Abstract
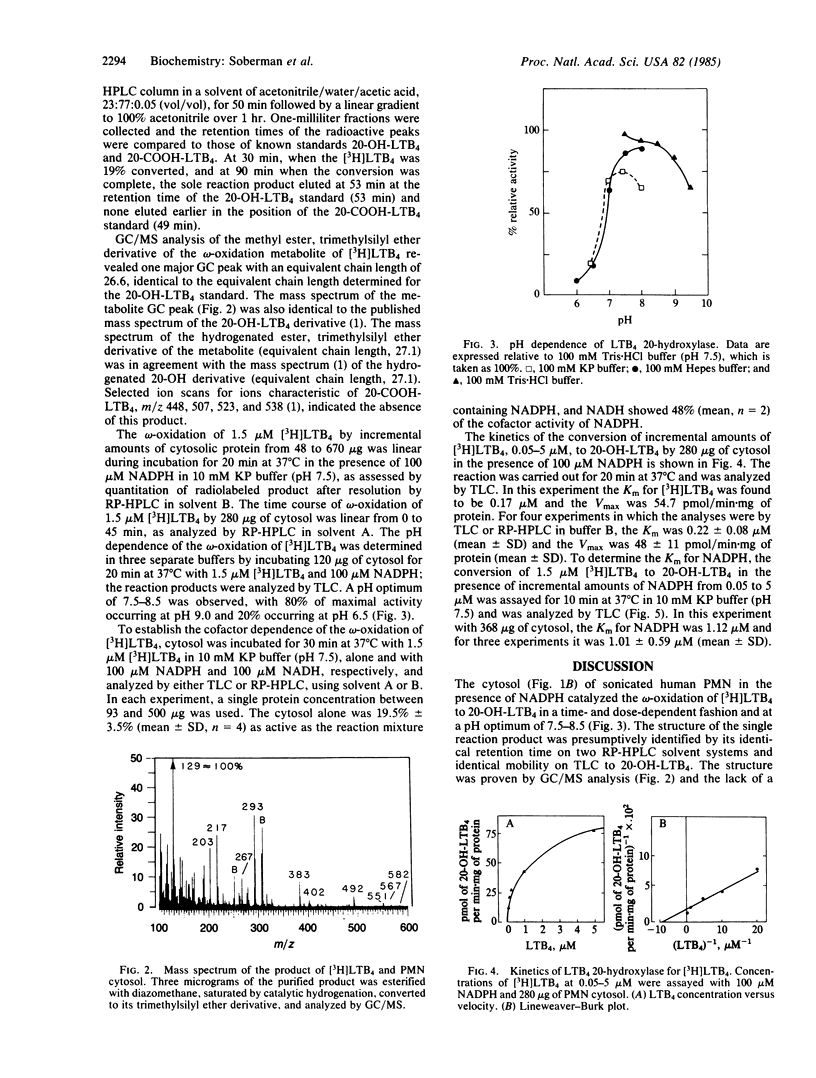
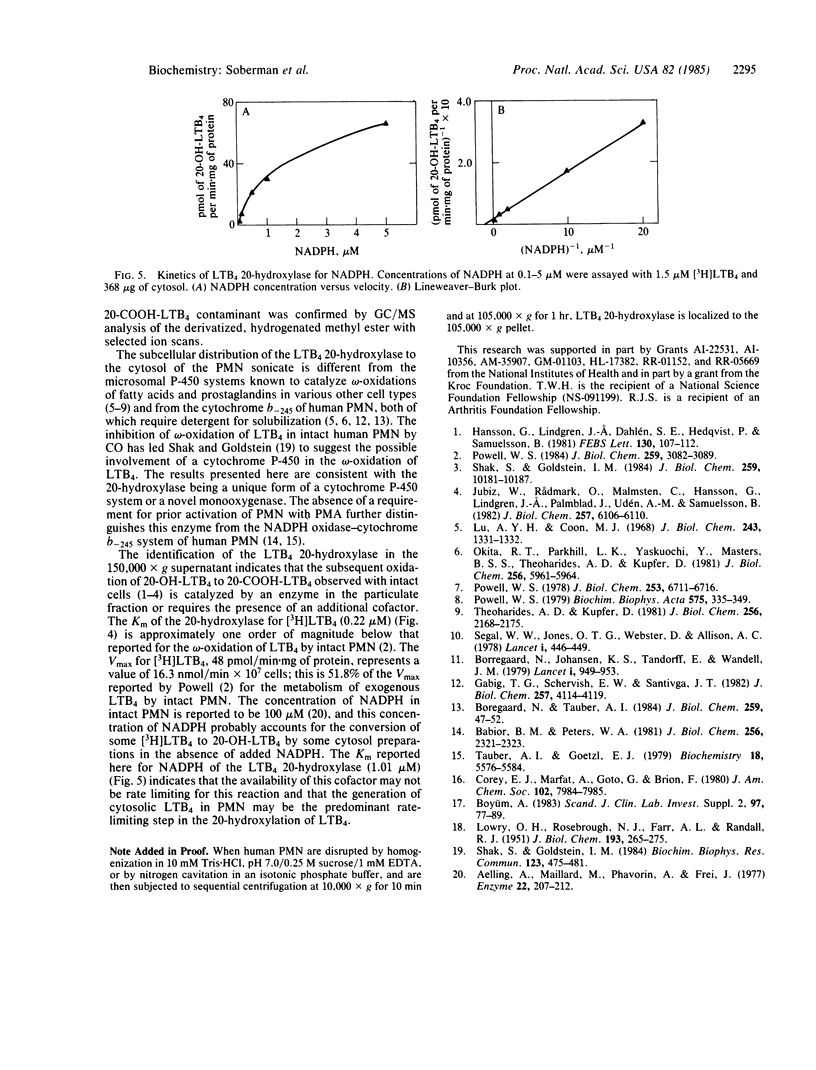
A single reaction product was formed during the incubation of 1.5 microM (5S,12R)-dihydroxy-6,14-cis-8,10-trans-[3H]icosatetraenoic acid (leukotriene B4, LTB4) for 30 min at 37 degrees C in 10 mM potassium phosphate buffer (pH 7.5) with 100 microM NADPH and the 150,000 X g supernatant of sonicated human polymorphonuclear leukocytes (PMN). The reaction product exhibited the same mobility on reversed-phase HPLC (RP-HPLC) and TLC as standard 20-hydroxy-LTB4 (20-OH-LTB4). When the omega-oxidation product of [3H]LTB4 was eluted from a Sep-Pak, resolved by RP-HPLC, and analyzed by GC/MS, its structure was determined to be solely 20-OH-LTB4. The Km of the 20-hydroxylase for [3H]LTB4 at its optimal pH of 7.5 was 0.22 +/- 0.08 microM (mean +/- SD, n = 4) and the Vmax was 48 +/- 11 pmol/min X mg of protein (mean +/- SD, n = 4). When the concentration of [3H]LTB4 was fixed at 1.5 microM, the Km for NADPH was 1.01 +/- 0.59 microM (mean +/- SD, n = 3). The location in the 150,000 X g supernatant of the LTB4 20-hydroxylase distinguishes it from the cytochrome P-450 system of liver, lung, and kidney microsomes and from the NADPH oxidase-cytochrome b-245 system of the human PMN. The LTB4 20-hydroxylase is either a unique cytochrome P-450 or other monooxygenase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aellig A., Maillard M., Phavorin A., Frei J. The energy metabolism of the leukocyte. IX. Changes in the concentration of the coenzymes NAD, NADH, NADP, and NADPH in polymorphonuclear leukocytes during phagocytosis of Staphylococcus albus and due to the action of phospholipase C. Enzyme. 1977;22(3):207–212. [PubMed] [Google Scholar]
- Babior B. M., Peters W. A. The O2--producing enzyme of human neutrophils. Further properties. J Biol Chem. 1981 Mar 10;256(5):2321–2323. [PubMed] [Google Scholar]
