Abstract
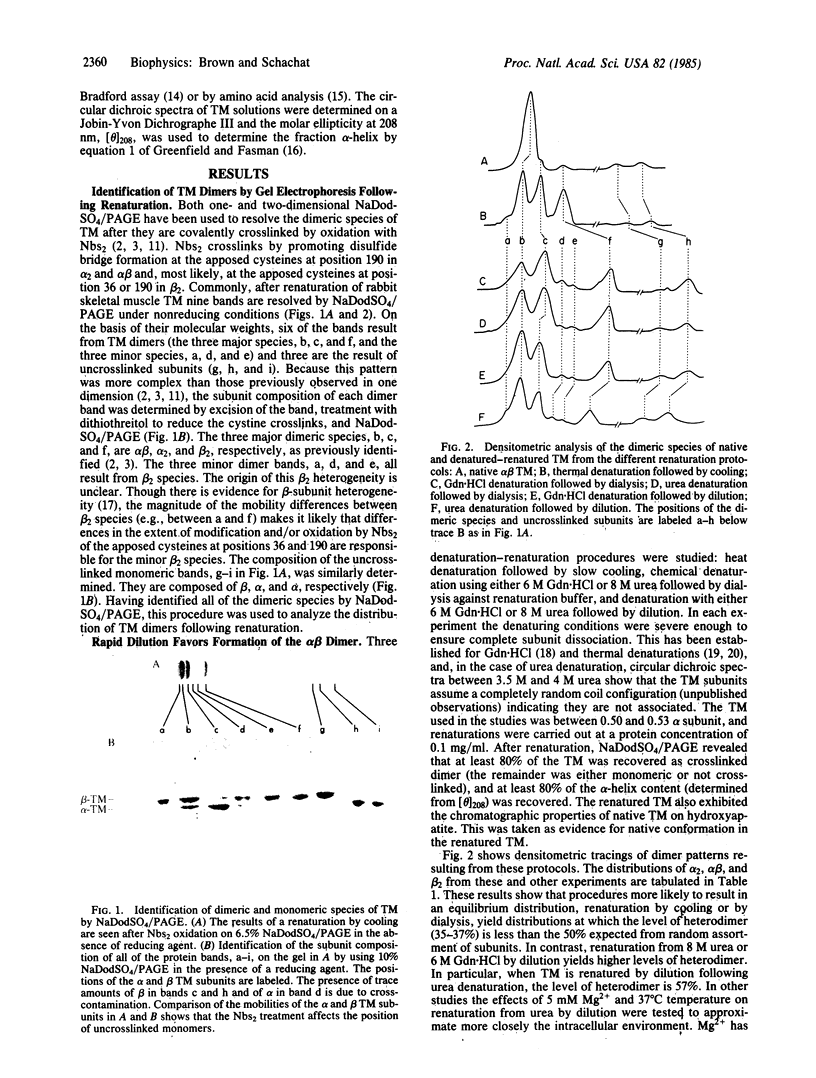
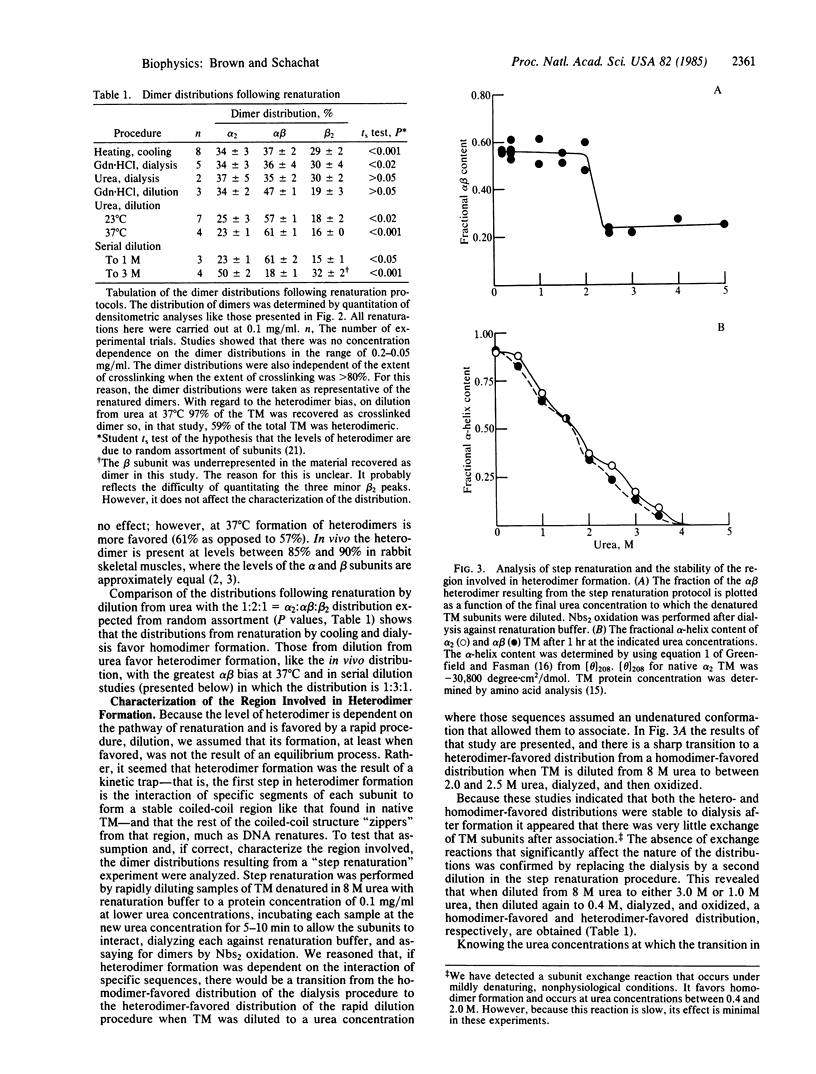
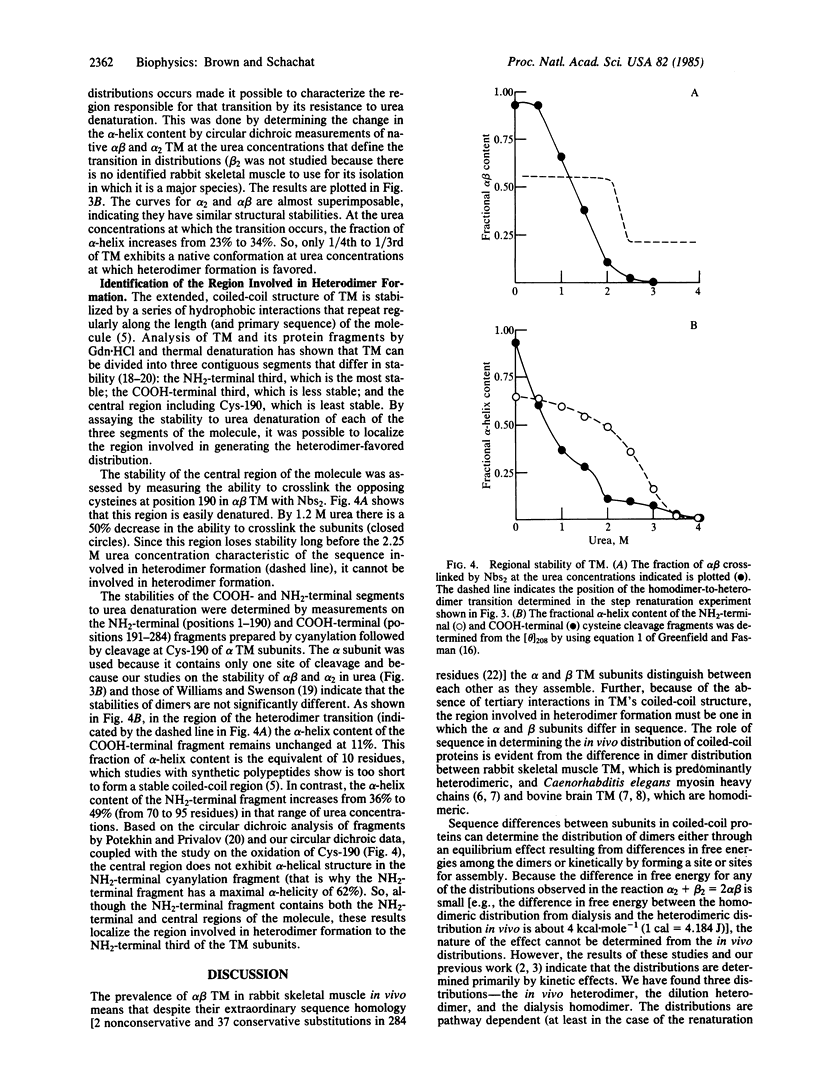
The observation that the alpha beta heterodimer is the predominant species of tropomyosin in rabbit skeletal muscles has led to the suggestion that this species assembles preferentially. To understand the molecular basis of this assembly process, we have studied renaturation under conditions that favor heterodimer formation. When skeletal muscle tropomyosin composed of equal amounts of alpha and beta subunits is renatured either by cooling or by dialysis a distribution that favors homodimers is generated. In contrast, rapid renaturation by dilution from urea favors the heterodimer. Further analysis of this latter renaturation procedure with cysteine-cleavage fragments of tropomyosin using circular dichroic measurements shows that as few as 30 residues in the NH2-terminal third of each tropomyosin subunit are involved in the initial interaction that results in heterodimer formation. Based on the density of sequence substitutions between the alpha and beta subunits, that region probably includes residues 36-64.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bretscher A., Weber K. Tropomyosin from bovine brain contains two polypeptide chains of slightly different molecular weights. FEBS Lett. 1978 Jan 1;85(1):145–148. doi: 10.1016/0014-5793(78)81267-8. [DOI] [PubMed] [Google Scholar]
