Abstract
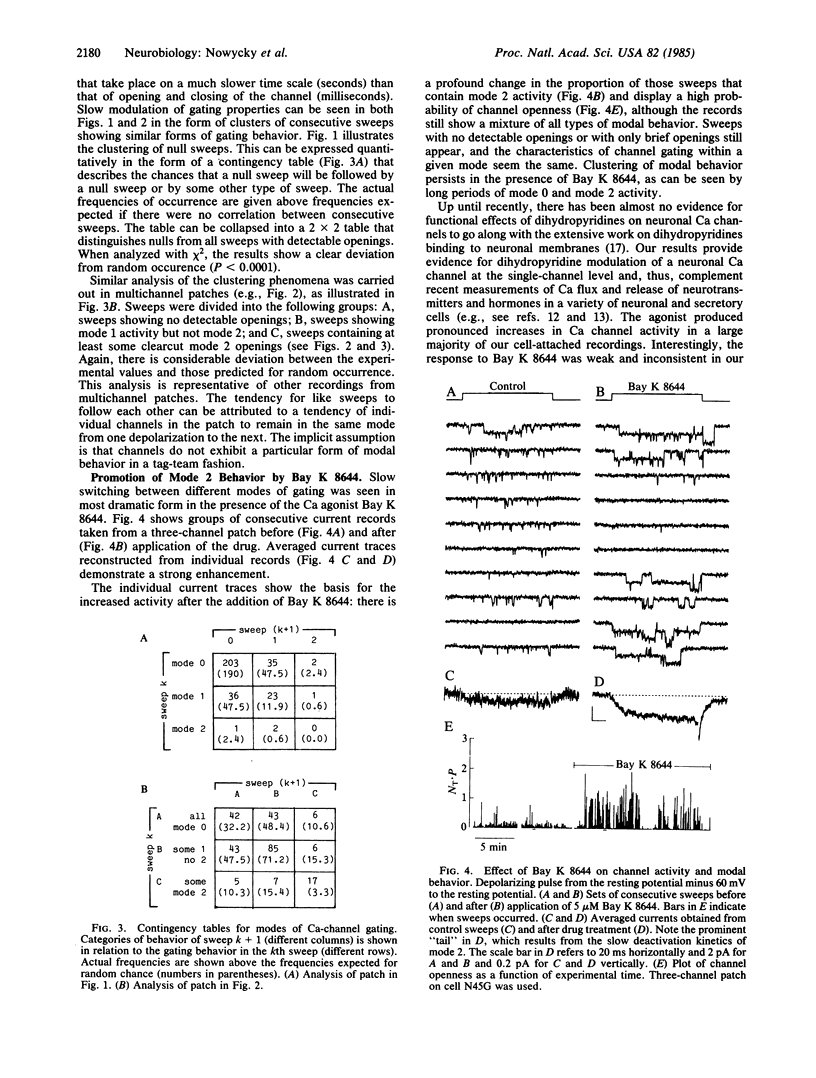
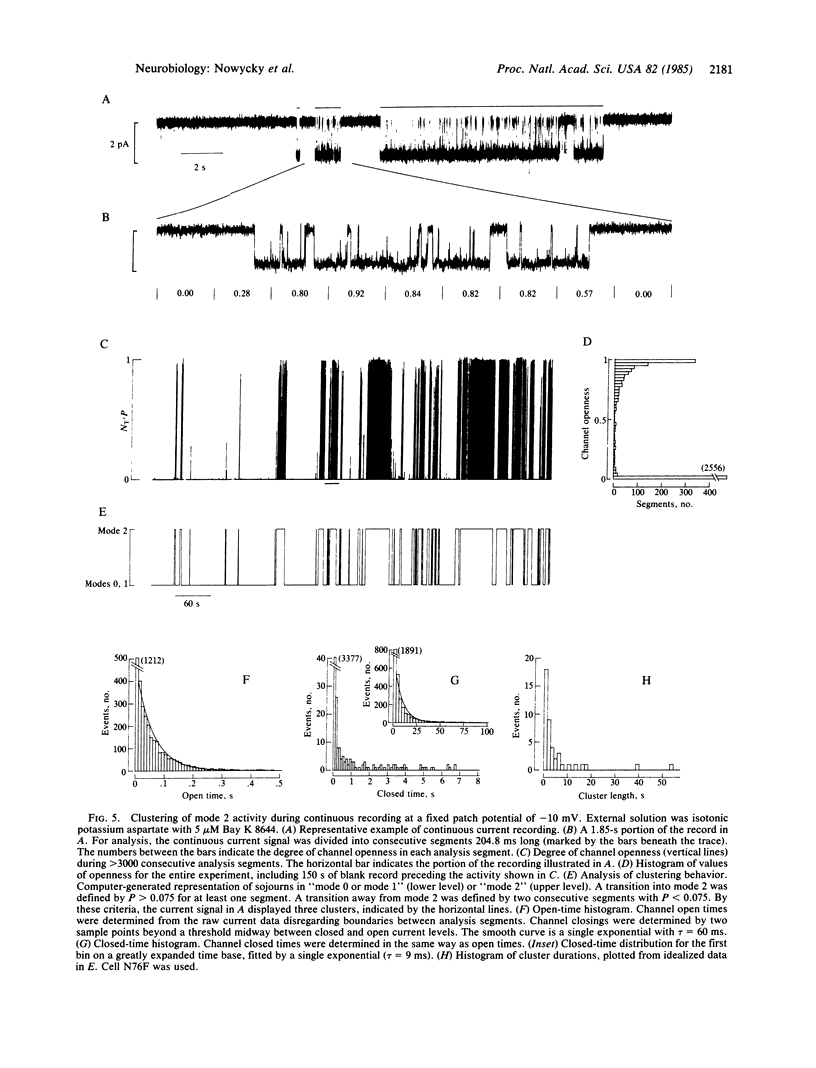
A large-conductance calcium channel in chicken dorsal root ganglion neurons was studied with patch-clamp recordings of unitary currents. In addition to the conventional pattern of Ca-channel gating previously described in neurons ("mode 1"), we observed a different form of gating behavior ("mode 2"). Unlike the brief (approximately equal to 1 ms) openings in mode 1, mode 2 openings tend to be longer (greater than 10 ms) and often outlast the test pulse. In mode 2, the probability of channel openness (P) is high at relatively negative potentials where P in mode 1 is low. Mode 2 activity appears much less often than mode 1 activity in the absence of drug. However, the balance is strongly shifted in favor of mode 2 by the dihydropyridine Ca agonist Bay K 8644, an effect that underlies a marked enhancement of Ca-channel activity. This is the first evidence for dihydropyridine control of neuronal Ca-channel function at the single-channel level. Sweeps showing mode 1 or mode 2 gating appeared interspersed with sweeps with no openings, during which the channel was unavailable for opening ("null mode" or "mode 0"). Two approaches showed that switching between all three modes occurred on a time scale of seconds: (i) channels tended to remain in the same mode from one sweep to the next, with pulses at 0.25 Hz; and (ii) steady depolarizations in Bay K 8644 produced clusters of mode 2 openings lasting several seconds. Changes in the rates of switching might be important in neurochemical modulation of Ca channels. Bay K 8644 and other dihydropyridine Ca agonists might be useful experimental tools for manipulating transmitter release, neurite extension, and other neuronal functions dependent on intracellular Ca.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown A. M., Camerer H., Kunze D. L., Lux H. D. Similarity of unitary Ca2+ currents in three different species. Nature. 1982 Sep 9;299(5879):156–158. doi: 10.1038/299156a0. [DOI] [PubMed] [Google Scholar]
- Carbone E., Lux H. D. A low voltage-activated, fully inactivating Ca channel in vertebrate sensory neurones. Nature. 1984 Aug 9;310(5977):501–502. doi: 10.1038/310501a0. [DOI] [PubMed] [Google Scholar]
