Abstract
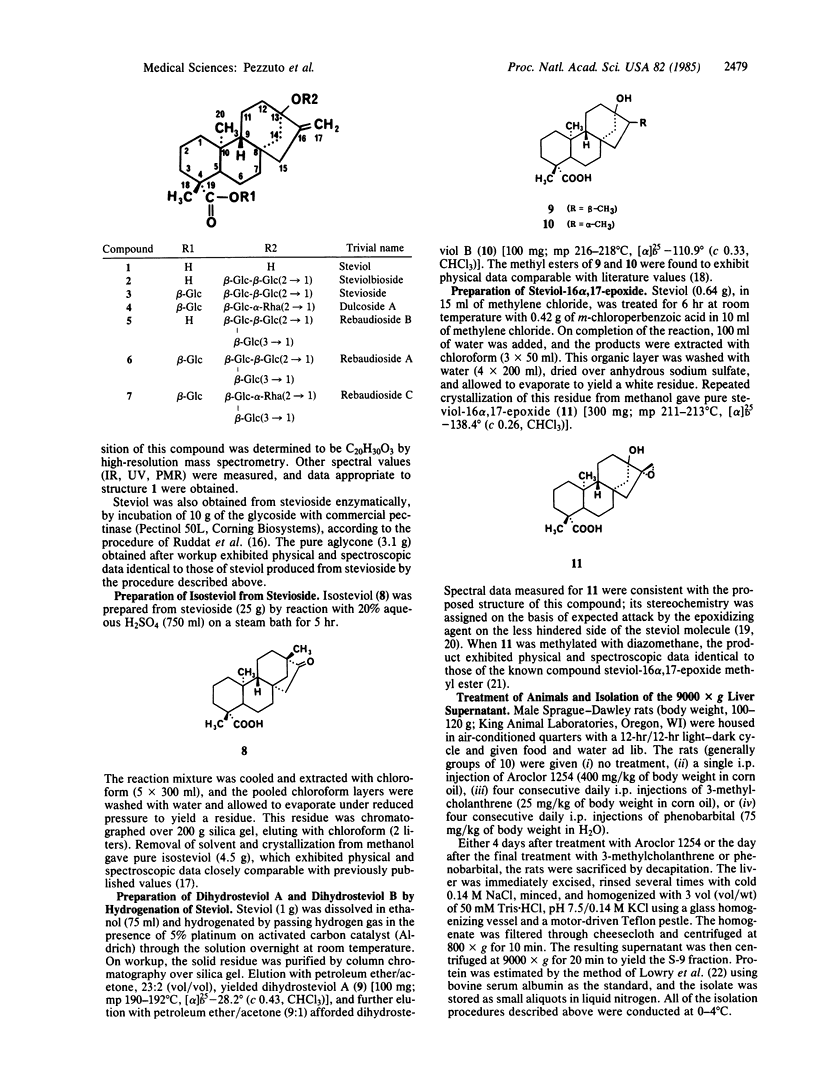
Stevioside, a constituent of Stevia rebaudiana, is commonly used as a noncaloric sugar substitute in Japan. Consistent with reports in the literature, we have found that stevioside is not mutagenic as judged by utilization of Salmonella typhimurium strain TM677, either in the presence or in the absence of a metabolic activating system. Similar negative results were obtained with several structurally related sweet-tasting glycosides. However, steviol, the aglycone of stevioside, was found to be highly mutagenic when evaluated in the presence of a 9000 X g supernatant fraction derived from the livers of Aroclor 1254-pretreated rats. Expression of mutagenic activity was dependent on both pretreatment of the rats with Aroclor 1254 and addition of NADPH; unmetabolized steviol was not active. The structurally related species, isosteviol, was not active regardless of metabolic activation. Similarly, chemical reduction of the unsaturated bond linking the carbon-16 and -17 positions of steviol resulted in the generation of two isomeric products, dihydrosteviol A and B, that were not mutagenic. In addition, ent-kaurenoic acid was found to be inactive. It is therefore clear that a metabolite of an integral component of stevioside is mutagenic; structural features of requisite importance for the expression of mutagenic activity include a hydroxy group at position 13 and an unsaturated bond joining the carbon atoms at positions 16 and 17. A potential metabolite of steviol, steviol-16 alpha,17-epoxide, was synthesized chemically and found to be ineffective as a direct-acting mutagen. Thus, although stevioside itself appears innocuous, it would seem prudent to expeditiously and unequivocally establish the human metabolic disposition of this substance.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvares A. P. Cytochrome P-450s: research highlights of the last two decades. Drug Metab Rev. 1981;12(2):431–436. doi: 10.3109/03602538108994039. [DOI] [PubMed] [Google Scholar]
- Ames B. N., Gurney E. G., Miller J. A., Bartsch H. Carcinogens as frameshift mutagens: metabolites and derivatives of 2-acetylaminofluorene and other aromatic amine carcinogens. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3128–3132. doi: 10.1073/pnas.69.11.3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames B. N., Sims P., Grover P. L. Epoxides of carcinogenic polycyclic hydrocarbons are frameshift mutagens. Science. 1972 Apr 7;176(4030):47–49. doi: 10.1126/science.176.4030.47. [DOI] [PubMed] [Google Scholar]
- Brown J. P. A review of the genetic effects of naturally occurring flavonoids, anthraquinones and related compounds. Mutat Res. 1980 May;75(3):243–277. doi: 10.1016/0165-1110(80)90029-9. [DOI] [PubMed] [Google Scholar]
