Abstract
Globoid-cell leukodystrophy (GLD) or Krabbe disease is a lysosomal disease caused by β-galactocerebrosidase (GALC) deficiency resulting in a rapidly progressive neurodegenerative disorder. Unfortunately, the only available treatment is hematopoietic bone marrow transplantation, which prevents its fulminant manifestation but without treating further neurological manifestations. Here we describe the development of a cellular high-throughput screening (HTS) assay using GLD patient fibroblasts to screen for small molecules that enhance the residual mutant GALC enzymatic activity. Small molecules have substantial therapeutic potential in GLD as they are more prone to cross the blood-brain barrier, reaching the neuronal affected cells. The transformation of primary skin fibroblasts with SV40 large T antigen showed to maintain the biochemical characteristics of the GLD cells and generates sufficient cells for the HTS. Using a specific fluorescent substrate, residual GALC activity from a SV40-transformed GLD patient fibroblast was measurable in high-dense microplates plates. The pilot quantitative HTS against a small compound collection showed robust statistics. The small molecules that showed active concentration-response curves were further studied in primary GLD fibroblasts. This cell-based HTS assay demonstrates the feasibility of employing live-GLD patient cells to identify therapeutic agents that can be potentially be used for the treatment of this progressive neurodegenerative disease.
Keywords: β-galactocerebrosidase, high-throughput screening, small molecules, Krabbe Disease
INTRODUCTORY STATEMENT
Globoid-cell leukodystrophy (GLD), mostly known as Krabbe disease (MIM#245200), is an autosomal recessive lysosomal storage disease (LSD) caused by the deficiency of β-galatocerebrosidase (GALC; EC 3.2.1.46). In the general population, the disease incidence varies between 1/100,000–250,000 live births, but in two closed small populations the severe form of GLD is approximately 1/100–150 [1]. The infantile form predominates and is manifested within the first months of life with irritability, fisted hands, poor head control evolving to feeding disturbances, uncontrollable seizures and loss of vision. Death usually occurs by 1 to 3 years of age [2]. Late onset forms of GLD represent approximately 10% of cases, and include the juvenile and adult onsets that predominantly present with motor weakness, sensory and motor neuropathy, cognitive impairment and psychiatric/behavior disturbances [3; 4]. Biochemically, the deficient enzyme, GALC, is a 644-amino acid peptide in its mature form whose crystal structure has been recently elucidated giving insights into the effects of disease-causing mutations [5]. GALC is a soluble lysosomal hydrolase and has galactosylceramide and galactosylsphingosine (psychosine) as natural substrates. Both are important metabolites during myelination, when the galactosylation of sphingosine generates psychosine, which is rapidly metabolized into sphingosine by removal the galactose moiety by GALC [6]. In brain specimens obtained from GLD patients, psychosine levels are substantially elevated [7]. At elevated concentrations, psychosine becomes cytotoxic, in particular to oligodendrocytes, the myelin-forming cells [6]. Therefore, the biochemical disturbances in GLD are manifested by different degrees of brain demyelination resulting in a broad neurological spectrum of GLD [6]. In LSDs, most of the FDA-approved therapies are based on enzyme replacement therapy (ERT), which treat non-neurological symptoms of only six of these disorders [8]. Unfortunately, in GLD, a primarily neurological disease, ERT will not be efficacious given that ERT agents as large-size molecules are unable to cross the blood-brain barrier (BBB). Hematopoietic stem cell transplantation can prevent the rapid and fulminant neurological course of the infantile form of GLD [9]. However, transplanted GLD patients have shown to develop further neurological symptoms related to GLD [10]. On the other hand, patients with late onset forms usually show a residual GALC enzymatic activity resultant from a misfolded and unstable GALC that is directed to the ER-associated degradation (ERAD). Missense mutations represent the majority of the over 70 mutations in the GALC gene reported in GLD [2; 5]. Based on the crystal structure of GALC, nearly 70% of disease-associated missense mutations are predicted to cause instability and misfolding of GALC, and consequently being target to the ERAD [5]. In GLD, as in most LSDs, symptoms associated with the disease are only manifested if a mutation encodes a GALC mutant that works below a critical threshold, which is, in general, ~10% of wild type enzymatic activity [11]. Small molecules have been shown to assist the folding of misfolded mutant lysosomal enzymes resulting in enhancements of their residual enzymatic activity [12; 13; 14; 15; 16]. Thus, increases of the residual mutant GALC activity above the critical threshold should prevent the cytotoxic levels of psychosine, avoiding the oligodendrocyte apoptosis and the subsequent demyelination process. This will likely to reflect in disease stabilization and amelioration of GLD patient symptoms.
Small molecules are therapeutic agents that are more likely to cross the BBB. Recently, we described a patient cell-based assay having as a target a lysosomal enzyme deficient in a neurological LSD [17]. Herein, we report the first GLD patient cell-based assay having the lysosomal GALC as a target. In order to assess the residual GALC enzymatic activity, the cell-based HTS assay was performed using a fluorescent hexadecanoylamino-4-methylumbelliferyl (HMU)-based substrate, which is GALC-specific and is firstly utilized in a high-throughput setting [18]. Different from previous reported in vitro biochemical assays for GALC[19], the use of GLD patient fibroblasts already at the primary stage of the HTS provides the opportunity to examine the target protein (GALC) in a cellular milieu containing potentially disrupted biochemical and signaling pathways secondary to disease molecular processes. Thus, the developed HTS assay for GALC will be important to identify small molecules that can be potential therapeutic agents to treat this devastating neurodegenerative LSD.
MATERIAL AND METHODS
Chemical reagents and equipments
Synthetic fluorescent substrate 6-hexadecanoylamino-4- methylumbelliferyl-β-D-galactoside (HMUGal; FW 592) and standard 6-hexadecanoylamino-4-methylumbelliferyl (HMU; FW 430) were purchased from Moscerdam and used for GALC assays [18]. Other 4-methylumbelliferyl-based fluorescent substrates were used for lysosomal enzyme assays: 4-methylumbelliferyl-β-D-galactopyranoside (β-galactosidase), 4-methylumbelliferyl-(2-acetamido-2-deoxy)-β-D-glucopyranoside (total β-hexosaminidase) and 4-Methylumbelliferyl β-D-glucopyranoside (glucocerebrosidase) were used. All these synthetic substrates were purchased from Sigma-Aldrich Inc.. Chemicals including sodium acetate, sodium chloride, sodium hydroxide, sodium taurocholate, oleic acid, citric acid, chloroform, methanol, sodium phosphate dihydrate, Triton-X100 and serum albumin (human and bovine) were all purchased from Sigma-Aldrich Inc. Bradford protein assays [20] were performed using a commercially available kit from Thermo-Fischer Inc.. Molecular kits for PCRs and reverse transcriptase-PCRs were purchased from Invitrogen Inc. In order to identify and confirm mutations in the GALC gene of GLD patient cell lines used in assays, oligonucleotides for sequencing of genomic and cDNA were designed as previously described [1], and synthesized and purchased from Sigma-Aldrich and IDT Corp.. Library of Pharmacological Active Compounds (LOPAC) for the pilot HTS assay was received from Sigma-Aldrich Inc. All LOPAC compounds were diluted in dimethyl sulfoxide (DMSO). Microplates used in the cellular assay were black clear and flat-bottom 96-well (BD Comp.) and 384-well plates (Greiner CellStar®). The 1,536-well plates used were black, clear and flat-bottom and purchased from Greiner Bio-One Inc.. For cell counting, Beckman Coulter Z1 Coulter Particle Counter (Beckman Coulter Inc.) was used. Tissue culture medium containing resuspended cells were diluted 1:10 in the Z-pak Balanced Electrolyte Solution Isoton II Diluent (Beckman Coulter Inc.) prior injecting into the Cell Counter. All microplates used were tissue culture treated. SpectraMAX Gemini XS was used to measure fluorescence from both 96- and 384-well plates. The GALC assay performed in 1,536-well plates required the Thermo Scientific MultiDrop Combi - to seed cells into the plates and the Perkin Elmer 1430 ultraHTS Wallace Microplate Imager ViewLux, used for fluorescence reading.
