Abstract
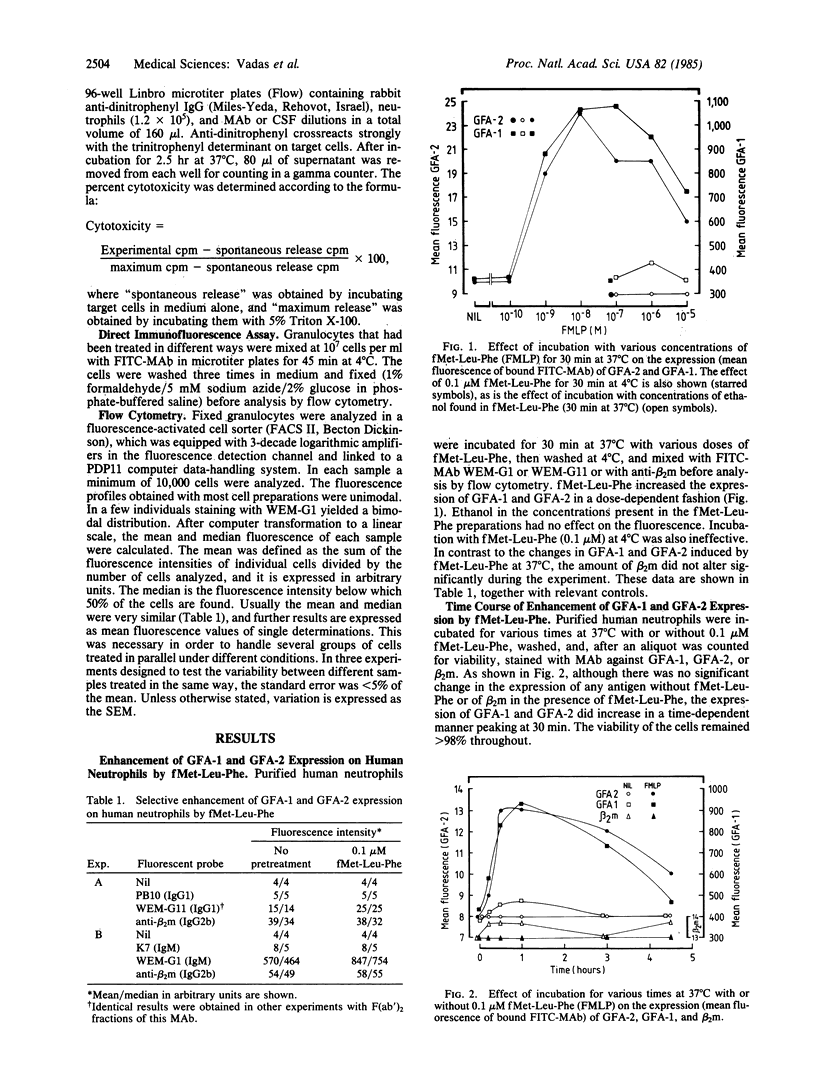
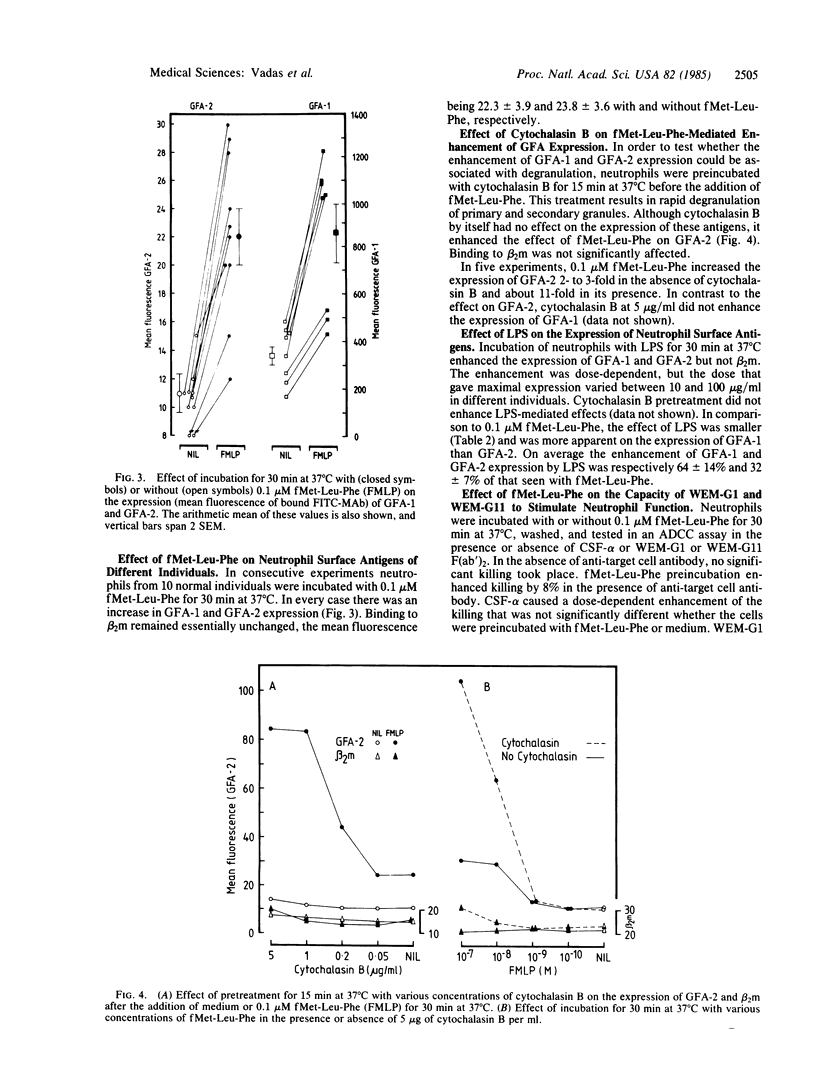
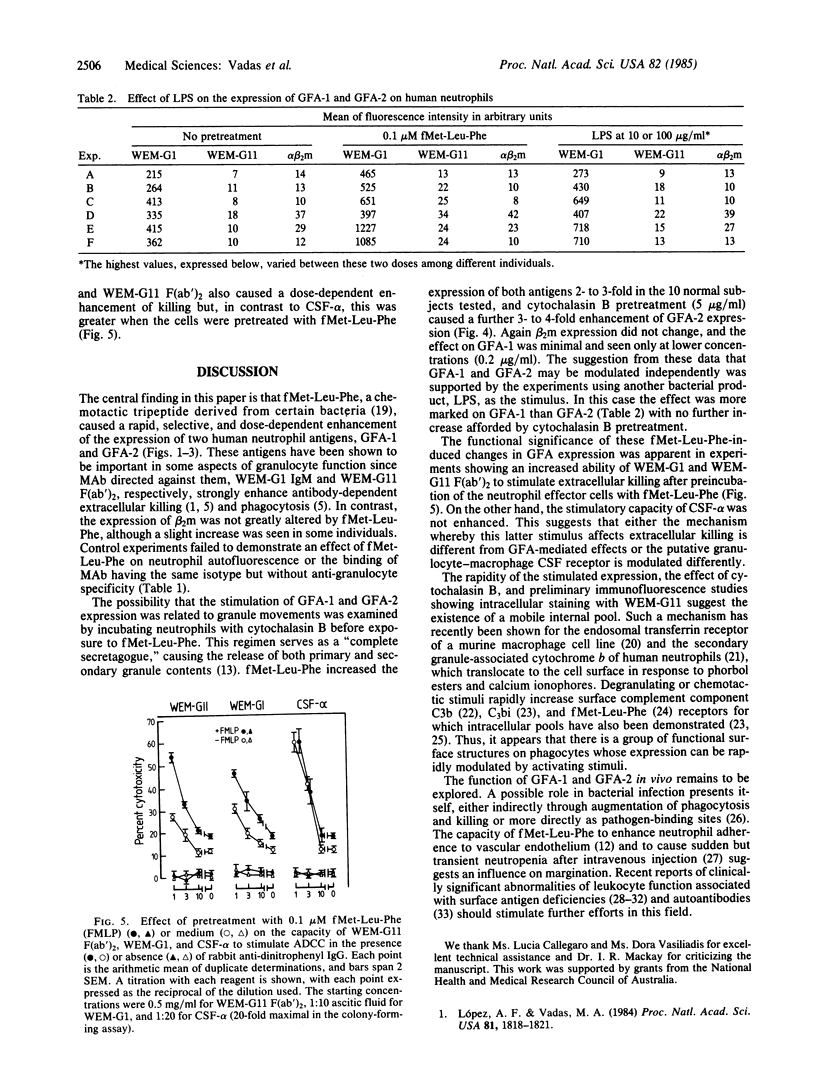
The regulation of expression of two human granulocyte functional antigens (GFA-1 and GFA-2) was examined. N-formylmethionylleucylphenylalanine (fMet-Leu-Phe) caused a rapid, dose-dependent enhancement of the expression of these antigens, 2- to 4-fold within 30 min, but not of another surface structure, beta 2-microglobulin. Pretreatment of the cells with cytochalasin B at 5 micrograms/ml further enhanced the effect of fMet-Leu-Phe on the expression of GFA-2, raising its surface expression 11-fold. Lipopolysaccharide also stimulated the expression of GFA-1 and GFA-2. The effect of lipopolysaccharide was less than that of fMet-Leu-Phe and was more marked on GFA-1 than on GFA-2. Pretreatment of neutrophils with fMet-Leu-Phe not only stimulated their cytotoxic activity against antibody-coated target cells but also increased their capacity to be stimulated by monoclonal antibodies to GFA-1 and GFA-2. These findings show that the expression of functional surface structures on human neutrophils is subject to rapid and selective regulation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. C., Schmalstieg F. C., Arnaout M. A., Kohl S., Tosi M. F., Dana N., Buffone G. J., Hughes B. J., Brinkley B. R., Dickey W. D. Abnormalities of polymorphonuclear leukocyte function associated with a heritable deficiency of high molecular weight surface glycoproteins (GP138): common relationship to diminished cell adherence. J Clin Invest. 1984 Aug;74(2):536–551. doi: 10.1172/JCI111451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaout M. A., Pitt J., Cohen H. J., Melamed J., Rosen F. S., Colten H. R. Deficiency of a granulocyte-membrane glycoprotein (gp150) in a boy with recurrent bacterial infections. N Engl J Med. 1982 Mar 25;306(12):693–699. doi: 10.1056/NEJM198203253061201. [DOI] [PubMed] [Google Scholar]
- Bentwood B. J., Henson P. M. The sequential release of granule constitutents from human neutrophils. J Immunol. 1980 Feb;124(2):855–862. [PubMed] [Google Scholar]
