Abstract
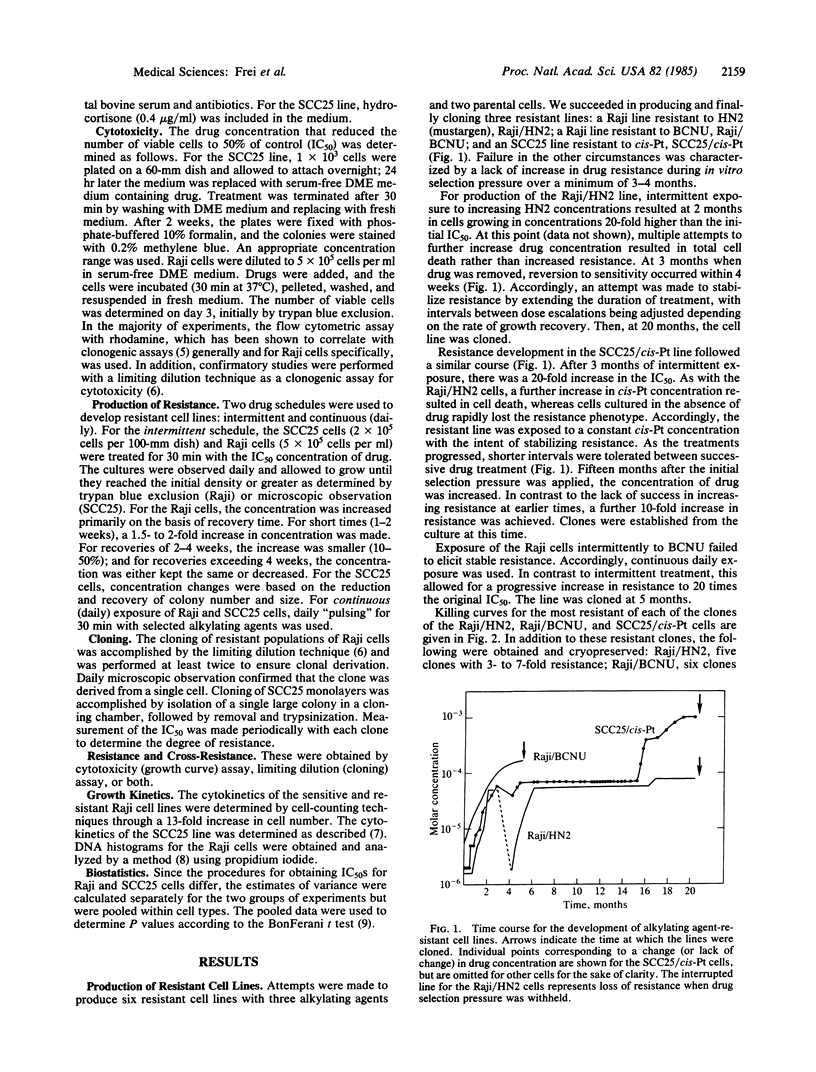
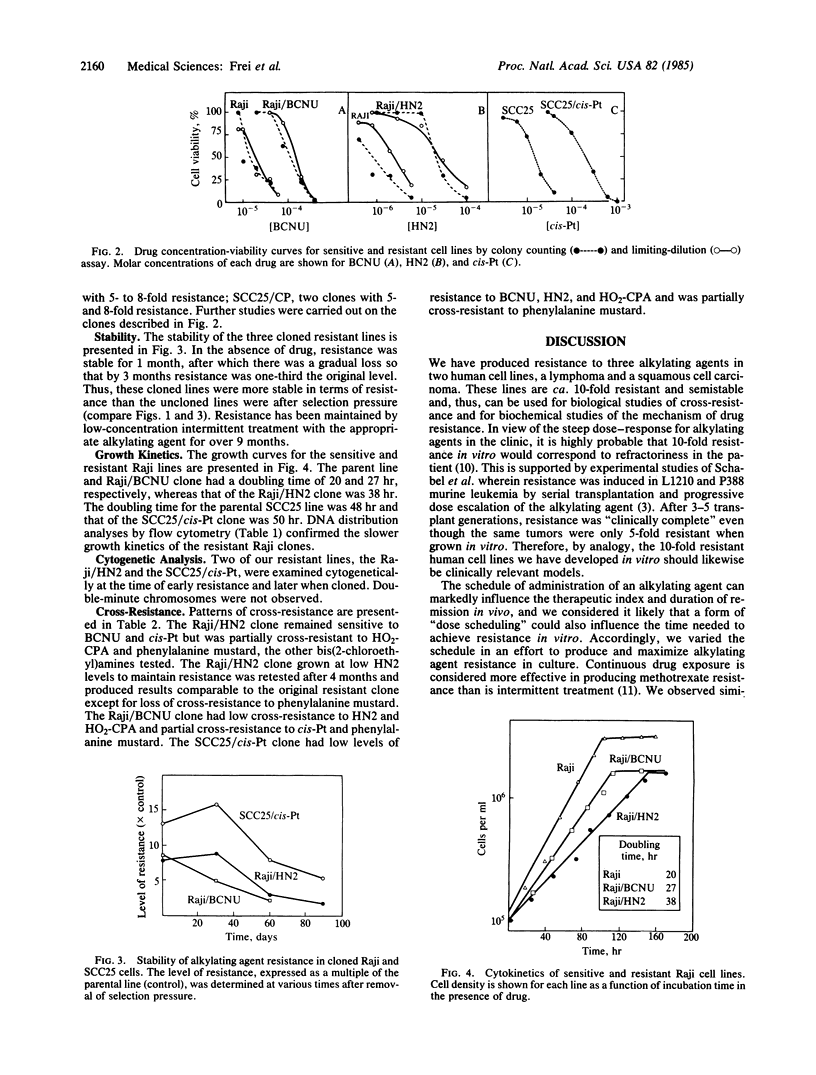
Development of in vitro resistance to HN2 (also called mustargen or mechlorethamine hydrochloride), N,N'-bis(2-chloroethyl)-N-nitrosourea (BCNU), and cisplatin [cis-diamminedichloroplatinum(II)] was achieved in two human cell lines, the Raji/Burkitt lymphoma and a squamous cell carcinoma of the tongue. A 10- to 20-fold increase in resistance relative to the parental line was achieved in 3-4 months of continuous selection pressure. At this time, further increase in selection pressure resulted in cell death, while removal of drug led to rapid loss of resistance. However, by holding selection pressure constant over 8-12 months, semistable clones ranging in resistance up to 8- to 12-fold were obtained. The half-life for resistance loss upon removal of drug was 2-3 months. In the presence of intermittent low concentrations of the alkylating agent, resistance has been maintained in excess of 9 months. With one exception, the growth kinetics of the resistant clones were slightly slower than those of the parental lines. Cross-resistance studies were performed against HN2, BCNU, cisplatin, phenylalanine mustard, and hydroperoxycyclophosphamide. There was, in general, a lack of cross-resistance. We conclude that stable resistance to alkylating agents is produced with difficulty. We propose that these semistable cloned human tumor lines represent clinically relevant models for the study of alkylating agent resistance and that the cross-resistance patterns among these cells have important therapeutic and mechanistic implications.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Kellems R. E., Bertino J. R., Schimke R. T. Selective multiplication of dihydrofolate reductase genes in methotrexate-resistant variants of cultured murine cells. J Biol Chem. 1978 Mar 10;253(5):1357–1370. [PubMed] [Google Scholar]
- Bernal S. D., Shapiro H. M., Chen L. B. Monitoring the effect of anti-cancer drugs on L1210 cells by a mitochondrial probe, rhodamine-123. Int J Cancer. 1982 Aug 15;30(2):219–224. doi: 10.1002/ijc.2910300215. [DOI] [PubMed] [Google Scholar]
- Byfield J. E., Calabro-Jones P. M. Carrier-dependent and carrier-independent transport of anti-cancer alkylating agents. Nature. 1981 Nov 19;294(5838):281–283. doi: 10.1038/294281a0. [DOI] [PubMed] [Google Scholar]