- Bronson D. D., Schachat F. H. Heterogeneity of contractile proteins. Differences in tropomyosin in fast, mixed, and slow skeletal muscles of the rabbit. J Biol Chem. 1982 Apr 10;257(7):3937–3944. [PubMed] [Google Scholar]
- Cummins P., Perry S. V. The subunits and biological activity of polymorphic forms of tropomyosin. Biochem J. 1973 Aug;133(4):765–777. doi: 10.1042/bj1330765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield N., Fasman G. D. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry. 1969 Oct;8(10):4108–4116. doi: 10.1021/bi00838a031. [DOI] [PubMed] [Google Scholar]
- Jacobson G. R., Schaffer M. H., Stark G. R., Vanaman T. C. Specific chemical cleavage in high yield at the amino peptide bonds of cysteine and cystine residues. J Biol Chem. 1973 Oct 10;248(19):6583–6591. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehrer S. S. Effects of an interchain disulfide bond on tropomyosin structure: intrinsic fluorescence and circular dichroism studies. J Mol Biol. 1978 Jan 15;118(2):209–226. doi: 10.1016/0022-2836(78)90413-8. [DOI] [PubMed] [Google Scholar]
- Lehrer S. S. Intramolecular crosslinking of tropomyosin via disulfide bond formation: evidence for chain register. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3377–3381. doi: 10.1073/pnas.72.9.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak A. S., Lewis W. G., Smillie L. B. Amino acid sequences of rabbit skeletal beta- and cardiac tropomyosins. FEBS Lett. 1979 Sep 15;105(2):232–234. doi: 10.1016/0014-5793(79)80618-3. [DOI] [PubMed] [Google Scholar]