- Borregaard N., Heiple J. M., Simons E. R., Clark R. A. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J Cell Biol. 1983 Jul;97(1):52–61. doi: 10.1083/jcb.97.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley S. G. Cellular and molecular mechanisms of action of bacterial endotoxins. Annu Rev Microbiol. 1979;33:67–94. doi: 10.1146/annurev.mi.33.100179.000435. [DOI] [PubMed] [Google Scholar]
- Buchanan M. R., Crowley C. A., Rosin R. E., Gimbrone M. A., Jr, Babior B. M. Studies on the interaction between GP-18-0-deficient neutrophils and vascular endothelium. Blood. 1982 Jul;60(1):160–165. [PubMed] [Google Scholar]
- Buys S. S., Keogh E. A., Kaplan J. Fusion of intracellular membrane pools with cell surfaces of macrophages stimulated by phorbol esters and calcium ionophores. Cell. 1984 Sep;38(2):569–576. doi: 10.1016/0092-8674(84)90511-7. [DOI] [PubMed] [Google Scholar]
- Crowley C. A., Curnutte J. T., Rosin R. E., André-Schwartz J., Gallin J. I., Klempner M., Snyderman R., Southwick F. S., Stossel T. P., Babior B. M. An inherited abnormality of neutrophil adhesion. Its genetic transmission and its association with a missing protein. N Engl J Med. 1980 May 22;302(21):1163–1168. doi: 10.1056/NEJM198005223022102. [DOI] [PubMed] [Google Scholar]
- Dana N., Todd R. F., 3rd, Pitt J., Springer T. A., Arnaout M. A. Deficiency of a surface membrane glycoprotein (Mo1) in man. J Clin Invest. 1984 Jan;73(1):153–159. doi: 10.1172/JCI111186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T., Collins L. A. Increased expression of C3b receptors on polymorphonuclear leukocytes induced by chemotactic factors and by purification procedures. J Immunol. 1983 Jan;130(1):370–375. [PubMed] [Google Scholar]
- Fletcher M. P., Gallin J. I. Degranulating stimuli increase the availability of receptors on human neutrophils for the chemoattractant f-met-leu-phe. J Immunol. 1980 Apr;124(4):1585–1588. [PubMed] [Google Scholar]
- Fletcher M. P., Gallin J. I. Human neutrophils contain an intracellular pool of putative receptors for the chemoattractant N-formyl-methionyl-leucyl-phenylalanine. Blood. 1983 Oct;62(4):792–799. [PubMed] [Google Scholar]