- Borregaard N., Johansen K. S., Taudorff E., Wandall J. H. Cytochrome b is present in neutrophils from patients with chronic granulomatous disease. Lancet. 1979 May 5;1(8123):949–951. doi: 10.1016/s0140-6736(79)91722-7. [DOI] [PubMed] [Google Scholar]
- Borregaard N., Tauber A. I. Subcellular localization of the human neutrophil NADPH oxidase. b-Cytochrome and associated flavoprotein. J Biol Chem. 1984 Jan 10;259(1):47–52. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Gabig T. G., Schervish E. W., Santinga J. T. Functional relationship of the cytochrome b to the superoxide-generating oxidase of human neutrophils. J Biol Chem. 1982 Apr 25;257(8):4114–4119. [PubMed] [Google Scholar]
- Hansson G., Lindgren J. A., Dahlén S. E., Hedqvist P., Samuelsson B. Identification and biological activity of novel omega-oxidized metabolites of leukotriene B4 from human leukocytes. FEBS Lett. 1981 Jul 20;130(1):107–112. doi: 10.1016/0014-5793(81)80676-x. [DOI] [PubMed] [Google Scholar]
- Jubiz W., Rådmark O., Malmsten C., Hansson G., Lindgren J. A., Palmblad J., Udén A. M., Samuelsson B. A novel leukotriene produced by stimulation of leukocytes with formylmethionylleucylphenylalanine. J Biol Chem. 1982 Jun 10;257(11):6106–6110. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lu A. Y., Coon M. J. Role of hemoprotein P-450 in fatty acid omega-hydroxylation in a soluble enzyme system from liver microsomes. J Biol Chem. 1968 Mar 25;243(6):1331–1332. [PubMed] [Google Scholar]
- Okita R. T., Parkhill L. K., Yasukochi Y., Masters B. S., Theoharides A. D., Kupfer D. The omega- and (omega-1)-hydroxylase activities of prostaglandins A1 and E1 and lauric acid by pig kidney microsomes and a purified kidney cytochrome P-450. J Biol Chem. 1981 Jun 25;256(12):5961–5964. [PubMed] [Google Scholar]
- Powell W. S. Metabolism of prostaglandins, prostaglandin analogs and thromboxane B2 by lung and liver microsomes from pregnant rabbits. Biochim Biophys Acta. 1979 Dec 18;575(3):335–349. doi: 10.1016/0005-2760(79)90102-4. [DOI] [PubMed] [Google Scholar]