- Cavalié A., Ochi R., Pelzer D., Trautwein W. Elementary currents through Ca2+ channels in guinea pig myocytes. Pflugers Arch. 1983 Sep;398(4):284–297. doi: 10.1007/BF00657238. [DOI] [PubMed] [Google Scholar]
- Dunlap K., Fischbach G. D. Neurotransmitters decrease the calcium conductance activated by depolarization of embryonic chick sensory neurones. J Physiol. 1981 Aug;317:519–535. doi: 10.1113/jphysiol.1981.sp013841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman S. B., Miller R. J. Calcium channel activation: a different type of drug action. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5580–5583. doi: 10.1073/pnas.81.17.5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García A. G., Sala F., Reig J. A., Viniegra S., Frías J., Fontériz R., Gandía L. Dihydropyridine BAY-K-8644 activates chromaffin cell calcium channels. Nature. 1984 May 3;309(5963):69–71. doi: 10.1038/309069a0. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984 Oct 11;311(5986):538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- Kokubun S., Reuter H. Dihydropyridine derivatives prolong the open state of Ca channels in cultured cardiac cells. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4824–4827. doi: 10.1073/pnas.81.15.4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux H. D., Nagy K. Single channel Ca2+ currents in Helix pomatia neurons. Pflugers Arch. 1981 Sep;391(3):252–254. doi: 10.1007/BF00596179. [DOI] [PubMed] [Google Scholar]
- Miller R. J., Freedman S. B. Are dihydropyridine binding sites voltage sensitive calcium channels? Life Sci. 1984 Mar 26;34(13):1205–1221. doi: 10.1016/0024-3205(84)90543-5. [DOI] [PubMed] [Google Scholar]
- Reuter H. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature. 1983 Feb 17;301(5901):569–574. doi: 10.1038/301569a0. [DOI] [PubMed] [Google Scholar]
- Schramm M., Thomas G., Towart R., Franckowiak G. Novel dihydropyridines with positive inotropic action through activation of Ca2+ channels. Nature. 1983 Jun 9;303(5917):535–537. doi: 10.1038/303535a0. [DOI] [PubMed] [Google Scholar]
- Tsien R. W. Calcium channels in excitable cell membranes. Annu Rev Physiol. 1983;45:341–358. doi: 10.1146/annurev.ph.45.030183.002013. [DOI] [PubMed] [Google Scholar]