Cells, tissue culture conditions and fibroblast transformation
Cultured fibroblasts were obtained from Cell Core Bank from Kennedy Krieger Intellectual and Developmental Disability Research Center, Johns Hopkins Medical Institutions, National Hospital of Omuta, Furuoka, Japan and the cell bank at Telethon Network of Genetic Biobanks, Genova, Italy. The cell lines are unidentified, and obtained through informed consents under human subject protection regulations of local Institutional Review Boards (IRB) regulations. Primary and transformed fibroblasts were cultured using Dulbecco's Modified Eagle Medium (DMEM; Medtech Inc.) with phenol red with 10% fetal calf serum (FCS; Gemini Biologicals Inc.). No antibiotics were used in routine tissue culture procedures. Cells were cultured using traditional incubators with 5% CO2 at 37°C. Cells were washed at least twice in phosphate-buffered saline (PBS) before being harvested. Trypsin PBS solution (0.05%) was used to dissociate cells. Cell lysates for GALC enzymatic assays were obtained by re-suspending cells in sodium phosphate buffer (10 mM; pH 6) with protease inhibitors cocktail (Halt Protease Inhibitor Cocktail - Thermo Fisher). Harvested cell pellets were lysed by freeze-thaw method without the use of detergents. Glycerol reaching 10% was added into pre-lysis solution. Cultured primary GLD patient skin fibroblasts were transformed using pSV3-neo plasmid containing the simian virus (SV) 40 large T cell antigen [21]. The plasmid pSV3-neo (ATCC Inc.) has 8.60 kb size with markers ampR, G418R, SV40 promoter, pBR322 early promoter, and pMB1 replicon. Firstly, primary cells were cultured in 75 cm2-flasks, and once these flasks became confluent, cells were dissociated using 0.05% trypsin. An average of 7×106 cells in DMEM containing 10% FCS medium were transfected with 30 µg pSV3 plasmid by electroporation using Gene Pulser Xcell Electroporation Systems (Biorad Inc.). Cuvettes of 4 mm were used for these experiments. Approximately 4–6 weeks were taken to obtaining initial clones of SV40 transformed cells.
Cellular GALC enzymatic activity assays in 96-well and 384-well plates
The GALC cellular enzymatic activity was done using HMUGal substrate as described earlier [18]. During miniaturization process, multi-well plate GALC assays were performed in 96-well plates using both cultured primary and SV40-transformed fibroblasts. These cells were initially cultured in 75 cm2 tissue culture treated flasks up to full confluence. After washing the 75 cm2 flask twice with PBS, cells were re-suspended with 0.05% Trypsin and diluted with non-phenol red DMEM 10% FCS to ~300–500 cells/µL concentration. Each well of 96-well plate received 100 µL of the cell suspension medium of primary fibroblasts. Cells were seeded one day before cells were lysed and GALC assay was started. Fibroblasts from controls with wild type (WT) GALC and GLD patients with different GALC mutants were cultured in 96-well plates. After removing the medium, substrate citrate buffer (0.4 mM HMUGal; pH 5.2) containing sodium taurocholate (TC; 1mg/mL) was dispensed to each well (25 µL/well of the 96-well plates). Incubation for a 17h-period at 37°C was performed. GALC reaction was stopped using 150 µL of 0.5M NaHCO3/0.5MNa2CO3 buffer (pH 10.7) with 0.25% TX-100. The fluorescence signal was read in SpectraMAX Gemini (Molecular Devices) at 404/460 nm excitation/emission pair. For the GALC assays performed in 384-well plates, the SV40T cells from control (GALC-WT) and GLD patient (GALC-G270D) were also cultured to confluence in tissue cultured treated 75 cm2-flasks, before being dissociated with Trypsin-PBS 0.05% solution and subsequent diluted in 10% FCS DMEM (non-phenol red) medium to achieve ~ 1×103 cells/µL. On average 20–25×103 cells were seeded in each well of 384-well plates. After 24–36 hs in culture, 20 µL of the previously described HMUGal substrate buffer was dispensed per well already containing 20 µL of culture medium (DMEM). The 384-well plates were incubated for 17hs at 37°C. A volume of 70 µL of the GALC stopping-reaction solution (pH 10.5) was dispensed per well. Fluorescence was read at the same excitation and emission above described with SpectraMAX Gemini XS.
Cellular GALC enzymatic activity assay in 1,536-well plates
The miniaturization to 1,536-well plate was performed using 128.0/85MM, tissue culture Greiner black clear and flat-bottom plates. On average 3–4×103 cells in 2 µL of culture medium were seeded per well of a 1,536-well plate. Cells were seed into the plates 16–24 hs before the treatment with LOPAC began. Substrate/lysis buffer as described above but with a 0.2 mM HMUGal substrate buffer was used at a volume of 2 µL/well. After 17 hs incubation at 37°C, 6 µL/well of the same stop solution above described was added in each well and fluorescence was measured using ViewLux (PerkinElmer) at 406 nm excitation and 450 nm emission. The assay was executed using the Thermo Scientific Multidrop Combi to dispense DMEM medium with re-suspended cells and assay solutions including substrate/lysis and stop solution. The volumes and details are found in Table 1.
Table 1.
HTS Assays Conditions in 1,536-well plate
| Step | Parameters | Value | Time | Description |
|---|---|---|---|---|
| 1 | Dispense of cells in 1,536-wells | 2 µL | - | SV40T fibroblasts from a control (GALC-WT) and GLD patient (GALC-G270D) were seeded into multiple 1,536-well plates. Wells without cells (only medium) were placed in columns 1–2. Control cells were placed in columns 3 and 4. GLD patient cells are seeded into columns 5–48. Cells cultured in DMEM medium with 10% FCS at 37°C and 5% CO2 |
| 2 | Incubation | - | 12h | 37°C and 5% CO2 |
| 3 | Pin dispense | 23 nL | - | LOPAC compounds are diluted in DMSO |
| 4 | Incubation | - | 48h | 37°C at 5%CO2 |
| 5 | Dispense | 2 µL | - | 0.2 m HMUGal – substrate buffer – final [HMUGal] of 0.1 mM |
| 7 | Incubation | - | 17h | GALC assay performed at 37°C |
| 8 | Dispense | 6 µL | - | Stop reaction buffer – 0.5 M NaHCO3/0.5 M NaCO3 + 1 mg/mL of TCD |
| 9 | Fluorescence Readout | Ex=404 nm; Em=450 nm | - | *CCD-based Viewlux reader |
Charged-couple device (CCD)
Quantitative cell-based GALC HTS pilot assay
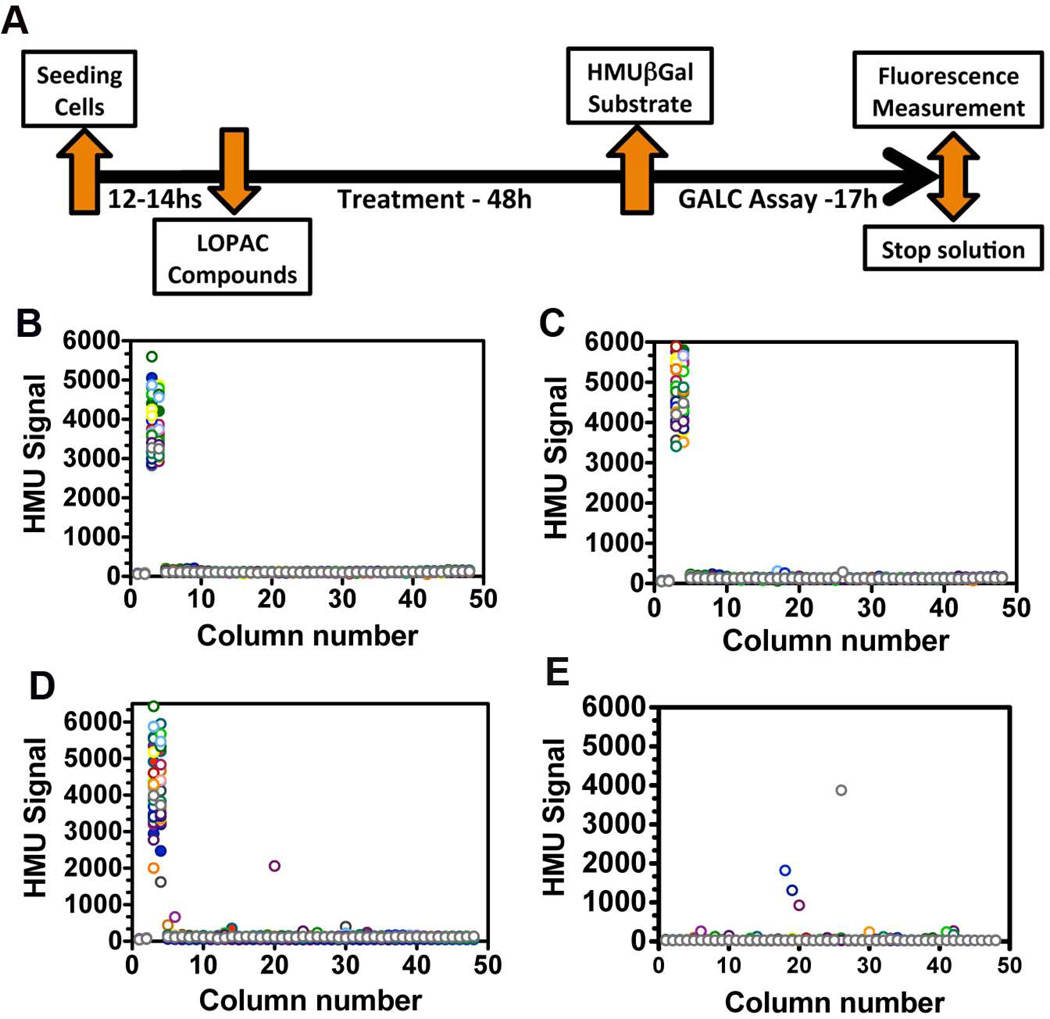
A collection of 1,280 pharmacological compounds from LOPAC (Sigma- Aldrich Inc.) was used to perform a pilot HTS. One single 1,536-well plate is able to accommodate the entire LOPAC library and necessary controls. SV40T fibroblasts from a GLD patient (GALC-G270D) and control (GALC-WT) were used in the pilot HTS. Six different concentrations of LOPAC library were tested: 4.6, 9.2, 23, 46, 115 and 230 µM. Compounds were transferred to 1,536-well plates via Kalypsys Pin-Tool equipped with a 1,536-pin array (V & P Scientific, Palo Alto, CA). The pin used was FP1S10 (0.457) mm diameter (FP1; V & P Scientific) with a withdrawal speed of 0.5 cm/s, dispensing 23 nL into wells containing 2 µL of culture medium [22]. Using this pin-tool transfer protocol, the concentration indicated above was achieved with single- or double-tool transfer from the three prepared stock concentrations in three LOPAC stock concentrations of 0.4, 2 and 10 mM. For instance, concentrations of 4.6 µM and 9.2 µM were achieved through single or double-pinning of the 0.4 mM LOPAC stock. In a similar fashion, the 2 mM LOPAC stock was used to reach treatment concentrations of 23 µM (single-pinning) and 46 µM (double-pinning), and the 10 mM LOPAC stock to prepare the 115 µM (single-pinning) and 230 µM (double-pinning) treatment concentrations. One additional 1,536-well plate, in which cells were treated with only DMSO, was performed as a control plate. Cells were then exposed for 48h-treatment period with LOPAC compounds (one compound/well) at the six different concentrations. After treatment, substrate/lysis buffer was dispensed in each well as above described and depicted in figure 4 and Table 2.