- Croy R. G., Wogan G. N. Quantitative comparison of covalent aflatoxin-DNA adducts formed in rat and mouse livers and kidneys. J Natl Cancer Inst. 1981 Apr;66(4):761–768. [PubMed] [Google Scholar]
- Gelboin H. V. Benzo[alpha]pyrene metabolism, activation and carcinogenesis: role and regulation of mixed-function oxidases and related enzymes. Physiol Rev. 1980 Oct;60(4):1107–1166. doi: 10.1152/physrev.1980.60.4.1107. [DOI] [PubMed] [Google Scholar]
- Groopman J. D., Croy R. G., Wogan G. N. In vitro reactions of aflatoxin B1-adducted DNA. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5445–5449. doi: 10.1073/pnas.78.9.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinghorn A. D., Soejarto D. D., Nanayakkara N. P., Compadre C. M., Makapugay H. C., Hovanec-Brown J. M., Medon P. J., Kamath S. K. A phytochemical screening procedure for sweet ent-kaurene glycosides in the genus Stevia. J Nat Prod. 1984 May-Jun;47(3):439–444. doi: 10.1021/np50033a007. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lu A. Y., West S. B. Multiplicity of mammalian microsomal cytochromes P-45. Pharmacol Rev. 1979 Dec;31(4):277–295. [PubMed] [Google Scholar]
- Pezzuto J. M., Lau P. P., Luh Y., Moore P. D., Wogan G. N., Hecht S. M. There is a correlation between the DNA affinity and mutagenicity of several 3-amino-1-methyl-5H-pyrido[4,3-b]indoles. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1427–1431. doi: 10.1073/pnas.77.3.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddat M., Heftmann E., Lang A. Biosynthesis of steviol. Arch Biochem Biophys. 1965 Jun;110(3):496–499. doi: 10.1016/0003-9861(65)90441-8. [DOI] [PubMed] [Google Scholar]
- Sakamoto I., Kohda H., Murakami K., Tanaka O. [Quantitative analysis of stevioside (author's transl)]. Yakugaku Zasshi. 1975 Dec;95(12):1507–1510. doi: 10.1248/yakushi1947.95.12_1507. [DOI] [PubMed] [Google Scholar]
- Skopek T. R., Liber H. L., Kaden D. A., Thilly W. G. Relative sensitivities of forward and reverse mutation assays in Salmonella typhimurium. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4465–4469. doi: 10.1073/pnas.75.9.4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skopek T. R., Liber H. L., Krolewski J. J., Thilly W. G. Quantitative forward mutation assay in Salmonella typhimurium using 8-azaguanine resistance as a genetic marker. Proc Natl Acad Sci U S A. 1978 Jan;75(1):410–414. doi: 10.1073/pnas.75.1.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingard R. E., Jr, Brown J. P., Enderlin F. E., Dale J. A., Hale R. L., Seitz C. T. Intestinal degradation and absorption of the glycosidic sweeteners stevioside and rebaudioside A. Experientia. 1980 May 15;36(5):519–520. doi: 10.1007/BF01965774. [DOI] [PubMed] [Google Scholar]


