Abstract
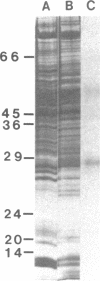
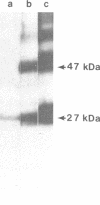
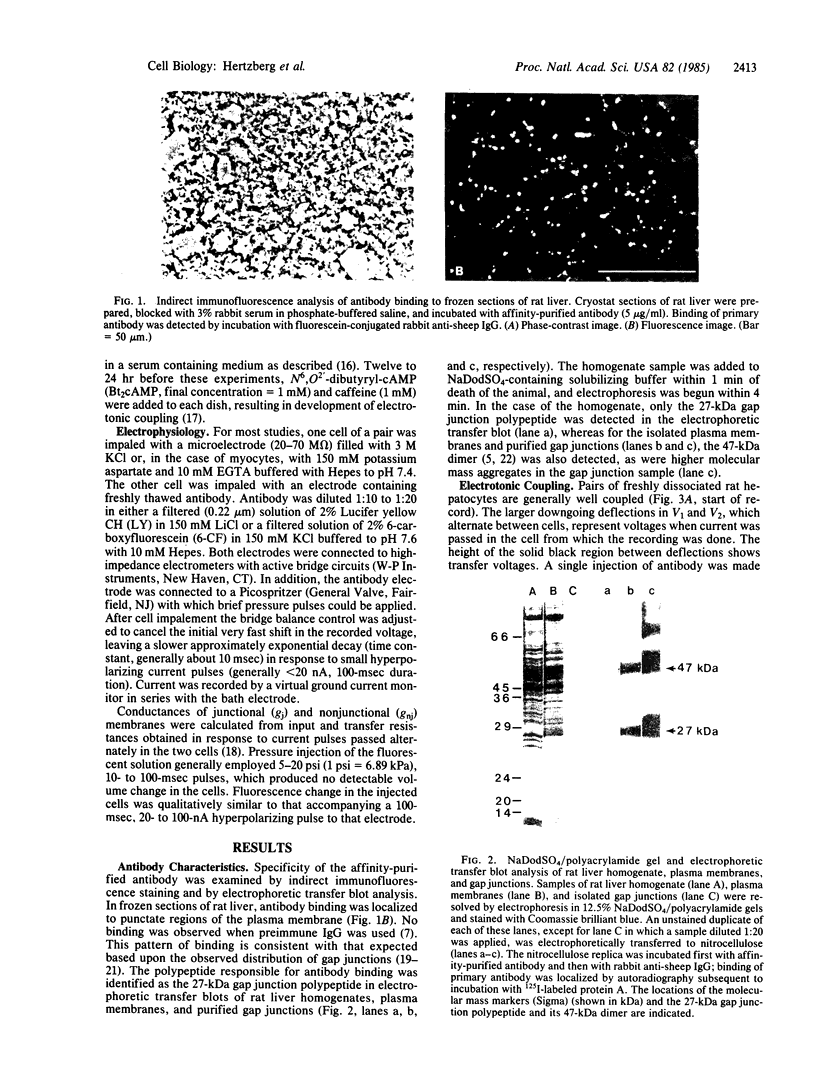
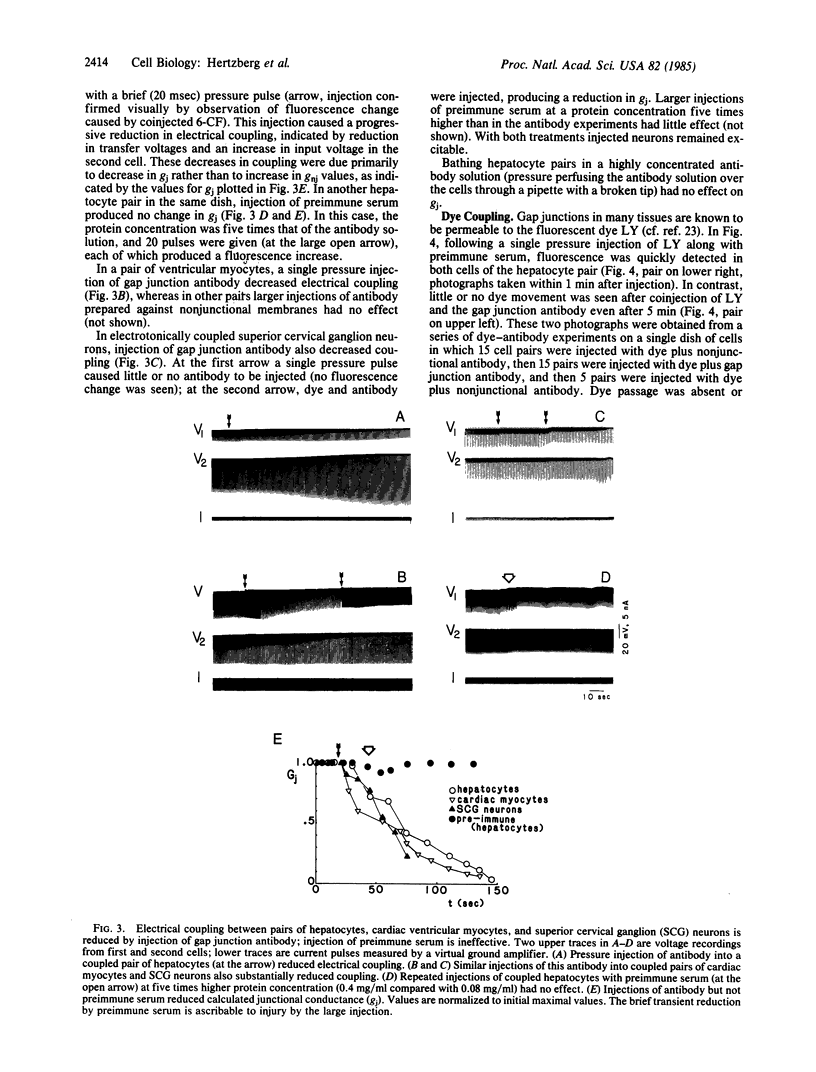
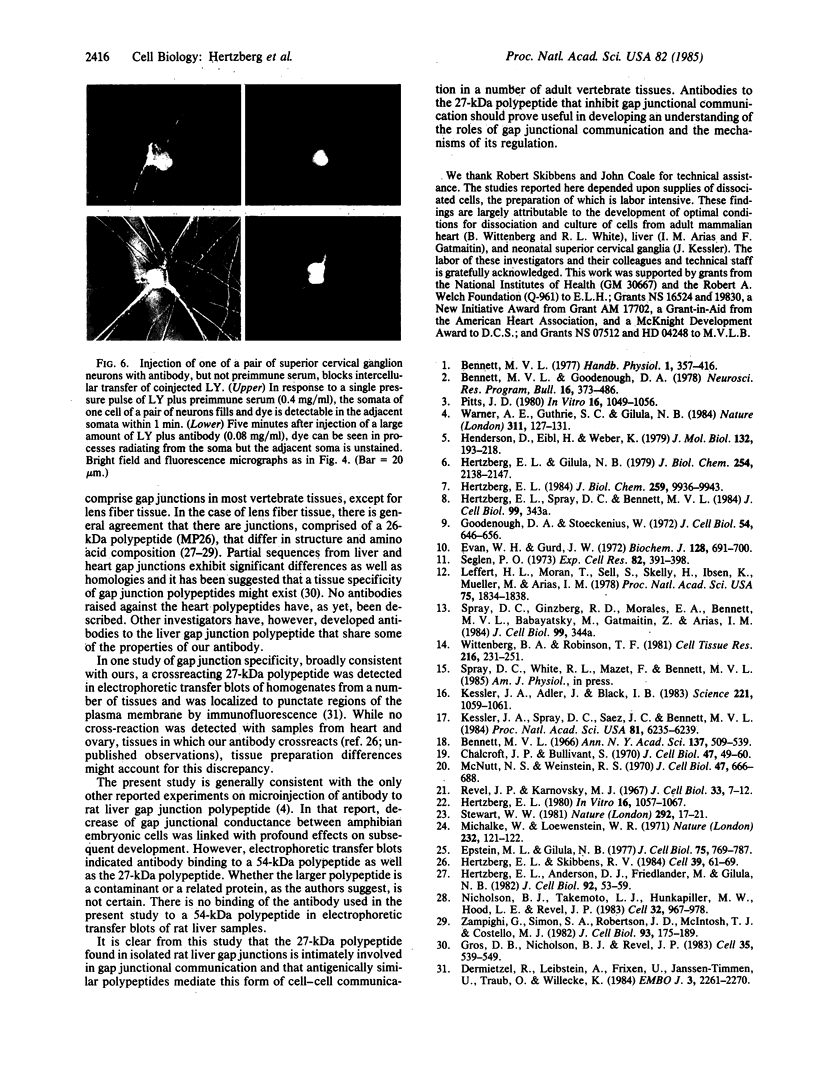
Antibody raised against isolated rat liver gap junctions was microinjected into coupled cells in culture to assess its influence on gap junctional conductance. A rapid inhibition of fluorescent dye transfer and electrical coupling was produced in pairs of freshly dissociated adult rat hepatocytes and myocardial cells as well as in pairs of superior cervical ganglion neurons from neonatal rats cultured under conditions in which electrotonic synapses form. The antibodies have been shown by indirect immunofluorescence to bind to punctate regions of the plasma membrane in liver. By immunoreplica analysis of rat liver homogenates, plasma membranes, and isolated gap junctions resolved on NaDodSO4/polyacrylamide gels, binding was shown to be specific for the 27-kDa major polypeptide of gap junctions. This and similar antibodies should provide a tool for further investigation of the role of cell-cell communication mediated by gap junctions and indicate that immunologically similar polypeptides comprise gap junctions in adult mammalian cells derived from all three germ layers.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett M. V., Goodenough D. A. Gap junctions, electrotonic coupling, and intercellular communication. Neurosci Res Program Bull. 1978 Sep;16(3):1–486. [PubMed] [Google Scholar]
- Bennett M. V. Physiology of electrotonic junctions. Ann N Y Acad Sci. 1966 Jul 14;137(2):509–539. doi: 10.1111/j.1749-6632.1966.tb50178.x. [DOI] [PubMed] [Google Scholar]