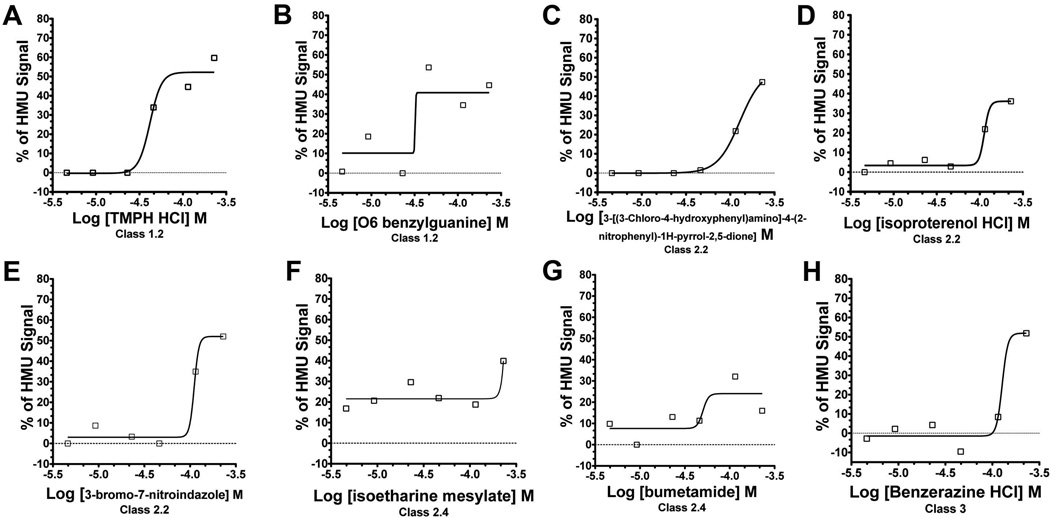
Figure 4. Cell-based GALC HTS design and scatter plot results from the quantitative pilot HTS against the LOPAC library.
(A) The time-line of events of the live-cell HTS assay. Since the HTS assay was quantitative, the 1,280 small molecules from the LOPAC were tested in each plate. Scattered plot results from the HTS against LOPAC is shown in panels B-E. Each color represents a different column of 1,536-well plate. In each panel, column numbers 1 and 2 represent HMU fluorescence signals from wells without cells (only medium); columns 3 and 4 depicts HMU signals from wells with control cells with wild type (WT) GALC (highest HMU signals), which were not exposed to small molecules. Columns 5 to 44 contained GLD patient cells (GALC-G270D) treated with LOPAC library. In columns 45-48, GLD patient cells received only the solvent of the chemical compounds (DMSO). The 1,536-well plates treated with the solvent only DMSO (0.57%; B), the lowest (4.6 µM; C) and the highest (230 µM; D) LOPAC concentrations. (E) Scatter plot of a 1,536-well plate containing only medium (no cells) and with the highest LOPAC treatment concentration (230 µM).
Table 2.
Small molecules from LOPAC that showed active concentration-response curves (CRCs) of class 1 to 3 in the pilot HTS.
| Small Molecule Name | Molecular Weight |
CAS number | Label | CRC class |
|---|---|---|---|---|
| 2,2,6,6-tetramethylpiperidin-4-yl heptanoate HCl (TMPH) | 305.88 | 849461-91-2 | SM-1 | 1.2 |
| O6-benzylguanine | 241.25 | 19916-73-5 | SM-2 | 1.4 |
| 3-[(3-Chloro-4-hydroxyphenyl)amino]-4-(2-nitrophenyl)-1H-pyrrol-2,5-dione or SB415286 | 359.72 | 264218-23-7 | SM-3 | 2.2 |
| isoproterenol HCl or (−)-Isoprenaline hydrochloride, (−)-N-Isopropyl-L-noradrenaline hydrochloride, (R)-3,4-Dihydroxy-α-(isopropylaminomethyl)benzyl alcohol hydrochloride | 247.72 | 5984-95-2 | SM-4 | 2.2 |
| guanabenz acetate salt | 291.13 | 23256-50-0 | SM-5 | 2.2 |
| 4-cyclohexylmethoxy-2,6-diamino-5-nitrosopyrimidine or NU6027 | 251.28 | 220036-08-8 | SM-6 | 2.2 |
| 3-Bromo-7-nitroindazole | 242.03 | 74209-34-0 | SM-7 | 2.2 |
| isoproterenol (+)-bitartrate salt | 361.34 | 54750-10-6 | SM-8 | 2.2 |
| 4-chloro-DL-phenylalanine methyl ester HCl | 250.12 | 14173-40-1 | SM-9 | 2.2 |
| edrophonium chloride | 201.69 | 116-38-1 | SM-10 | 2.4 |
| bromopheriramine maleate | 435.31 | 980-71-2 | SM-11 | 2.4 |
| isoetharine mesylate salt or 4-[1-Hydroxy-2-[(1-methylethyl)amino]butyl]-1,2-benzenediol mesylate salt | 335.42 | 7279-75-6 | SM-12 | 2.4 |
| bumetanide or 3-(Aminosulfonyl)-5-(butylamino)-4-phenoxybenzoic acid | 364.42 | 28395-03-1 | SM-13 | 2.4 |
| benserazide HCl or DL-serine 2-(2,3,4-trihydroxybenzyl)hydrazide HCl | 293.70 | 14919-77-8 | SM-14 | 3 |
| pyridostigmine HCl or 3-(Dimethylaminocarbonyloxy)-1-methylpyridinium bromide | 261.12 | 101-26-8 | SM-15 | 3 |
| R(−)-2,10,11-Trihydroxy-N-propylnoraporphine hydrobromide hydrate | 392.29* | 79640-85-0* | SM-16 | 3 |
| nialamide or N-Benzyl-β-(isonicotinylhydrazino)propionamide NIsonicotinoyl-N’-[β-(N-benzylcarboxamido)ethyl]hydrazine Pyridine-4-carboxylic 2-[2-(benzylcarbamoyl)ethyl]hydrazide | 298.34 | 51-12-7 | SM-17 | 3 |
| (+/−)-cis-Piperidine-2 | 173.17 | 46026-75-9 | SM-18 | 3 |
| alloxapine or benzo[g]pteridine-2,4(1H,3H)-dione Isoalloxazine | 214.18 | 490-59-5 | SM-19 | 3 |
SM, small molecule;
anhydrous base
Validation of selected small molecules
Primary cultured skin fibroblasts from control (GALC-WT) and GLD patients were seeded into 96- and 384-well plates as described above. Candidate compounds presenting concentration-response curves (CRCs) of classes 1, 2 and 3 in the HTS were tested in primary cells from 2 different GLD patients with GALC-G270D and GALC-G553R mutants. The following compounds were purchased from Sigma-Aldrich Inc. and used in these assays: 2,2,6,6-tetramethylpiperidin-4-yl heptanoate (TMPH) HCl, O6-benzylguanine, 3-[(3-Chloro-4-hydroxyphenyl)amino]-4-(2-nitrophenyl)-1H-pyrrol-2,5-dione or SB415286, isoproterenol HCl, guanabenz acetate salt, 4-cyclohexylmethoxy-2,6-diamino-5-nitrosopyrimidine or NU6027, 3-bromo-7-nitroindazole, isoproterenol (+)-bitartrate salt, 4-chloro-DL-phenylalanine methyl ester HCl, isoetharine mesylate salt, bumetanide, benserazide HCl, pyridostigmine HCl and nialamide. All compounds were diluted in dimethyl sulfoxide (DMSO) initially to a stock-concentration of 50 mM. Cells were grow to confluence in 384-well plates and then treated in quadruplicates in 5 concentrations: 1.9, 5.6, 16.7, 50 and 150 µM in DMEM with 10% additional FCS (10%) for 5 days. After five days, medium was removed, cells were washed twice with PBS followed by GALC assay in cultured plates using fluorescent HMUGal substrate buffer as earlier described. HMUGal was standardized by average of protein level/well, which was measured by Bradford method [20].
