo JL, Swirski FK, Shtatland T, Kohler RH, Jaffer FA, Aikawa M, Weissleder R. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation. 2007;116:2841–2850. doi: 10.1161/CIRCULATIONAHA.107.732867. [DOI] [PubMed] [Google Scholar]
- 20.Abdelbaky A, Corsini E, Figueroa AL, Fontanez S, Subramanian S, Ferencik M, Brady TJ, Hoffmann U, Tawakol A. Focal Arterial Inflammation Precedes Subsequent Calcification in the Same Location: A Longitudinal FDG-PET/CT Study. Circ Cardiovasc Imaging. 2013;6:747–754. doi: 10.1161/CIRCIMAGING.113.000382. [DOI] [PubMed] [Google Scholar]
- 21.Abedin M, Lim J, Tang TB, Park D, Demer LL, Tintut Y. N-3 fatty acids inhibit vascular calcification via the p38-mitogen-activated protein kinase and peroxisome proliferator-activated receptor-gamma pathways. Circ Res. 2006;98:727–729. doi: 10.1161/01.RES.0000216009.68958.e6. [DOI] [PubMed] [Google Scholar]
- 22.Kageyama A, Matsui H, Ohta M, Sambuichi K, Kawano H, Notsu T, Imada K, Yokoyama T, Kurabayashi M. Palmitic acid induces osteoblastic differentiation in vascular smooth muscle cells through ACSL3 and NF-kappaB, novel targets of eicosapentaenoic acid. PLoS One. 2013;8:e68197. doi: 10.1371/journal.pone.0068197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun Y, Byon CH, Yuan K, Chen J, Mao X, Heath JM, Javed A, Zhang K, Anderson PG, Chen Y. Smooth muscle cell-specific runx2 deficiency inhibits vascular calcification. Circ Res. 2012;111:543–552. doi: 10.1161/CIRCRESAHA.112.267237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hessle L, Johnson KA, Anderson HC, Narisawa S, Sali A, Goding JW, Terkeltaub R, Millan JL. Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc Natl Acad Sci U S A. 2002;99:9445–9449. [Google Scholar]
- 25.Yadav MC, Simao AM, Narisawa S, Huesa C, McKee MD, Farquharson C, Millan JL. Loss of skeletal mineralization by the simultaneous ablation of PHOSPHO1 and alkaline phosphatase function: a unified model of the mechanisms of initiation of skeletal calcification. J Bone Miner Res. 2011;26:286–297. doi: 10.1002/jbmr.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiffer-Moreira T, Yadav MC, Zhu D, Narisawa S, Sheen C, Stec B, Cosford ND, Dahl R, Farquharson C, Hoylaerts MF, Macrae VE, Millan JL. Pharmacological inhibition of PHOSPHO1 suppresses vascular smooth muscle cell calcification. J Bone Miner Res. 2013;28:81–91. doi: 10.1002/jbmr.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang Z, Wang A, Yuan F, Yan Z, Liu B, Chu JS, Helms JA, Li S. Differentiation of multipotent vascular stem cells contributes to vascular diseases. Nat Commun. 2012;3:875. doi: 10.1038/ncomms1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen AT, Gomez D, Bell RD, et al. Smooth muscle cell plasticity: fact or fiction? Circ Res. 2013;112:17–22. doi: 10.1161/CIRCRESAHA.112.281048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang Z, Wang A, Wang D, Li S. Smooth muscle cells: to be or not to be? Response to Nguyen et Al. Circ Res. 2013;112:23–26. doi: 10.1161/CIRCRESAHA.112.281055. [DOI] [PubMed] [Google Scholar]
- 30.Wong MN, Nguyen TP, Chen TH, Hsu JJ, Zeng X, Saw A, Demer EM, Zhao X, Tintut Y, Demer LL. Preferred mitotic orientation in pattern formation by vascular mesenchymal cells. Am J Physiol Heart Circ Physiol. 2012;303:H1411–1417. doi: 10.1152/ajpheart.00625.