- Mak A. S., Smillie L. B., Stewart G. R. A comparison of the amino acid sequences of rabbit skeletal muscle alpha- and beta-tropomyosins. J Biol Chem. 1980 Apr 25;255(8):3647–3655. [PubMed] [Google Scholar]
- Potekhin S. A., Privalov P. L. Co-operative blocks in tropomyosin. J Mol Biol. 1982 Aug 15;159(3):519–535. doi: 10.1016/0022-2836(82)90299-6. [DOI] [PubMed] [Google Scholar]
- Schachat F. H., Harris H. E., Epstein H. F. Two homogeneous myosins in body-wall muscle of Caenorhabditis elegans. Cell. 1977 Apr;10(4):721–728. doi: 10.1016/0092-8674(77)90106-4. [DOI] [PubMed] [Google Scholar]
- Schachat F., Garcea R. L., Epstein H. F. Myosins exist as homodimers of heavy chains: demonstration with specific antibody purified by nematode mutant myosin affinity chromatography. Cell. 1978 Oct;15(2):405–411. doi: 10.1016/0092-8674(78)90009-0. [DOI] [PubMed] [Google Scholar]
- Williams D. L., Jr, Swenson C. A. Tropomyosin stability: assignment of thermally induced conformational transitions to separate regions of the molecule. Biochemistry. 1981 Jun 23;20(13):3856–3864. doi: 10.1021/bi00516a029. [DOI] [PubMed] [Google Scholar]
- Zak R., Martin A. F., Prior G., Rabinowitz M. Comparison of turnover of several myofibrillar proteins and critical evaluation of double isotope method. J Biol Chem. 1977 May 25;252(10):3430–3435. [PubMed] [Google Scholar]