Other lysosomal enzyme assays
Measurements of total β-hexosaminidase (Hex), β-galactosidase and glucocerebrosidase enzymatic activity were performed using cultured cell lysates from primary and SV40T fibroblasts as previously described [12; 17].
Cytotoxicity assay
Small molecules with active CRCs were later tested for toxicity effect in primary cells of controls and GLD patients. After 48h treatment course with different concentrations of selected small molecules, cell viability assays were performed in 96-well plates utilizing CellQuant-Blue™ reagents as protocol and described earlier [17]. This is a non-radioactive fluorescent assay that is available through BioAssay Systems.
Data Analysis
Where applicable, data are expressed as the mean ± standard deviation (S.D.). Comparisons of parametric data were analyzed in the use of conventional parametric statistical methods including two-tail Student’s t test. The statistical test Z’ factor was used to measure the quality of the assay and its robustness for HTS screening [23]. This single statistic takes into consideration both signal-to-noise and reproducibility. Assays with a Z’-factor > 0.5 are considered to be robust enough for HTS [23]. Coefficient of variation (CV) and signal-to-noise ratios were also calculated when comparing signals from the enzymatic activity present in the control (GALC-WT) and the GLD patient cells (GALC-G270D). Concentration-curve response (CRCs) analysis for compound dose responses was performed utilizing non-linear regression curve fit using Prism version 5.0d.
RESULTS
Characterization of transformed GLD patient fibroblasts used in the HTS
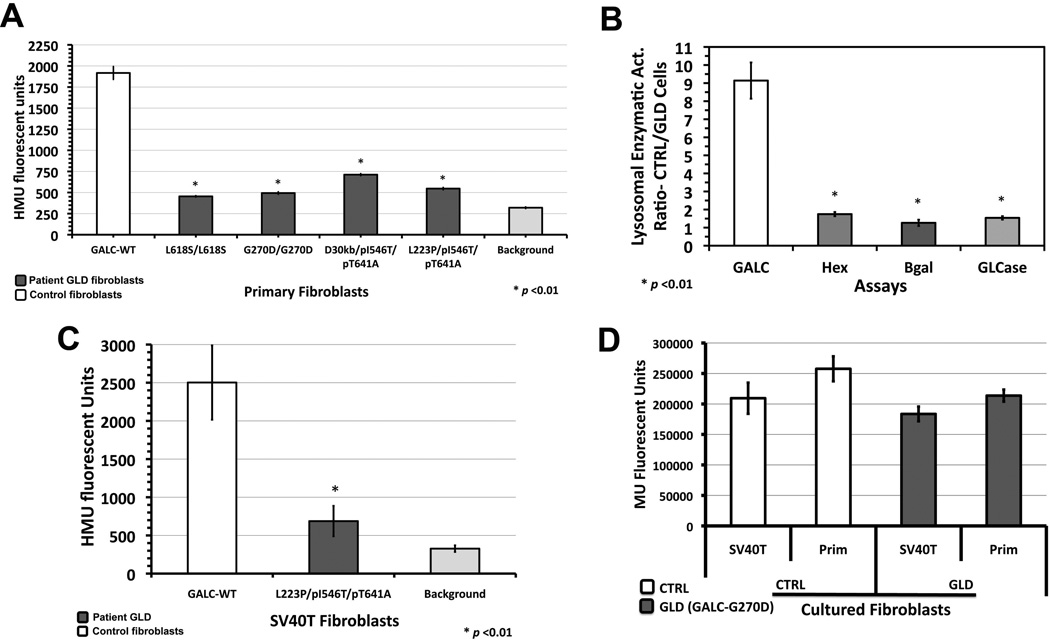
The diagnosis of GLD is established by measurement of GALC activity in peripheral leukocytes or cultured fibroblasts [2]. The GALC enzymatic assay is done using either the radioactive substrate, H3-galactose-ceramide [24] or the fluorescent substrate, 6-hexadecanoylamino-4-methylumbelliferyl-β-D-galactopyranoside (HMUGal) [18]. Based on its sensitivity and specificity, HMUGal was utilized to assay the enzymatic activity of GALC in primary skin fibroblasts from GLD patients with late onset forms of GLD (juvenile and adult) [2]. A collection of skin fibroblast lines from GLD patients with different clinical forms was assembled and the levels of residual GALC enzymatic activity were determined in 96-well plate format (Fig.1A and B). The cellular GALC assay was able to distinguish the residual enzymatic activity present in different GLD patients’ cells with different mutations (Fig.1A). In order to pursue a large-scale HTS campaign and assure sufficient number of cells, the GLD patient primary fibroblasts were successfully transformed with large T antigen from simian virus-40 (SV40). Transformed (SV40T) cells showed conservation of several biochemical aspects of primary cells they were originated. When culturing SV40T from controls and GLD fibroblasts, a general increase in the HMU fluorescence signal per well was observed (Fig.1C). No specific alterations in the levels of GALC enzymatic activity as well as other lysosomal enzymes were observed (Fig.1D). These cells showed an increased rate of proliferation in comparison to primary cells, as they are less inhibited by contact. In 75-cm2 flasks, SV40T fibroblasts reach confluence within 1–2 days, and their primary fibroblasts counterparts require on average 3–5 days. Once flasks are confluent, SV40T and primary fibroblasts were trypsinized, re-suspended in cultured medium (same volume) and automatic cell counting was performed for each cell line. The cell-number ratio of SV40T/primary cells was 2.89±0.07 and 3.39±0.59 for controls and GLD patient (GALC-G270D) cells, respectively.
Figure 1. Comparison of primary and SV40 transformed GLD patient fibroblasts.
(A) Using fluorescent synthetic substrate HMUGal, primary cultured skin fibroblasts from controls with wild type (WT) GALC showed a higher enzymatic activity than several GLD patient fibroblasts with described GALC mutants. (B) The ratio of enzymatic activity of other lysosomal enzymes control/GLD cells including β-hexosaminidase (total), β-galactosidase (Bgal) and glucocerebrosidase (GLCase) was close to 1 demonstrating that other lysosomal enzymes are intact in GLD primary cultured cells. (C) SV40-transformed GLD patient fibroblasts preserved the deficient GALC activity with a measurable enzymatic activity. (D) When SV40 transformed cells were compared to primary cells from which they originated, no significant difference of lysosomal enzymatic activity levels were observed as shown by glucocerebrosidase activity using synthetic substrate 4-methylumbelliferyl β-D glucopyranoside (MUGlc).
Miniaturization of cell-based GALC assay using SV40 transformed GLD patient fibroblasts
Considering the advantages of using a cellular assay in the HTS assay for small molecules, a cell line with consistent and measurable levels of residual GALC activity is crucial for the success of the HTS. The SV40T fibroblasts with GALC-G270D mutant showed consistent levels of residual GALC activity. Based on the crystal structure of GALC [5], the G270D missense mutation results in an acidic side chain introduced near the loop of β-sandwich, which can result in significant instability and misfolding [5]. This mutation represents nearly 50% of patients with adult onset clinical presentation [2]. On the basis of the above data, SV40T GLD patient cell line with GALC-G270D was subsequently selected for the cell-based HTS.
