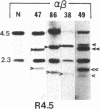
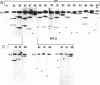
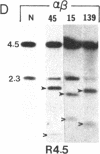
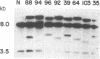
Abstract
There is considerable evidence that links the activation of cellular genes to oncogenesis. We previously reported that structural rearrangements in the cellular oncogene c-erbB correlate with the development of erythroblastosis induced by avian leukosis virus (ALV). c-erbB recently has been shown to be related to the gene encoding epidermal growth factor receptor. We now have characterized the detailed mechanisms of c-erbB activation by ALV proviruses. We report here that the ALV proviral integration sites are clustered 5' to the region where homology to v-erbB starts, suggesting that interruption in this region of c-erbB is important for its activation. The proviruses are oriented in the same transcriptional direction as c-erbB and usually are full-size. The latter finding is in contrast to the frequent deletions observed within the c-myc-linked proviruses in B-cell lymphomas. We have also identified a second c-erbB allele, which differs from the previously known allele primarily by a deletion in an intron region. This allele is also oncogenic upon mutation by an ALV provirus.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Gottesman M. Promoter occlusion: transcription through a promoter may inhibit its activity. Cell. 1982 Jul;29(3):939–944. doi: 10.1016/0092-8674(82)90456-1. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Cellular oncogenes and retroviruses. Annu Rev Biochem. 1983;52:301–354. doi: 10.1146/annurev.bi.52.070183.001505. [DOI] [PubMed] [Google Scholar]
- Cullen B. R., Lomedico P. T., Ju G. Transcriptional interference in avian retroviruses--implications for the promoter insertion model of leukaemogenesis. Nature. 1984 Jan 19;307(5948):241–245. doi: 10.1038/307241a0. [DOI] [PubMed] [Google Scholar]
- Downward J., Yarden Y., Mayes E., Scrace G., Totty N., Stockwell P., Ullrich A., Schlessinger J., Waterfield M. D. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequences. Nature. 1984 Feb 9;307(5951):521–527. doi: 10.1038/307521a0. [DOI] [PubMed] [Google Scholar]
- Fung Y. K., Crittenden L. B., Kung H. J. Orientation and position of avian leukosis virus DNA relative to the cellular oncogene c-myc in B-lymphoma tumors of highly susceptible 15I5 X 7(2) chickens. J Virol. 1982 Nov;44(2):742–746. doi: 10.1128/jvi.44.2.742-746.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung Y. K., Fadly A. M., Crittenden L. B., Kung H. J. On the mechanism of retrovirus-induced avian lymphoid leukosis: deletion and integration of the proviruses. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3418–3422. doi: 10.1073/pnas.78.6.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung Y. K., Lewis W. G., Crittenden L. B., Kung H. J. Activation of the cellular oncogene c-erbB by LTR insertion: molecular basis for induction of erythroblastosis by avian leukosis virus. Cell. 1983 Jun;33(2):357–368. doi: 10.1016/0092-8674(83)90417-8. [DOI] [PubMed] [Google Scholar]
- Hayward W. S., Neel B. G., Astrin S. M. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature. 1981 Apr 9;290(5806):475–480. doi: 10.1038/290475a0. [DOI] [PubMed] [Google Scholar]
- Hohn B., Murray K. Packaging recombinant DNA molecules into bacteriophage particles in vitro. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3259–3263. doi: 10.1073/pnas.74.8.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. R., Chen W. S., Kruiger W., Stolarsky L. S., Weber W., Evans R. M., Verma I. M., Gill G. N., Rosenfeld M. G. Expression cloning of human EGF receptor complementary DNA: gene amplification and three related messenger RNA products in A431 cells. Science. 1984 May 25;224(4651):843–848. doi: 10.1126/science.6326261. [DOI] [PubMed] [Google Scholar]
- Merlino G. T., Xu Y. H., Ishii S., Clark A. J., Semba K., Toyoshima K., Yamamoto T., Pastan I. Amplification and enhanced expression of the epidermal growth factor receptor gene in A431 human carcinoma cells. Science. 1984 Apr 27;224(4647):417–419. doi: 10.1126/science.6200934. [DOI] [PubMed] [Google Scholar]
- Neel B. G., Hayward W. S., Robinson H. L., Fang J., Astrin S. M. Avian leukosis virus-induced tumors have common proviral integration sites and synthesize discrete new RNAs: oncogenesis by promoter insertion. Cell. 1981 Feb;23(2):323–334. doi: 10.1016/0092-8674(81)90128-8. [DOI] [PubMed] [Google Scholar]
- Noori-Daloii M. R., Swift R. A., Kung H. J., Crittenden L. B., Witter R. L. Specific integration of REV proviruses in avian bursal lymphomas. Nature. 1981 Dec 10;294(5841):574–576. doi: 10.1038/294574a0. [DOI] [PubMed] [Google Scholar]
- Nusse R., van Ooyen A., Cox D., Fung Y. K., Varmus H. Mode of proviral activation of a putative mammary oncogene (int-1) on mouse chromosome 15. Nature. 1984 Jan 12;307(5947):131–136. doi: 10.1038/307131a0. [DOI] [PubMed] [Google Scholar]
- Payne G. S., Bishop J. M., Varmus H. E. Multiple arrangements of viral DNA and an activated host oncogene in bursal lymphomas. Nature. 1982 Jan 21;295(5846):209–214. doi: 10.1038/295209a0. [DOI] [PubMed] [Google Scholar]
- Payne G. S., Courtneidge S. A., Crittenden L. B., Fadly A. M., Bishop J. M., Varmus H. E. Analysis of avian leukosis virus DNA and RNA in bursal tumours: viral gene expression is not required for maintenance of the tumor state. Cell. 1981 Feb;23(2):311–322. doi: 10.1016/0092-8674(81)90127-6. [DOI] [PubMed] [Google Scholar]
- Saito H., Hayday A. C., Wiman K., Hayward W. S., Tonegawa S. Activation of the c-myc gene by translocation: a model for translational control. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7476–7480. doi: 10.1073/pnas.80.24.7476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeant A., Saule S., Leprince D., Begue A., Rommens C., Stehelin D. Molecular cloning and characterization of the chicken DNA locus related to the oncogene erbB of avian erythroblastosis virus. EMBO J. 1982;1(2):237–242. doi: 10.1002/j.1460-2075.1982.tb01153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A. Conversion of RNA to DNA in mammals: Alu-like elements and pseudogenes. Nature. 1983 Feb 10;301(5900):471–472. doi: 10.1038/301471a0. [DOI] [PubMed] [Google Scholar]
- Shih C. K., Linial M., Goodenow M. M., Hayward W. S. Nucleotide sequence 5' of the chicken c-myc coding region: localization of a noncoding exon that is absent from myc transcripts in most avian leukosis virus-induced lymphomas. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4697–4701. doi: 10.1073/pnas.81.15.4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Taub R., Moulding C., Battey J., Murphy W., Vasicek T., Lenoir G. M., Leder P. Activation and somatic mutation of the translocated c-myc gene in burkitt lymphoma cells. Cell. 1984 Feb;36(2):339–348. doi: 10.1016/0092-8674(84)90227-7. [DOI] [PubMed] [Google Scholar]
- Vennström B., Bishop J. M. Isolation and characterization of chicken DNA homologous to the two putative oncogenes of avian erythroblastosis virus. Cell. 1982 Jan;28(1):135–143. doi: 10.1016/0092-8674(82)90383-x. [DOI] [PubMed] [Google Scholar]