- Goding J. W. Conjugation of antibodies with fluorochromes: modifications to the standard methods. J Immunol Methods. 1976;13(3-4):215–226. doi: 10.1016/0022-1759(76)90068-5. [DOI] [PubMed] [Google Scholar]
- Hoover R. L., Folger R., Haering W. A., Ware B. R., Karnovsky M. J. Adhesion of leukocytes to endothelium: roles of divalent cations, surface charge, chemotactic agents and substrate. J Cell Sci. 1980 Oct;45:73–86. doi: 10.1242/jcs.45.1.73. [DOI] [PubMed] [Google Scholar]
- Huang L. C., Civin C. I., Magnani J. L., Shaper J. H., Ginsburg V. My-1, the human myeloid-specific antigen detected by mouse monoclonal antibodies, is a sugar sequence found in lacto-N-fucopentaose III. Blood. 1983 May;61(5):1020–1023. [PubMed] [Google Scholar]
- Issekutz A. C., Lee K. Y., Biggar W. D. Enhancement of human neutrophil bactericidal activity by chemotactic factors. Infect Immun. 1979 May;24(2):295–301. doi: 10.1128/iai.24.2.295-301.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer N., Perez H. D., Goldstein I. M. An immunoglobulin (IgG) inhibitor of polymorphonuclear leukocyte motility in a patient with recurrent infection. N Engl J Med. 1980 Nov 27;303(22):1253–1258. doi: 10.1056/NEJM198011273032202. [DOI] [PubMed] [Google Scholar]
- Lehmeyer J. E., Snyderman R., Johnston R. B., Jr Stimulation of neutrophil oxidative metabolism by chemotactic peptides: influence of calcium ion concentration and cytochalasin B and comparison with stimulation by phorbol myristate acetate. Blood. 1979 Jul;54(1):35–45. [PubMed] [Google Scholar]
- López A. F., Vadas M. A. Stimulation of human granulocyte function by monoclonal antibody WEM-G1. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1818–1821. doi: 10.1073/pnas.81.6.1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marasco W. A., Phan S. H., Krutzsch H., Showell H. J., Feltner D. E., Nairn R., Becker E. L., Ward P. A. Purification and identification of formyl-methionyl-leucyl-phenylalanine as the major peptide neutrophil chemotactic factor produced by Escherichia coli. J Biol Chem. 1984 May 10;259(9):5430–5439. [PubMed] [Google Scholar]