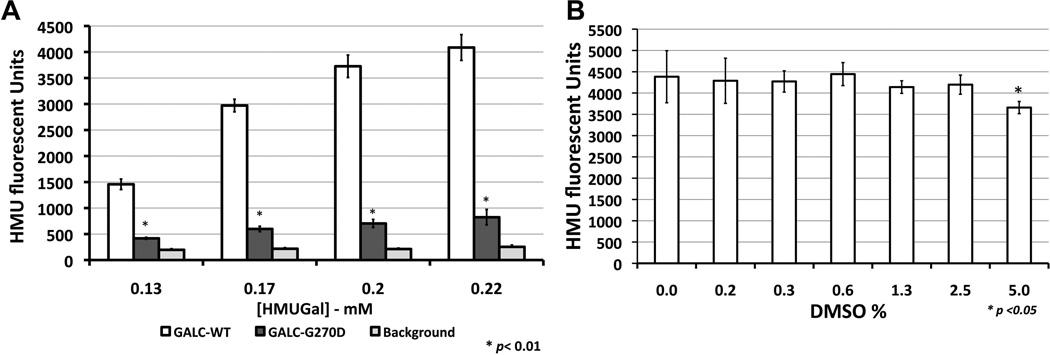
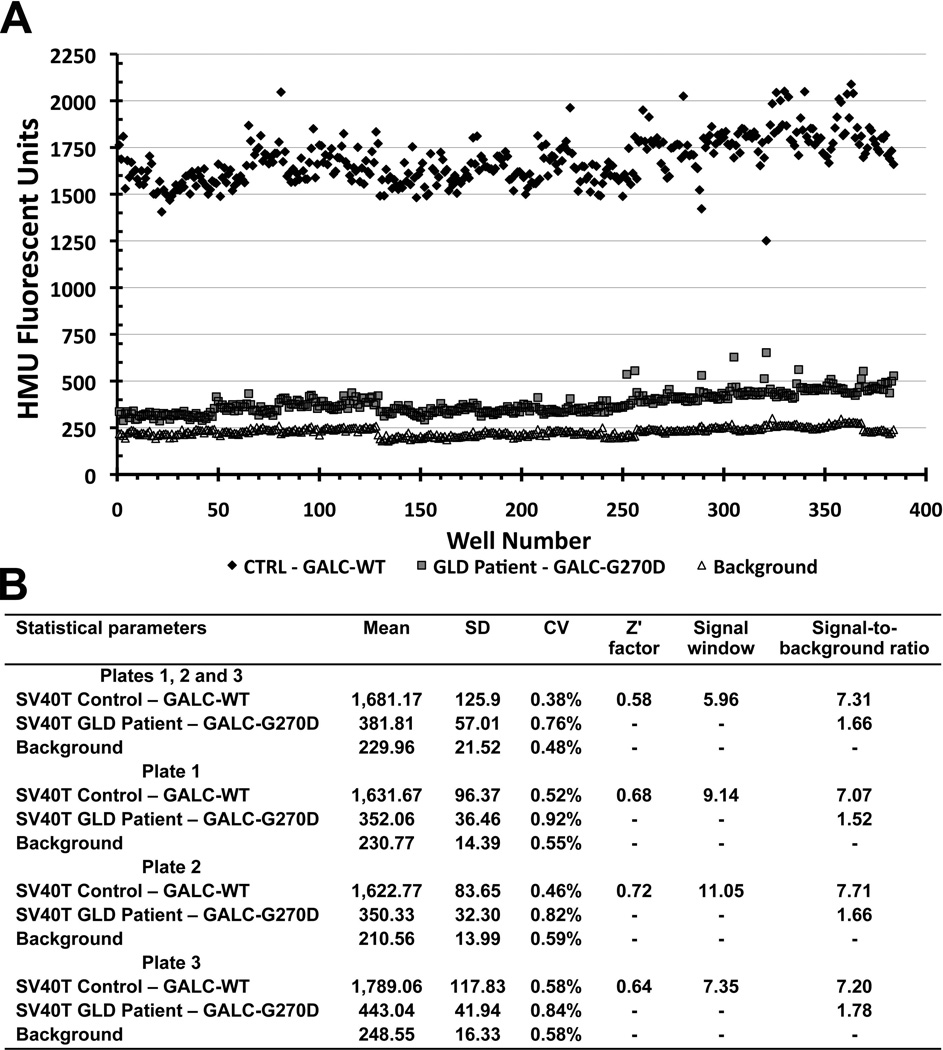
In order to adapt the assay into a multi-well plate setting and HTS adaptive protocol prioritizing reagent addition-only and no reagent removals steps, the ideal HMUGal substrate concentration was determined in citrate-buffer at pH 5.2 (Fig.2A). Once flasks become confluent, cells were re-suspended and transferred to 384-well black clear and flat-bottom microplates. In the initial experiments, on average 20–25×103 cells were dispensed per well. The substrate buffer needed to be slightly modified from the one routinely used in biochemical diagnostic assays [18]. In the high-throughput setting, the HMUGal buffer was dispensed directly into wells containing cells in culture medium. Sodium taurocholate is used routinely to stabilize the GALC enzyme. Here, we slightly increased sodium taurocholate concentration in the HMU substrate buffer to 1 mg/mL, promoting cell lysis during the incubation period for 17hs at 37°C. After stopping the assay with sodium carbonate buffer (0.5M; pH 10.7), HMU fluorescence signals were measured at λexcitation 404 nm and λemission 450 nm. The tolerance assay to DMSO (solvent in which compounds are dissolved) showed that stability of the GALC assay at concentrations of 2.5% or lower of DMSO in the assay solution (Fig.2B). Plate uniformity assessment was performed to test interplate assay variability (Fig.3). Three 384-well plates were prepared with fresh reagents in separate days (Fig.3B-D). The combined analysis showed a Z’ factor of 0.58 and along with coefficient of variation, signal window and signal-to-background ratio reflected the robustness of the cell-based biochemical GALC assay (Fig.3A and 3E). In addition, the SV40T GLD patient cells (GALC-G270D) showed consistent levels of residual GALC activity (Fig.3A). A slight drift was observed in SV40T GLD patient fibroblasts in plate 1 likely due to lack of full homogenization of the harvested cells in culture DMEM medium seeding into the multi-well plates (Fig.S1).
Figure 2. Development of GALC assay in multi-well plates.
Optimization of the assay was performed in multi-well plates. (A) Substrate concentration assays were performed using cultured SV40T skin fibroblasts in 384-well plates. The cells were cultured to full well-confluence and before starting the GALC assay. Substrate concentrations in the assay reaction over 0.2 mM of HMUGal generate elevated signal-to-background ratios (>5). GALC enzymatic activity from control cells with wild type (GALC-WT) produced higher HMU fluorescence signal (white bars). HMUGal substrate was able to detect a small residual activity of SV40T mutant GALC (GALC-G270D – dark gray columns) above the background, which represent fluorescence signals from wells with culture medium only (no cells). (B) The DMSO assay was performed in SV40T control cells (GALC-WT) demonstrated that GALC activity is reduced as DMSO concentration is increased in reaction solution. The DMSO concentrations of 2.5% or lower showed not to affect GALC enzymatic activity.
Figure 3. Plate uniformity assessment of the proposed GALC assay in 384-well plates.
The SV40T fibroblasts from control (CTRL) and a GLD patient (GALC-G270D/G270D) were cultured in three 384-well plates. (A) Scatter plot is shown representing assays performed in three interleaved 384-well plates on different days using freshly prepared reagents. CTRL, GLD patient cells and wells containing no cells (only medium) were displayed in interleaved plate format (in different column-positions of each plate). In scatter plot A (combined analysis of plates1, 2 and 3), the response was plotted against well number, ordered firstly by row and then by column. In CTRL cells, GALC-WT activity (black diamonds) generated consistently higher HMU fluorescence signals than those from GLD patient cells (with GALC-G270D; gray squares). Residual GALC-G270D activity was measurable above background signal (white triangles). (B) Table describes the mean, standard deviation (SD), coefficient of variation (CV), Z’ factor, Signal Window (SW) and signal to background from the 3 interleaved combined analysis along with the individual analysis of each plate. Consistency of the results of the 3 different interleaved-plates was observed. For the purpose of calculations (Z’ factor and signal window), HMU fluorescence signal obtained from CTRL (GALC-WT) cells was considered as maximum, and fluorescence signal from GLD patient cells (G270D-GALC) as minimum. The Z’ factor value for the plate uniformity assessment was 0.58.
Pilot HTS GALC in 1,536-well plates using a small collection of drug library
Based on assay parameters established for 384-well plates, the cell-based biochemical HTS assay for GALC activity was adapted to 1,536-well plates (48 columns with 32 wells each). The conditions for 1,536-well plate assay are described in Table 1. In order to assess the robustness of the HTS assay developed, a pilot screen was done of the Library of Pharmacologically Active Compounds (LOPAC) containing 1,280 small molecules. The specific time-points and assay design are depicted in figure 4A. The SV40T GLD patient fibroblasts (GALC-G270D) were used and seeded in columns 5 to 44 of 1,536-well microplates. Since no specific small molecule that increases GALC-G270D enzymatic activity is known, SV40T control fibroblasts harboring the GALC-WT were used as control and seeded in columns 3 and 4 of each plate. Columns 1 and 2 contained only culture medium (no cells) and were used as blank-wells. Columns 45–48 contained GLD cells exposed only to DMSO, solvent used for LOPAC compounds. On average 4,000 cells were dispensed per well. Using this plate design, all the 1,280 compounds of the LOPAC could be accommodated in one single 1,536-well plate: columns 5 to 44. Six different concentrations of the LOPAC compounds were tested: 4.6, 9.2, 23, 46, 115 and 230 µM. SV40T GLD patient fibroblasts were treated for 48hs with the LOPAC concentrations described above. One additional plate was prepared with the GLD patient cells in columns 5–44 treated only with DMSO. This plate was used as a reference for treated plates. No false-positives were observed in the plate treated only with DMSO (Fig.4B). False-positives were defined as plate-wells with HMU fluorescence signals above 3 times the standard deviations from signal of the entire DMSO plate (Fig.4B). The assay showed robust statistical parameters with an average Z’ factor of 0.58, signal-to-background ratio of 34-fold and coefficient of variation (CV) of 3.05% for control cells (GALC-WT) and 0.76% for GLD patient cells (GALC-G270D). Figure 4 shows the scatter plots from the plate treated only with DMSO (Fig.4B) and the plates treated with the lowest (4.6 µM; Fig.4C) and highest (230 µM; Fig.4D) concentrations of the LOPAC. In order to identify for small molecules that carry inherent fluorescence at the HTS conditions, a 1,536-well plate containing no cells and only the highest treatment concentration of small molecules (230 µM) was prepared and assayed in parallel (Fig.4E). The plot results from the remaining four concentrations (9.2, 23, 46 and 115 µM) are shown in supplemental data (Fig.S2).