- Powell W. S. Properties of leukotriene B4 20-hydroxylase from polymorphonuclear leukocytes. J Biol Chem. 1984 Mar 10;259(5):3082–3089. [PubMed] [Google Scholar]
- Powell W. S. omega-Oxidation of prostaglandins by lung and liver microsomes. Changes in enzyme activity induced by pregnancy, pseudopregnancy, and progesterone treatment. J Biol Chem. 1978 Oct 10;253(19):6711–6716. [PubMed] [Google Scholar]
- Segal A. W., Jones O. T., Webster D., Allison A. C. Absence of a newly described cytochrome b from neutrophils of patients with chronic granulomatous disease. Lancet. 1978 Aug 26;2(8087):446–449. doi: 10.1016/s0140-6736(78)91445-9. [DOI] [PubMed] [Google Scholar]
- Shak S., Goldstein I. M. Carbon monoxide inhibits omega-oxidation of leukotriene B4 by human polymorphonuclear leukocytes: evidence that catabolism of leukotriene B4 is mediated by a cytochrome P-450 enzyme. Biochem Biophys Res Commun. 1984 Sep 17;123(2):475–481. doi: 10.1016/0006-291x(84)90255-9. [DOI] [PubMed] [Google Scholar]
- Shak S., Goldstein I. M. Omega-oxidation is the major pathway for the catabolism of leukotriene B4 in human polymorphonuclear leukocytes. J Biol Chem. 1984 Aug 25;259(16):10181–10187. [PubMed] [Google Scholar]
- Tauber A. I., Goetzl E. J. Structural and catalytic properties of the solubilized superoxide-generating activity of human polymorphonuclear leukocytes. Solubilization, stabilization in solution, and partial characterization. Biochemistry. 1979 Dec 11;18(25):5576–5584. doi: 10.1021/bi00592a009. [DOI] [PubMed] [Google Scholar]
- Theoharides A. D., Kupfer D. Evidence for different hepatic microsomal monooxygenases catalyzing omega- and (omega-1)-hydroxylations of prostaglandins E1 and E2. Effects of inducers of monooxygenase on the kinetic constants of prostaglandin hydroxylation. J Biol Chem. 1981 Mar 10;256(5):2168–2175. [PubMed] [Google Scholar]