- Day R. S., 3rd, Ziolkowski C. H., Scudiero D. A., Meyer S. A., Lubiniecki A. S., Girardi A. J., Galloway S. M., Bynum G. D. Defective repair of alkylated DNA by human tumour and SV40-transformed human cell strains. Nature. 1980 Dec 25;288(5792):724–727. doi: 10.1038/288724a0. [DOI] [PubMed] [Google Scholar]
- Erickson L. C., Laurent G., Sharkey N. A., Kohn K. W. DNA cross-linking and monoadduct repair in nitrosourea-treated human tumour cells. Nature. 1980 Dec 25;288(5792):727–729. doi: 10.1038/288727a0. [DOI] [PubMed] [Google Scholar]
- Frei E., 3rd, Canellos G. P. Dose: a critical factor in cancer chemotherapy. Am J Med. 1980 Oct;69(4):585–594. doi: 10.1016/0002-9343(80)90472-6. [DOI] [PubMed] [Google Scholar]
- Frei E., 3rd, Rosowsky A., Wright J. E., Cucchi C. A., Lippke J. A., Ervin T. J., Jolivet J., Haseltine W. A. Development of methotrexate resistance in a human squamous cell carcinoma of the head and neck in culture. Proc Natl Acad Sci U S A. 1984 May;81(9):2873–2877. doi: 10.1073/pnas.81.9.2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg G. J., Begleiter A. Membrane transport of alkylating agents. Pharmacol Ther. 1980;8(2):237–274. doi: 10.1016/0163-7258(80)90048-0. [DOI] [PubMed] [Google Scholar]
- Goldenberg G. J., Vanstone C. L., Israels L. G., Iise D., Bihler I. Evidence for a transport carrier of nitrogen mustard in nitrogen mustard-sensitive and -resistant L5178Y lymphoblasts. Cancer Res. 1970 Sep;30(9):2285–2291. [PubMed] [Google Scholar]
- Karran P., Lindahl T., Griffin B. Adaptive response to alkylating agents involves alteration in situ of O6-methylguanine residues in DNA. Nature. 1979 Jul 5;280(5717):76–77. doi: 10.1038/280076a0. [DOI] [PubMed] [Google Scholar]
- Kartner N., Shales M., Riordan J. R., Ling V. Daunorubicin-resistant Chinese hamster ovary cells expressing multidrug resistance and a cell-surface P-glycoprotein. Cancer Res. 1983 Sep;43(9):4413–4419. [PubMed] [Google Scholar]
- Kaufman R. J., Brown P. C., Schimke R. T. Amplified dihydrofolate reductase genes in unstably methotrexate-resistant cells are associated with double minute chromosomes. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5669–5673. doi: 10.1073/pnas.76.11.5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishan A. Rapid flow cytofluorometric analysis of mammalian cell cycle by propidium iodide staining. J Cell Biol. 1975 Jul;66(1):188–193. doi: 10.1083/jcb.66.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T., Karran P., Demple B., Sedgwick B., Harris A. Inducible DNA repair enzymes involved in the adaptive response to alkylating agents. Biochimie. 1982 Aug-Sep;64(8-9):581–583. doi: 10.1016/s0300-9084(82)80091-6. [DOI] [PubMed] [Google Scholar]