Concentration-response curve analysis of pilot quantitative HTS GALC assay
The main reason for performing the pilot GLD cell-based HTS assay in multiple concentrations of the LOPAC library is to allow selection of small molecules based on their active concentration response-curves (CRCs) generated, i.e. a dose-response enhancement of GALC residual enzymatic activity [22]. Based on the classification of the CRCs previously described [17; 22] (Table S1 in supplemental data), 19 small molecules (SM) showed active CRC patterns within classes 1 to 3 (Table S1). In this HTS assay, an active CRC represents the percentages of HMU signal increase (y axis) as a function of the increasing SM concentrations (x axis) used to treat the SV40T GLD patient fibroblasts in the 1,536-well plates (Fig.5). Of note, small molecules that showed inherent fluorescence on 1,536-well plates containing no cells and the highest concentration of LOPAC (230 µM; Fig.5D) were excluded. Table 2 describes the small molecules with their respective CRCs. One small molecule showed active CRC of class 1.2 with partial efficacy <80% and good fit r2≥0.9 (SM-1; Fig.5A) and another showed active CRC of class 1.4 with also partial efficacy <80% but poor fit r2<0.9 (SM-2; Fig.5B). Eleven other small molecules showed active CRCs of class 2 (partial curves: only one asymptotes): 7 with CRCs of class 2.2 (Figs.5C, 5D and 5E) - partial efficacy (<80%) and good fit (r2≥0.9); 4 with CRCs of class 2.4 (Fig.5F and 5G) - partial efficacy (<80%) and poor fit (r2<0.9). Six other small molecules showed active CRCs of class 3: “single point-activity” as shown in SM-14 (Fig.5H). The remaining small molecules with active CRCs are depicted in figure S3 (supplemental data).
Figure 5. The concentration-response curve analysis from the GALC quantitative HTS assay against LOPAC library.
In the cell-based HTS for GALC using LOPAC concentrations ranging 4.6 - 230 µM, 19 small molecules (SMs) presented active concentration-response curves (CRCs). For each SM, a CRC is produced by the percentages of HMU signal increase (y axis) as a function of the different SM concentrations (x axis) used to treat the GLD patient fibroblasts. Two small molecules showed CRCs of class 1 (complete curves; high and partial efficacies): TMPH HCl (SM-1) – class 1.2 (A) and O6 benzylguanine (SM-2) – class 1.4 (B). Seven small molecules showed CRCs curve of class 2.2 (partial curve; r2≥0.9; efficacy≤80%) and three of them are shown: 3-[(3-Chloro-4-hydroxyphenyl)amino]-4-(2-nitrophenyl)-1H-pyrrol-2,5-dione (SM-3;C); isoproterenol HCl (SM-4; D); 3-bromo-7-nitroindazole (SM-7; E). Four other SMs presented class 2.4 CRCs (partial curve; r2≤0.9; efficacy Min.-80%): including isoetharine mesylate (SM-12; F) and bumetamide (SM-13; G). Six molecules presented class 3 CRCs characterized by “single-point of activity”. Benzerazine HCl (SM-14; H) is one of the SMs showing class 3 CRCs.
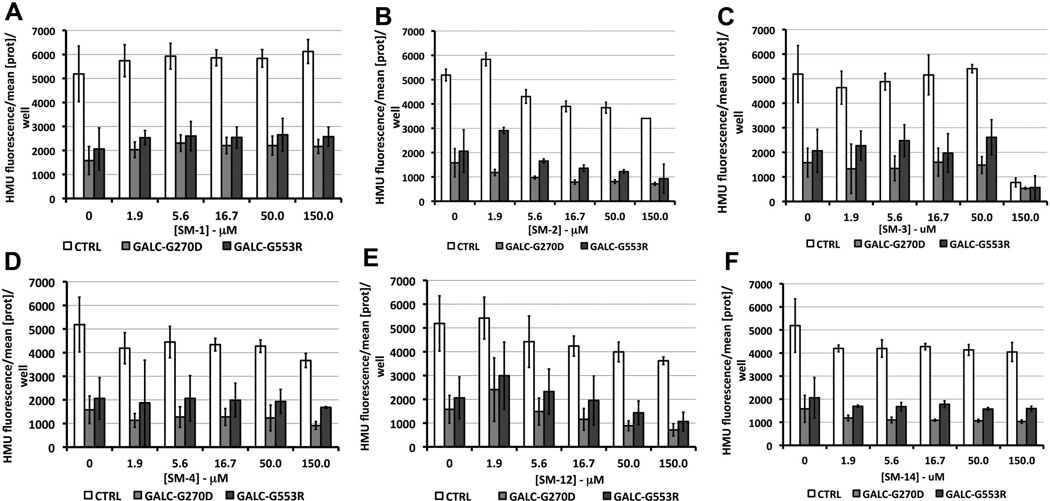
Confirmation of small molecule with active CRCs in primary GLD patient fibroblasts
Since this is a novel cell-based HTS assay for GALC enhancers, no specific cut-off and criteria for selection of the small molecules with active CRCs is known. Therefore, all small molecules from class 1 and 2.2 along with representatives of classes 2.4 and 3 were studied further. Primary cells from controls and two GLD fibroblasts cells containing GALC-G270D and GALC-G553R, which is also predicted to result in misfolded GALC [5], were tested at multiple concentrations of the small molecules ranging from 1.9 to 150 µM (Fig.6). Slight increases in the residual enzymatic activity of GALC-G270D, GALC-G553R and GALC-WT were observed with TMPH HCl (SM-1) and O-6 benzylguanine (SM-2), two small molecules with CRCs of class 1 (Figs.6A and 6B). However, none of these enzymatic activity enhancements were statistically significant. Other small molecules with CRCs of class 2.2, 2.4 and 3 showed no significant enhancements and some displayed a degree of cytotoxicity reflected by reductions of GALC enzyme activity (Figs.6C), which was later confirmed in cell-viability assays (Fig.S5 in supplemental data). The effects of GALC enzymatic activity in control and GLD primary cells of other small molecules with CRCs of class 2 and 3 are shown in Fig.S4 in the supplemental data.
Figure 6. Treatment of primary fibroblasts from GLD patients and control fibroblasts with small molecules with active concentration-response curves.
Several of the small molecules (SMs) that demonstrated active concentration-response curves (CRCs) of classes 1 to 3 were used to treat two primary fibroblast lines from GLD patients with GALC-G270D and GALCG553R mutants. A primary fibroblast control with wild type GALC-WT was also tested. Two compounds presented CRCs of class 1, TMPH HCl (SM-1; A) and O6-benzylguanine (SM-2; B). Other SMs tested and represented here showed CRCs of class 2.2 including 3-[(3-Chloro-4-hydroxyphenyl)amino]-4-(2-nitrophenyl)-1H-pyrrol-2,5-dione (SM-3; C) and isoproterenol HCl (SM-4; D). Two representatives of SMs with CRCs of class 2.4, isoetharine mesylate (SM-12; E) and CRCs of class 3, benserazine HCl (SM-14; F), are also shown in this figure. In each primary fibroblast cell line, the average level of protein concentration per well each 96-well plate was used to standardize the HMU fluorescence signals. None of the increases of HMU fluorescence signals compared to non-treated cells were statistically significant (p<0.05).
DISCUSSION
GLD (or Krabbe disease) is a devastating neurological LSDs caused by the deficiency of GALC, a lysosomal hydrolase that removes the galactose moiety in the β-anomeric configuration from specific glycosphingolipids including galactosylceramide and galactosylsphingosine, known as psychosine [6]. The progressive loss of oligodendrocytes and myelin, and subsequent reactive astrocytic gliosis and infiltration of the unique “globoid cells” are the pathologic hallmarks which labeled the name of “globoid-cell leukodystrophy” for this LSD [25]. As in other LSDs, clinical problems are not manifested unless GALC mutations encode a mutant GALC that functions below a critical threshold [11; 26]. In GLD patients, GALC residual enzymatic activity is detectable as mostly GALC missense mutations encode misfolded mutant enzyme that retains a low percentage of catalytic activity, although still below the critical threshold (usually ~10% of wild type enzyme) [11]. Therefore, based on the pathogenesis of GLD, enhancements of mutant GALC residual activity just above the critical threshold can prevent the psychosine elevation, loss of oligodendrocytes and subsequent demyelination. Clinically, this will potentially arrest and/or reverse the neurological disease process in GLD.