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen TH, Hsu JJ, Zhao X, Guo C, Wong MN, Huang Y, Li Z, Garfinkel A, Ho CM, Tintut Y, Demer LL. Left-right symmetry breaking in tissue morphogenesis via cytoskeletal mechanics. Circ Res. 2012;110:551–559. doi: 10.1161/CIRCRESAHA.111.255927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng SL, Shao JS, Behrmann A, Krchma K, Towler DA. Dkk1 and MSX2-Wnt7b signaling reciprocally regulate the endothelial-mesenchymal transition in aortic endothelial cells. Arterioscler Thromb Vasc Biol. 2013;33:1679–1689. doi: 10.1161/ATVBAHA.113.300647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yao Y, Jumabay M, Ly A, Radparvar M, Cubberly MR, Bostrom KI. A role for the endothelium in vascular calcification. Circ Res. 2013;113:495–504. doi: 10.1161/CIRCRESAHA.113.301792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang S, Eto H, Kato H, Doi K, Kuno S, Kinoshita K, Ma H, Tsai CH, Chou WT, Yoshimura K. Comparative Characterization of Stromal Vascular Cells Derived from Three Types of Vascular Wall and Adipose Tissue. Tissue Eng Part A. 2013;19:2724–2734. doi: 10.1089/ten.TEA.2013.0057. [DOI] [PubMed] [Google Scholar]
- 35.Ueland T, Dahl CP, Gullestad L, Aakhus S, Broch K, Skardal R, Vermeer C, Aukrust P, Schurgers LJ. Circulating levels of non-phosphorylated undercarboxylated matrix Gla protein are associated with disease severity in patients with chronic heart failure. Clin Sci (Lond) 2011;121:119–127. doi: 10.1042/CS20100589. [DOI] [PubMed] [Google Scholar]
- 36.Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- 37.Bostrom K, Tsao D, Shen S, Wang Y, Demer LL. Matrix GLA protein modulates differentiation induced by bone morphogenetic protein-2 in C3H10T1/2 cells. J Biol Chem. 2001;276:14044–14052. doi: 10.1074/jbc.M008103200. [DOI] [PubMed] [Google Scholar]
- 38.Wallin R, Cain D, Hutson SM, Sane DC, Loeser R. Modulation of the binding of matrix Gla protein (MGP) to bone morphogenetic protein-2 (BMP-2) Thromb Haemost. 2000;84:1039–1044. [PubMed] [Google Scholar]
- 39.Khavandgar Z, Roman H, Li J, Lee S, Vali H, Brinckmann J, Davis EC, Murshed M. Elastin haploinsufficiency impedes the progression of arterial calcification in MGP-deficient mice. J Bone Miner Res. 2013 doi: 10.1002/jbmr.2039. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 40.Schurgers LJ, Joosen IA, Laufer EM, et al. Vitamin K-antagonists accelerate atherosclerotic calcification and induce a vulnerable plaque phenotype. PLoS One. 2012;7:e43229. doi: 10.1371/journal.pone.0043229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shea MK, Booth SL, Miller ME, Burke GL, Chen H, Cushman M, Tracy RP, Kritchevsky SB. Association between circulating vitamin K1 and coronary calcium progression in community-dwelling adults: the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr. 2013;98:197–208. doi: 10.3945/ajcn.112.056101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boxma PY, van den Berg E, Geleijnse JM, Laverman GD, Schurgers LJ, Vermeer C, Kema IP, Muskiet FA, Navis G, Bakker SJ, de Borst MH. Vitamin k intake and plasma desphospho-uncarboxylated matrix Gla-protein levels in kidney transplant recipients. PLoS One. 2012;7:e47991. doi: 10.1371/journal.pone.0047991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCabe KM, Booth SL, Fu X, Shobeiri N, Pang JJ, Adams MA, Holden RM. Dietary vitamin K and therapeutic warfarin alter the susceptibility to vascular calcification in experimental chronic kidney disease. Kidney Int. 2013;83:835–844. doi: 10.1038/ki.2012.477. [DOI] [PubMed] [Google Scholar]
- 44.Furie B, Bouchard BA, Furie BC. Vitamin K-dependent biosynthesis of gamma-carboxyglutamic acid. Blood. 1999;93:1798–1808. [PubMed] [Google Scholar]
- 45.Kruger T, Oelenberg S, Kaesler N, et al. Warfarin Induces Cardiovascular Damage in Mice. Arterioscler Thromb Vasc Biol. 2013;33:2618–2624. doi: 10.1161/ATVBAHA.113.302244. [DOI] [PubMed] [Google Scholar]
- 46.Nurminskaya M, Kaartinen MT. Transglutaminases in mineralized tissues. Front Biosci. 2006;11:1591–1606. doi: 10.2741/1907. [DOI] [PubMed] [Google Scholar]