- Chalcroft J. P., Bullivant S. An interpretation of liver cell membrane and junction structure based on observation of freeze-fracture replicas of both sides of the fracture. J Cell Biol. 1970 Oct;47(1):49–60. doi: 10.1083/jcb.47.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermietzel R., Leibstein A., Frixen U., Janssen-Timmen U., Traub O., Willecke K. Gap junctions in several tissues share antigenic determinants with liver gap junctions. EMBO J. 1984 Oct;3(10):2261–2270. doi: 10.1002/j.1460-2075.1984.tb02124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein M. L., Gilula N. B. A study of communication specificity between cells in culture. J Cell Biol. 1977 Dec;75(3):769–787. doi: 10.1083/jcb.75.3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. H., Gurd J. W. Preparation and properties of nexuses and lipid-enriched vesicles from mouse liver plasma membranes. Biochem J. 1972 Jul;128(3):691–700. doi: 10.1042/bj1280691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough D. A., Stoeckenius W. The isolation of mouse hepatocyte gap junctions. Preliminary chemical characterization and x-ray diffraction. J Cell Biol. 1972 Sep;54(3):646–656. doi: 10.1083/jcb.54.3.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros D. B., Nicholson B. J., Revel J. P. Comparative analysis of the gap junction protein from rat heart and liver: is there a tissue specificity of gap junctions? Cell. 1983 Dec;35(2 Pt 1):539–549. doi: 10.1016/0092-8674(83)90188-5. [DOI] [PubMed] [Google Scholar]
- Henderson D., Eibl H., Weber K. Structure and biochemistry of mouse hepatic gap junctions. J Mol Biol. 1979 Aug 5;132(2):193–218. doi: 10.1016/0022-2836(79)90391-7. [DOI] [PubMed] [Google Scholar]
- Hertzberg E. L. A detergent-independent procedure for the isolation of gap junctions from rat liver. J Biol Chem. 1984 Aug 10;259(15):9936–9943. [PubMed] [Google Scholar]