Small molecule based-therapies, which are more likely to cross the BBB, have a substantial role in treating the brain disease in LSDs. Specific small molecules have shown to enhance the levels of the deficient lysosomal enzymes in several LSDs including GM2 gangliosidosis [12; 27] and Gaucher disease [12; 13; 14; 15; 16] by assisting the folding of mutant enzymes preventing their early degradation by ERAD. Other small molecules for Fabry disease and GM1 gangliosidosis had been characterized earlier [28]. Here a cell-based HTS assay using a representative GLD patient cell line (GALC-G270D) is firstly reported for the discovery of small molecules that promote enhancements of the mutant GALC enzymatic activity. The main advantage of the developed cell-based HTS assay is the possibility to identify small molecules that promote enhancements of the residual GALC-G270D activity levels by diverse mechanisms. Small molecules that assist the folding of unstable the mutant GALC-G270D by direct interactions, functioning as pharmacological chaperones (PCs), can be identified by the developed HTS assay. Other small molecules that increase the GALC-G270D levels by interactions with alternative pathways, critical for the protein folding (ER folding pathways) and degradation (ERAD) will be also identified [29]. These small molecules are known as proteostasis regulators (PRs) [29]. Another advantage of the reported HTS assay is the utilization of GLD patient cells already in the primary screen stage, enabling access to several potentially altered molecular pathways and consequently targets that will be exposed to the tens or hundred thousands small molecules. Thus, through this HTS assay, novel molecular pathways that interact indirectly with the GALC mutant folding, degradation and maturation pathways may be identified which, after characterization, can be used as therapeutic targets. The transformation of primary fibroblasts with pSV3 plasmid carrying the SV40 large T-antigen was necessary to provide the high number of cells required to a quantitative HTS of large chemical libraries. The SV40T cells from GLD patients and controls conserved the biochemical characteristics of primary cells as described earlier [21]. By using a fluorescent substrate, HMUGal, routinely used in the biochemical diagnosis of GLD, a robust HTS assay was developed using a GLD patient fibroblast carrying GALC-G270D mutant. Based on the current GALC crystal structure [5], the G270D mutation along with several others disease-associated missense mutations buried within the structure of GALC are predicted to severely affect its folding and stability. These GALC mutants are amenable to be rescued by small molecules [5]. One small molecule, α-lobeline, showed increases of nearly 50% of residual enzymatic activity of the GALC-D528N mutant, but failed to do so in other GALC mutants [19]. It is important to state that the GLD patient cell line with the GALC-G270D was chosen for the HTS as it is one the most common GALC mutants associated with the late onset GLD forms and showed consistently measurable residual enzymatic activity confirmed in high-throughput assay settings [2; 3] (Fig.3). In the plate uniformity assessment, consistent Z-factors >0.5 were observed (Fig.3A and 3E). The slight drift was observed in SV40T GLD patient fibroblasts in plate 1 (Fig.S3A) may be due cell seeding problems. When preparing multi-well plates, after trypsinazing and re-suspending from 75-cm2 tissue culture flasks, fibroblasts not fully homogenized in the tissue culture medium can result in non-uniform cell seeding, which can explain the increased GALC activity in some columns of the multi-well plate.
The developed GLD patient cellular assay showed robustness and feasibility when automated in 1,536-well plates for a quantitative pilot HTS against a small compound collection (LOPAC). The SV40T GLD patient fibroblasts were treated for 48h because small molecules that either function as PCs or PRs require a minimum time to assist the folding of misfolded GALC mutant, evading early degradation (ERAD) and increasing its level in the lysosomal compartment [12; 29]. The 1,536-well plate containing no cells and only the highest treatment concentration of small molecules (230 µM; Fig.4E) was able to detect some small molecules with inherent fluorescence. However, some compound fluorescence signals were not observed in the plate with SV40T GLD patient cells, which was also treated with 230 µM of LOPAC (Fig.4D). In fact, after GALC assay was performed in fibroblasts treated with some of the small molecule hits from LOPAC, quenching of the compound inherent fluorescence was observed (Fig.S6). This observation may explain the differences between the plate with only medium (Fig.4E) and the one with SV40T fibroblasts (Fig.4D). The plot results from the remaining four concentrations (9.2, 23, 46 and 115 µM) are shown in supplemental data (Fig.S2). The pilot HTS identified 19 small molecules demonstrating active CRCs of classes 1 to 3 (Figs.5 and S3), however none of those were confirmed to increase the residual GALC activity in two primary fibroblasts lines from GLD patients (Figs.6 and Fig.S4). Since this is the first patient cell-based HTS assay for GALC, the selection criteria of candidate “hits” has not been established. The treatment of primary fibroblasts from GLD patients with these small molecules, considering their respective CRC classes, generated informative conclusions. First, only two small molecules presented CRCs of class of class 1 (complete curve with two asymptotes) and one of them (SM-1) showed a good curve fitness (r2>0.9) (Table S1, supplemental data) [22]. Both small molecules showed partial efficacies (<80%), here represented as % of HMU signal enhancements (Figs.6A and 6B). These results indicate that small molecules showing CRCs of class 1 with efficacies within 30–60% are unlikely to be confirmed as “hits”. In addition, none of the eleven small molecules presenting CRCs of class 2 (partial curves with one asymptote) and nor the six molecules with CRCs of class 3 (single-point activity) were confirmed as GALC enhancers in primary GLD patient cells. Given the considerable number of representatives within these two CRC classes identified in the pilot HTS, CRCs of class 2 and 3 are unlikely to correspond to potential “hits”, in particular with CRC presenting partial efficacies (<80%). Since none of the 19 small molecules in the cell-based GALC HTS assay showed active CRCs with complete efficacy (>80%) based on the established CRC classification (Table S1, supplemental data) [22], efficacy may be a key CRCs parameter in the developed assay that can be useful to prioritize and further selected small molecule candidates when screening other libraries. Therefore, prioritizing small molecules with active CRCs with higher efficacies, i.e. 100% or even 200% activation, may produce a higher validation rate. Additionally, screening large libraries will increase the likelihood of identifying the small molecules with active CRCs with higher efficacies. Second, in previous studies of HTS assays using recombinant or purified lysosomal enzymes, small molecule were only confirmed as “hits” and subsequently PCs, when tested at different concentrations in cultured primary fibroblasts from patients with different mutant enzymes [12; 13]. Third, as the developed HTS utilizes GLD patient cells already in the primary stage, substantial selectivity is added to the HTS assay. Only small molecules showing dose-response enhancements of residual GALC resulting in active CRCs are identified. Therefore, given small size of chemical compound collection, which is designed for pilot HTS, a low “hit” rate is expected as previously described [17].
In summary, this study describes the development of the first cell-based HTS assay to identify small molecule “enhancers” of GALC using directly GLD patient cells at the primary stage of the screen. The SV40 transformation of primary skin fibroblasts from GLD patients conserved the residual GALC enzymatic activity levels, which was measurable in different high-density microplates. The synthetic fluorescent substrate HMUGal, used in diagnosis of GLD, was useful in measuring consistently the residual enzymatic activity of GALC mutants from different patient cell fibroblasts. The use of GLD patient cells in a quantitative HTS assay will allow the use of CRCs analysis to identify and prioritize the evaluation of potential therapeutic small molecules, as disease-cells are directly exposed to different concentrations of a specific compound library. The implementation of the cell-based HTS assay for GALC enhancers in large and structurally diverse small molecule libraries will identify therapeutic agents to treat this devastating neurological LSD.
Supplementary Material
ACKNOWLEDGEMENT
We are in dept with Elizabeth Wohler B.Sc. and Denise Batista Ph.D. who assisted in locating the cell lines in Cell Bank from Kennedy Krieger Institute, which is funded by Kennedy Krieger lntellectual and Developmental Disabilities Research Center. We are grateful for the assistance of Cassandra Obie B.Sc. and David Valle M.D. for initial assistance to establish the cultured fibroblast lines. One of the cell lines used from patients affected by Krabbe disease was obtained from G. Gaslini Institute - Telethon Genetic Biobank Network (Project No. GTB07001). We are also thankful for Mirella Filocamo Ph.D., Head of Lab Diagnosi Pre e Post-natale Malattie Metaboliche, Coordinator, Telethon Genetic Biobank Network and Gaslini Institute, Genova, Italy.