- Nicola N. A., Metcalf D., Johnson G. R., Burgess A. W. Separation of functionally distinct human granulocyte-macrophage colony-stimulating factors. Blood. 1979 Sep;54(3):614–627. [PubMed] [Google Scholar]
- O'Flaherty J. T., Showell H. J., Ward P. A. Neutropenia induced by systemic infusion of chemotactic factors. J Immunol. 1977 May;118(5):1586–1589. [PubMed] [Google Scholar]
- Parham P. On the fragmentation of monoclonal IgG1, IgG2a, and IgG2b from BALB/c mice. J Immunol. 1983 Dec;131(6):2895–2902. [PubMed] [Google Scholar]
- Skubitz K. M., Pessano S., Bottero L., Ferrero D., Rovera G., August J. T. Human granulocyte surface molecules identified by murine monoclonal antibodies. J Immunol. 1983 Oct;131(4):1882–1888. [PubMed] [Google Scholar]
- Snyderman R., Goetzl E. J. Molecular and cellular mechanisms of leukocyte chemotaxis. Science. 1981 Aug 21;213(4510):830–837. doi: 10.1126/science.6266014. [DOI] [PubMed] [Google Scholar]
- Urdal D. L., Brentnall T. A., Bernstein I. D., Hakomori S. I. A granulocyte reactive monoclonal antibody, 1G10, identifies the Gal beta 1-4 (Fuc alpha 1-3)GlcNAc (X determinant) expressed in HL-60 cells on both glycolipid and glycoprotein molecules. Blood. 1983 Nov;62(5):1022–1026. [PubMed] [Google Scholar]
- Vadas M. A., David J. R., Butterworth A., Pisani N. T., Siongok T. A. A new method for the purification of human eosinophils and neutrophils, and a comparison of the ability of these cells to damage schistosomula of Schistosoma mansoni. J Immunol. 1979 Apr;122(4):1228–1236. [PubMed] [Google Scholar]
- Vadas M. A., Nicola N. A., Metcalf D. Activation of antibody-dependent cell-mediated cytotoxicity of human neutrophils and eosinophils by separate colony-stimulating factors. J Immunol. 1983 Feb;130(2):795–799. [PubMed] [Google Scholar]
- Vadas M. A., Nicola N., Lopez A. F., Metcalf D., Johnson G., Pereira A. Mononuclear cell-mediated enhancement of granulocyte function in man. J Immunol. 1984 Jul;133(1):202–207. [PubMed] [Google Scholar]