- Hertzberg E. L., Anderson D. J., Friedlander M., Gilula N. B. Comparative analysis of the major polypeptides from liver gap junctions and lens fiber junctions. J Cell Biol. 1982 Jan;92(1):53–59. doi: 10.1083/jcb.92.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzberg E. L. Biochemical and immunological approaches to the study of gap junctional communication. In Vitro. 1980 Dec;16(12):1057–1067. doi: 10.1007/BF02619256. [DOI] [PubMed] [Google Scholar]
- Hertzberg E. L., Gilula N. B. Isolation and characterization of gap junctions from rat liver. J Biol Chem. 1979 Mar 25;254(6):2138–2147. [PubMed] [Google Scholar]
- Hertzberg E. L., Skibbens R. V. A protein homologous to the 27,000 dalton liver gap junction protein is present in a wide variety of species and tissues. Cell. 1984 Nov;39(1):61–69. doi: 10.1016/0092-8674(84)90191-0. [DOI] [PubMed] [Google Scholar]
- Kessler J. A., Adler J. E., Black I. B. Substance P and somatostatin regulate sympathetic noradrenergic function. Science. 1983 Sep 9;221(4615):1059–1061. doi: 10.1126/science.6192502. [DOI] [PubMed] [Google Scholar]
- Kessler J. A., Spray D. C., Saez J. C., Bennett M. V. Determination of synaptic phenotype: insulin and cAMP independently initiate development of electrotonic coupling between cultured sympathetic neurons. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6235–6239. doi: 10.1073/pnas.81.19.6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffert H., Moran T., Sell S., Skelly H., Ibsen K., Mueller M., Arias I. Growth state-dependent phenotypes of adult hepatocytes in primary monolayer culture. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1834–1838. doi: 10.1073/pnas.75.4.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNutt N. S., Weinstein R. S. The ultrastructure of the nexus. A correlated thin-section and freeze-cleave study. J Cell Biol. 1970 Dec;47(3):666–688. doi: 10.1083/jcb.47.3.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalke W., Loewenstein W. R. Communication between cells of different type. Nature. 1971 Jul 9;232(5306):121–122. doi: 10.1038/232121b0. [DOI] [PubMed] [Google Scholar]