- 47.Vyavahare NR, Hirsch D, Lerner E, Baskin JZ, Zand R, Schoen FJ, Levy RJ. Prevention of calcification of glutaraldehyde-crosslinked porcine aortic cusps by ethanol preincubation: mechanistic studies of protein structure and water-biomaterial relationships. J Biomed Mater Res. 1998;40:577–585. doi: 10.1002/(sici)1097-4636(19980615)40:4<577::aid-jbm9>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 48.Johnson KA, Polewski M, Terkeltaub RA. Transglutaminase 2 is central to induction of the arterial calcification program by smooth muscle cells. Circ Res. 2008;102:529–537. doi: 10.1161/CIRCRESAHA.107.154260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beazley KE, Banyard D, Lima F, Deasey SC, Nurminsky DI, Konoplyannikov M, Nurminskaya MV. Transglutaminase inhibitors attenuate vascular calcification in a preclinical model. Arterioscler Thromb Vasc Biol. 2013;33:43–51. doi: 10.1161/ATVBAHA.112.300260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beazley KE, Eghtesad S, Nurminskaya MV. Quercetin attenuates warfarin-induced vascular calcification in vitro independently from matrix Gla protein. J Biol Chem. 2013;288:2632–2640. doi: 10.1074/jbc.M112.368639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen NX, O'Neill K, Chen X, Kiattisunthorn K, Gattone VH, Moe SM. Transglutaminase 2 accelerates vascular calcification in chronic kidney disease. Am J Nephrol. 2013;37:191–198. doi: 10.1159/000347031. [DOI] [PubMed] [Google Scholar]
- 52.Li Q, Jiang Q, Uitto J. Ectopic mineralization disorders of the extracellular matrix of connective tissue: Molecular genetics and pathomechanisms of aberrant calcification. Matrix Biol. 2013 doi: 10.1016/j.matbio.2013.06.003. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pomozi V, Le Saux O, Brampton C, Apana A, Ilias A, Szeri F, Martin L, Monostory K, Paku S, Sarkadi B, Szakacs G, Varadi A. ABCC6 is a basolateral plasma membrane protein. Circ Res. 2013;112:e148–151. doi: 10.1161/CIRCRESAHA.111.300194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martin LJ, Lau E, Singh H, et al. ABCC6 localizes to the mitochondria-associated membrane. Circ Res. 2012;111:516–520. doi: 10.1161/CIRCRESAHA.112.276667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jiang Q, Endo M, Dibra F, Wang K, Uitto J. Pseudoxanthoma elasticum is a metabolic disease. J Invest Dermatol. 2009;129:348–354. doi: 10.1038/jid.2008.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Borst P, van de Wetering K, Schlingemann R. Does the absence of ABCC6 (multidrug resistance protein 6) in patients with Pseudoxanthoma elasticum prevent the liver from providing sufficient vitamin K to the periphery? Cell Cycle. 2008;7:1575–1579. doi: 10.4161/cc.7.11.6005. [DOI] [PubMed] [Google Scholar]
- 57.Wang J, Near S, Young K, Connelly PW, Hegele RA. ABCC6 gene polymorphism associated with variation in plasma lipoproteins. J Hum Genet. 2001;46:699–705. doi: 10.1007/s100380170003. [DOI] [PubMed] [Google Scholar]
- 58.Jiang Q, Li Q, Grand-Pierre AE, Schurgers LJ, Uitto J. Administration of vitamin K does not counteract the ectopic mineralization of connective tissues in Abcc6 (−/−) mice, a model for pseudoxanthoma elasticum. Cell Cycle. 2011;10:701–707. doi: 10.4161/cc.10.4.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gorgels TG, Waarsing JH, Herfs M, Versteeg D, Schoensiegel F, Sato T, Schlingemann RO, Ivandic B, Vermeer C, Schurgers LJ, Bergen AA. Vitamin K supplementation increases vitamin K tissue levels but fails to counteract ectopic calcification in a mouse model for pseudoxanthoma elasticum. J Mol Med (Berl) 2011;89:1125–1135. doi: 10.1007/s00109-011-0782-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nitschke Y, Baujat G, Botschen U, et al. Generalized arterial calcification of infancy and pseudoxanthoma elasticum can be caused by mutations in either ENPP1 or ABCC6. Am J Hum Genet. 