Abbreviation list
- BBB
blood-brain barrier
- CRC
concentration-response curve
- DMEM
Dulbecco's Modified Eagle Medium
- DMSO
dimethyl sulfoxide
- ER
endoplasmic reticulum
- ERAD
ER-associate degradation
- ERT
enzyme replacement therapy
- FCS
fetal calf serum
- GALC
β-galactocerebrosidase
- GLD
globoid-cell leukodystrophy
- HMU
6-hexadecanoylamino-4-methylumbelliferyl
- HMUGal
6-hexadecanoylamino-4-methylumbelliferyl-β-D-galactoside
- HTS
high throughput screening
- LOPAC
Library of Pharmacologically Active Compounds
- LSD
lysosomal storage disease
- PBS
phosphate-saline buffer
- PC
pharmacological chaperones
- PR
proteostasis regulators
- SM
small molecule
- SV40T
SV40-transformed
REFERENCES
- 1.Rafi MA, Luzi P, Zlotogora J, Wenger DA. Two different mutations are responsible for Krabbe disease in the Druze and Moslem Arab populations in Israel. Hum Genet. 1996;97:304–308. doi: 10.1007/BF02185759. [DOI] [PubMed] [Google Scholar]
- 2.Wenger DA, Rafi MA, Luzi P. Molecular genetics of Krabbe disease (globoid cell leukodystrophy): diagnostic and clinical implications. Hum Mutat. 1997;10:268–279. doi: 10.1002/(SICI)1098-1004(1997)10:4<268::AID-HUMU2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 3.Furuya H, Kukita Y, Nagano S, Sakai Y, Yamashita Y, Fukuyama H, Inatomi Y, Saito Y, Koike R, Tsuji S, Fukumaki Y, Hayashi K, Kobayashi T. Adult onset globoid cell leukodystrophy (Krabbe disease): analysis of galactosylceramidase cDNA from four Japanese patients. Hum Genet. 1997;100:450–456. doi: 10.1007/s004390050532. [DOI] [PubMed] [Google Scholar]
- 4.Fiumara A, Barone R, Arena A, Filocamo M, Lissens W, Pavone L, Sorge G. Krabbe leukodystrophy in a selected population with high rate of late onset forms: longer survival linked to c.121G>A (p.Gly41Ser) mutation. Clin Genet. 2010 doi: 10.1111/j.1399-0004.2010.01572.x. [DOI] [PubMed] [Google Scholar]
- 5.Deane JE, Graham SC, Kim NN, Stein PE, McNair R, Cachon-Gonzalez MB, Cox TM, Read RJ. Insights into Krabbe disease from structures of galactocerebrosidase. Proc Natl Acad Sci U S A. 2011;108:15169–15173. doi: 10.1073/pnas.1105639108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki K. Twenty five years of the "psychosine hypothesis": a personal perspective of its history and present status. Neurochem Res. 1998;23:251–259. doi: 10.1023/a:1022436928925. [DOI] [PubMed] [Google Scholar]
- 7.Svennerholm L, Vanier MT, Mansson JE. Krabbe disease: a galactosylsphingosine (psychosine) lipidosis. J Lipid Res. 1980;21:53–64. [PubMed] [Google Scholar]
- 8.Burrow TA, Hopkin RJ, Leslie ND, Tinkle BT, Grabowski GA. Enzyme reconstitution/replacement therapy for lysosomal storage diseases. Curr Opin Pediatr. 2007;19:628–635. doi: 10.1097/MOP.0b013e3282f161f2. [DOI] [PubMed] [Google Scholar]
- 9.Escolar ML, Poe MD, Provenzale JM, Richards KC, Allison J, Wood S, Wenger DA, Pietryga D, Wall D, Champagne M, Morse R, Krivit W, Kurtzberg J. Transplantation of umbilical-cord blood in babies with infantile Krabbe's disease. N Engl J Med. 2005;352:2069–2081. doi: 10.1056/NEJMoa042604. [DOI] [PubMed] [Google Scholar]
- 10.Duffner PK, Caviness VS, Jr, Erbe RW, Patterson MC, Schultz KR, Wenger DA, Whitley C. The long-term outcomes of presymptomatic infants transplanted for Krabbe disease: report of the workshop held on July 11 and 12, 2008, Holiday Valley, New York. Genet Med. 2009;11:450–454. doi: 10.1097/GIM.0b013e3181a16e04. [DOI] [PubMed] [Google Scholar]
- 11.Conzelmann E, Sandhoff K. Partial enzyme deficiencies: residual activities and the development of neurological disorders. Dev Neurosci. 1983;6:58–71. doi: 10.1159/000112332. [DOI] [PubMed] [Google Scholar]
- 12.Maegawa GH, Tropak M, Buttner J, Stockley T, Kok F, Clarke JT, Mahuran DJ. Pyrimethamine as a Potential Pharmacological Chaperone for Late-onset Forms of GM2 Gangliosidosis. J Biol Chem. 2007;282:9150–9161. doi: 10.1074/jbc.M609304200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maegawa GH, Tropak MB, Buttner JD, Rigat BA, Fuller M, Pandit D, Tang L, Kornhaber GJ, Hamuro Y, Clarke JT, Mahuran DJ. Identification and characterization of ambroxol as an enzyme enhancement agent for Gaucher disease. J Biol Chem. 2009;284:23502–23516. doi: 10.1074/jbc.M109.012393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marugan JJ, Zheng W, Motabar O, Southall N, Goldin E, Westbroek W, Stubblefield BK, Sidransky E, Aungst RA, Lea WA, Simeonov A, Leister W, Austin CP. Evaluation of quinazoline analogues as glucocerebrosidase inhibitors with chaperone activity. J Med Chem. 2011;54:1033–1058. doi: 10.1021/jm1008902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Motabar O, Goldin E, Leister W, Liu K, Southall N, Huang W, Marugan JJ, Sidransky E, Zheng W. A high throughput glucocerebrosidase assay using the natural substrate glucosylceramide. Anal Bioanal Chem. 2012;402:731–739. doi: 10.1007/s00216-011-5496-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng W, Padia J, Urban DJ, Jadhav A, Goker-Alpan O, Simeonov A, Goldin E, Auld D, LaMarca ME, Inglese J, Austin CP, Sidransky E. Three classes of glucocerebrosidase inhibitors identified by quantitative high-throughput screening are chaperone leads for Gaucher disease. Proc Natl Acad Sci U S A. 2007;104:13192–13197. doi: 10.1073/pnas.0705637104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geng H, Whiteley G, Ribbens J, Zheng W, Southall N, Hu X, Marugan JJ, Ferrer M, Maegawa GH. Novel Patient Cell-Based HTS Assay for Identification of Small Molecules for a Lysosomal Storage Disease. PLoS One. 2011;6:e29504. doi: 10.1371/journal.pone.0029504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiederschain G, Raghavan S, Kolodny E. Characterization of 6-hexadecanoylamino- 4-methylumbelliferyl-beta-D- galactopyranoside as fluorogenic substrate of galactocerebrosidase for the diagnosis of Krabbe disease. Clin Chim Acta. 1992;205:87–96. doi: 10.1016/s0009-8981(05)80003-8. [DOI] [PubMed] [Google Scholar]
- 19.Lee WC, Kang D, Causevic E, Herdt AR, Eckman EA, Eckman CB. Molecular characterization of mutations that cause globoid cell leukodystrophy and pharmacological rescue using small molecule chemical chaperones. J Neurosci. 2010;30:5489–5497. doi: 10.1523/JNEUROSCI.6383-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 21.Chatelut M, Harzer K, Christomanou H, Feunteun J, Pieraggi MT, Paton BC, Kishimoto Y, O'Brien JS, Basile JP, Thiers JC, Salvayre R, Levade T. Model SV40- transformed fibroblast lines for metabolic studies of human prosaposin and acid ceramidase deficiencies. Clin Chim Acta. 1997;262:61–76. doi: 10.1016/s0009-8981(97)06527-3. [DOI] [PubMed] [Google Scholar]
- 22.Inglese J, Auld DS, Jadhav A, Johnson RL, Simeonov A, Yasgar A, Zheng W, Austin CP. Quantitative high-throughput screening: a titration-based approach that efficiently identifies biological activities in large chemical libraries. Proc Natl Acad Sci U S A. 2006;103:11473–11478. doi: 10.1073/pnas.0604348103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang JH, Chung TD, Oldenburg KR. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J Biomol Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 24.Mansson JE, Svennerholm L. The use of galactosylceramides with uniform fatty acids as substrates in the diagnosis and carrier detection of Krabbe disease. Clin Chim Acta. 1982;126:127–133. doi: 10.1016/0009-8981(82)90028-6. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki K, Suzuki Y. Globoid cell leucodystrophy (Krabbe's disease): deficiency of galactocerebroside beta-galactosidase. Proc Natl Acad Sci U S A. 1970;66:302–309. doi: 10.1073/pnas.66.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hohenschutz C, Friedl W, Schlor KH, Waheed A, Conzelmann E, Sandhoff K, Propping P. Probable metachromatic leukodystrophy/pseudodeficiency compound heterozygote at the arylsulfatase A locus with neurological and psychiatric symptomatology. Am J Med Genet. 1988;31:169–175. doi: 10.1002/ajmg.1320310120. [DOI] [PubMed] [Google Scholar]
- 27.Clarke JT, Mahuran DJ, Sathe S, Kolodny EH, Rigat BA, Raiman JA, Tropak MB. An open-label Phase I/II clinical trial of pyrimethamine for the treatment of patients affected with chronic GM2 gangliosidosis (Tay-Sachs or Sandhoff variants) Mol Genet Metab. 2011;102:6–12. doi: 10.1016/j.ymgme.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishii S. Pharmacological chaperone therapy for Fabry disease. Proc Jpn Acad Ser B Phys Biol Sci. 2012;88:18–30. doi: 10.2183/pjab.88.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mu TW, Ong DS, Wang YJ, Balch WE, Yates JR, 3rd, Segatori L, Kelly JW. Chemical and biological approaches synergize to ameliorate protein-folding diseases. Cell. 2008;134:769–781. doi: 10.1016/j.cell.2008.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.