- Meyn R. E., Jenkins S. F., Thompson L. H. Defective removal of DNA cross-links in a repair-deficient mutant of Chinese hamster cells. Cancer Res. 1982 Aug;42(8):3106–3110. [PubMed] [Google Scholar]
- New treatment schedule with improved survival in childhood leukemia. Intermittent parenteral vs daily oral administration of methotrexate for maintenance of induced remission. Acute leukemia group B. JAMA. 1965 Oct 4;194(1):75–81. [PubMed] [Google Scholar]
- Redwood W. R., Colvin M. Transport of melphalan by sensitive and resistant L1210 cells. Cancer Res. 1980 Apr;40(4):1144–1149. [PubMed] [Google Scholar]
- Rheinwald J. G., Beckett M. A. Tumorigenic keratinocyte lines requiring anchorage and fibroblast support cultured from human squamous cell carcinomas. Cancer Res. 1981 May;41(5):1657–1663. [PubMed] [Google Scholar]
- Rheinwald J. G. Serial cultivation of normal human epidermal keratinocytes. Methods Cell Biol. 1980;21A:229–254. doi: 10.1016/s0091-679x(08)60769-4. [DOI] [PubMed] [Google Scholar]
- Schabel F. M., Jr, Trader M. W., Laster W. R., Jr, Wheeler G. P., Witt M. H. Patterns of resistance and therapeutic synergism among alkylating agents. Antibiot Chemother (1971) 1978;23:200–215. doi: 10.1159/000401484. [DOI] [PubMed] [Google Scholar]
- Sedgwick B., Lindahl T. A common mechanism for repair of O6-methylguanine and O6-ethylguanine in DNA. J Mol Biol. 1982 Jan 5;154(1):169–175. doi: 10.1016/0022-2836(82)90424-7. [DOI] [PubMed] [Google Scholar]
- Suzukake K., Petro B. J., Vistica D. T. Reduction in glutathione content of L-PAM resistant L1210 Cells confers drug sensitivity. Biochem Pharmacol. 1982 Jan 1;31(1):121–124. doi: 10.1016/0006-2952(82)90249-0. [DOI] [PubMed] [Google Scholar]
- Thomas C. B., Osieka R., Kohn K. W. DNA cross-linking by in vivo treatment with 1-(2-chloroethyl)-3-(4-methylcyclohexyl)-1-nitrosourea of sensitive and resistant human colon carcinoma xenograms in nude mice. Cancer Res. 1978 Aug;38(8):2448–2454. [PubMed] [Google Scholar]
- Vistica D. T., Toal J. N., Rabinovitz M. Amino acid-conferred protection against melphalan--characterization of melphalan transport and correlation of uptake with cytotoxicity in cultured L1210 murine leukemia cells. Biochem Pharmacol. 1978;27(24):2865–2870. doi: 10.1016/0006-2952(78)90202-2. [DOI] [PubMed] [Google Scholar]