2012;90:25–39. doi: 10.1016/j.ajhg.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Byon CH, Sun Y, Chen J, Yuan K, Mao X, Heath JM, Anderson PG, Tintut Y, Demer LL, Wang D, Chen Y. Runx2-upregulated receptor activator of nuclear factor kappaB ligand in calcifying smooth muscle cells promotes migration and osteoclastic differentiation of macrophages. Arterioscler Thromb Vasc Biol. 2011;31:1387–1396. doi: 10.1161/ATVBAHA.110.222547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maziere C, Salle V, Gomila C, Maziere JC. Oxidized low density lipoprotein enhanced RANKL expression in human osteoblast-like cells. Involvement of ERK, NFkappaB and NFAT. Biochim Biophys Acta. 2013;1832:1756–1764. doi: 10.1016/j.bbadis.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 63.Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL, Boyle WJ, Simonet WS. osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260–1268. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morony S, Tintut Y, Zhang Z, Cattley RC, Van G, Dwyer D, Stolina M, Kostenuik PJ, Demer LL. Osteoprotegerin inhibits vascular calcification without affecting atherosclerosis in ldlr(−/−) mice. Circulation. 2008;117:411–420. doi: 10.1161/CIRCULATIONAHA.107.707380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou S, Fang X, Xin H, Li W, Qiu H, Guan S. Osteoprotegerin inhibits calcification of vascular smooth muscle cell via down regulation of the Notch1-RBP-Jkappa/Msx2 signaling pathway. PLoS One. 2013;8:e68987. doi: 10.1371/journal.pone.0068987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Callegari A, Coons M, Ricks JL, Yang HL, Gross TS, Huber P, Rosenfeld ME, Scatena M. Bone Marrow- or Vessel Wall-Derived Osteoprotegerin Is Sufficient to Reduce Atherosclerotic Lesion Size and Vascular Calcification. Arterioscler Thromb Vasc Biol. 2013;33:2491–2450. doi: 10.1161/ATVBAHA.113.301755. [DOI] [PubMed] [Google Scholar]
- 67.Osako MK, Nakagami H, Shimamura M, Koriyama H, Nakagami F, Shimizu H, Miyake T, Yoshizumi M, Rakugi H, Morishita R. Cross-talk of receptor activator of nuclear factor-kappaB ligand signaling with renin-angiotensin system in vascular calcification. Arterioscler Thromb Vasc Biol. 2013;33:1287–1296. doi: 10.1161/ATVBAHA.112.301099. [DOI] [PubMed] [Google Scholar]
- 68.Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 69.Hu MC, Shi M, Zhang J, Quinones H, Griffith C, Kuro-o M, Moe OW. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2011;22:124–136. doi: 10.1681/ASN.2009121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lim K, Lu TS, Molostvov G, Lee C, Lam FT, Zehnder D, Hsiao LL. Vascular Klotho deficiency potentiates the development of human artery calcification and mediates resistance to fibroblast growth factor 23. Circulation. 2012;125:2243–2255. doi: 10.1161/CIRCULATIONAHA.111.053405. [DOI] [PubMed] [Google Scholar]
- 71.Scialla JJ, Lau WL, Reilly MP, et al. Fibroblast growth factor 23 is not associated with and does not induce arterial calcification. Kidney Int. 2013;83:1159–1168. doi: 10.1038/ki.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xiao Y, Peng C, Huang W, Zhang J, Xia M, Zhang Y, Ling W. Circulating fibroblast growth factor 23 is associated with angiographic severity and extent of coronary artery disease. PLoS One. 2013;8:e72545. doi: 10.1371/journal.pone.0072545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Everhart JE, Pettitt DJ, Knowler WC, Rose FA, Bennett PH. Medial arterial calcification and its association with mortality and complications of diabetes. Diabetologia. 1988;31:16–23. doi: 10.1007/BF00279127. [DOI] [PubMed] [Google Scholar]