- Nicholson B. J., Takemoto L. J., Hunkapiller M. W., Hood L. E., Revel J. P. Differences between liver gap junction protein and lens MIP 26 from rat: implications for tissue specificity of gap junctions. Cell. 1983 Mar;32(3):967–978. doi: 10.1016/0092-8674(83)90081-8. [DOI] [PubMed] [Google Scholar]
- Pitts J. D. The role of junctional communication in animal tissues. In Vitro. 1980 Dec;16(12):1049–1056. doi: 10.1007/BF02619255. [DOI] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of rat liver cells. 3. Enzymatic requirements for tissue dispersion. Exp Cell Res. 1973 Dec;82(2):391–398. doi: 10.1016/0014-4827(73)90357-1. [DOI] [PubMed] [Google Scholar]
- Stewart W. W. Lucifer dyes--highly fluorescent dyes for biological tracing. Nature. 1981 Jul 2;292(5818):17–21. doi: 10.1038/292017a0. [DOI] [PubMed] [Google Scholar]
- Warner A. E., Guthrie S. C., Gilula N. B. Antibodies to gap-junctional protein selectively disrupt junctional communication in the early amphibian embryo. Nature. 1984 Sep 13;311(5982):127–131. doi: 10.1038/311127a0. [DOI] [PubMed] [Google Scholar]
- Wittenberg B. A., Robinson T. F. Oxygen requirements, morphology, cell coat and membrane permeability of calcium-tolerant myocytes from hearts of adult rats. Cell Tissue Res. 1981;216(2):231–251. doi: 10.1007/BF00233618. [DOI] [PubMed] [Google Scholar]
- Zampighi G., Simon S. A., Robertson J. D., McIntosh T. J., Costello M. J. On the structural organization of isolated bovine lens fiber junctions. J Cell Biol. 1982 Apr;93(1):175–189. doi: 10.1083/jcb.93.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]