- 74.Menini S, Iacobini C, Ricci C, Blasetti Fantauzzi C, Salvi L, Pesce CM, Relucenti M, Familiari G, Taurino M, Pugliese G. The galectin-3/RAGE dyad modulates vascular osteogenesis in atherosclerosis. Cardiovasc Res. 2013;100:472–480. doi: 10.1093/cvr/cvt206. [DOI] [PubMed] [Google Scholar]
- 75.Nguyen N, Naik V, Speer MY. Diabetes mellitus accelerates cartilaginous metaplasia and calcification in atherosclerotic vessels of LDLr mutant mice. Cardiovasc Pathol. 2013;22:167–175. doi: 10.1016/j.carpath.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim HS, Chung W, Kim AJ, Ro H, Chang JH, Lee HH, Jung JY. Circulating levels of soluble receptor for advanced glycation end product (sRAGE) are inversely associated with vascular calcification in patients on hemodialysis independent of S100A12 (EN-RAGE) levels. Nephrology (Carlton) 2013 doi: 10.1111/nep.12166. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 77.Hofmann Bowman MA, Gawdzik J, Bukhari U, Husain AN, Toth PT, Kim G, Earley J, McNally EM. S100A12 in vascular smooth muscle accelerates vascular calcification in apolipoprotein E-null mice by activating an osteogenic gene regulatory program. Arterioscler Thromb Vasc Biol. 2011;31:337–344. doi: 10.1161/ATVBAHA.110.217745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fadini GP, Albiero M, Menegazzo L, Boscaro E, Agostini C, de Kreutzenberg SV, Rattazzi M, Avogaro A. Procalcific phenotypic drift of circulating progenitor cells in type 2 diabetes with coronary artery disease. Exp Diabetes Res. 2012;2012:921685. doi: 10.1155/2012/921685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Flammer AJ, Gossl M, Li J, Matsuo Y, Reriani M, Loeffler D, Simari RD, Lerman LO, Khosla S, Lerman A. Patients with an HbA1c in the prediabetic and diabetic range have higher numbers of circulating cells with osteogenic and endothelial progenitor cell markers. J Clin Endocrinol Metab. 2012;97:4761–4768. doi: 10.1210/jc.2012-2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Doyon M, Mathieu P, Moreau P. Decreased expression of gamma-carboxylase in diabetes-associated arterial stiffness: impact on matrix Gla protein. Cardiovasc Res. 2013;97:331–338. doi: 10.1093/cvr/cvs325. [DOI] [PubMed] [Google Scholar]
- 81.Sinha A, Vyavahare NR. High-glucose levels and elastin degradation products accelerate osteogenesis in vascular smooth muscle cells. Diab Vasc Dis Res. 2013;10:410–419. doi: 10.1177/1479164113485101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Saremi A, Bahn G, Reaven PD. Progression of vascular calcification is increased with statin use in the Veterans Affairs Diabetes Trial (VADT) Diabetes Care. 2012;35:2390–2392. doi: 10.2337/dc12-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mundy G, Garrett R, Harris S, Chan J, Chen D, Rossini G, Boyce B, Zhao M, Gutierrez G. Stimulation of bone formation in vitro and in rodents by statins. Science. 1999;286:1946–1949. doi: 10.1126/science.286.5446.1946. [DOI] [PubMed] [Google Scholar]
- 84.Liu Y, Drozdov I, Shroff R, Beltran LE, Shanahan CM. Prelamin A accelerates vascular calcification via activation of the DNA damage response and senescence-associated secretory phenotype in vascular smooth muscle cells. Circ Res. 2013;112:e99–109. doi: 10.1161/CIRCRESAHA.111.300543. [DOI] [PubMed] [Google Scholar]
- 85.Dai XY, Zhao MM, Cai Y, Guan QC, Zhao Y, Guan Y, Kong W, Zhu WG, Xu MJ, Wang X. Phosphate-induced autophagy counteracts vascular calcification by reducing matrix vesicle release. Kidney Int. 2013;83:1042–1051. doi: 10.1038/ki.2012.482. [DOI] [PubMed] [Google Scholar]