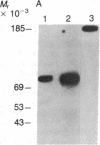
Abstract
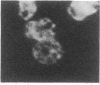
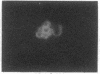
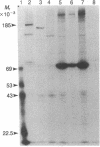
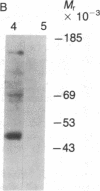
The asexual blood stages of the human malarial parasite Plasmodium falciparum produce many antigens, only some of which are important for protective immunity. Most of the putative protective antigens are believed to be expressed in schizonts and merozoites, the late stages of the asexual cycle. With the aim of cloning and characterizing genes for important parasite antigens, we used late-stage P. falciparum mRNA to construct a library of cDNA sequences inserted in the Escherichia coli expression vector pUC8. Nine thousand clones from the expression library were immunologically screened in situ with serum from Aotus monkeys immune to P. falciparum, and 95 clones expressing parasite antigens were identified. Mice were immunized with lysates from 49 of the bacterial clones that reacted with Aotus sera, and the mouse sera were tested for their reactivity with parasite antigens by indirect immunofluorescence, immunoprecipitation, and immunoblotting assays. Several different P. falciparum antigens were identified by these assays. Indirect immunofluorescence studies of extracellular merozoites showed that three of these antigens appear to be located on the merozoite surface. Thus, we have identified cDNA clones to three different P. falciparum antigens that may be important in protective immunity.
Full text
PDF


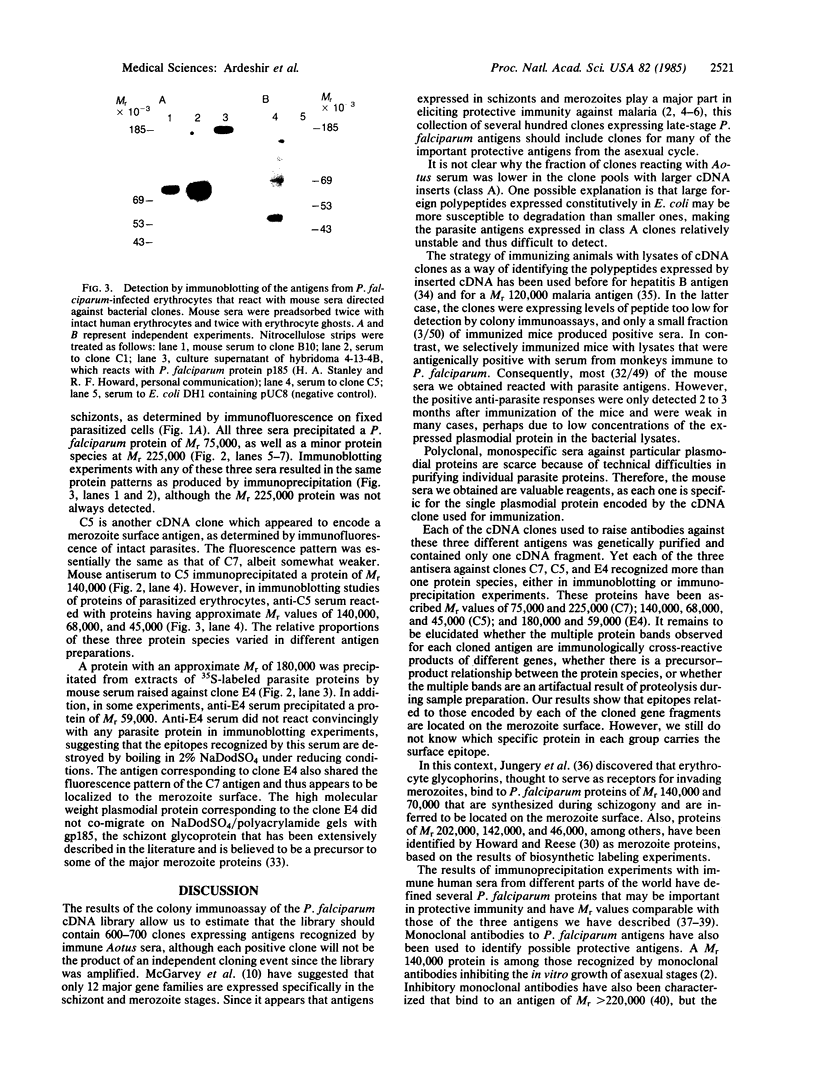

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G. V., Anders R. F., Knowles G. Differential effect of immunoglobulin on the in vitro growth of several isolates of Plasmodium falciparum. Infect Immun. 1983 Mar;39(3):1228–1235. doi: 10.1128/iai.39.3.1228-1235.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G. V., Coppel R. L., Vrbova H., Grumont R. J., Anders R. F. Plasmodium falciparum: comparative analysis of erythrocyte stage-dependent protein antigens. Exp Parasitol. 1982 Apr;53(2):279–284. doi: 10.1016/0014-4894(82)90070-4. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cochrane A. H., Santoro F., Nussenzweig V., Gwadz R. W., Nussenzweig R. S. Monoclonal antibodies identify the protective antigens of sporozoites of Plasmodium knowlesi. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5651–5655. doi: 10.1073/pnas.79.18.5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppel R. L., Brown G. V., Mitchell G. F., Anders R. F., Kemp D. J. Identification of a cDNA clone encoding a mature blood stage antigen of Plasmodium falciparum by immunization of mice with bacterial lysates. EMBO J. 1984 Feb;3(2):403–407. doi: 10.1002/j.1460-2075.1984.tb01820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppel R. L., Cowman A. F., Anders R. F., Bianco A. E., Saint R. B., Lingelbach K. R., Kemp D. J., Brown G. V. Immune sera recognize on erythrocytes Plasmodium falciparum antigen composed of repeated amino acid sequences. 1984 Aug 30-Sep 5Nature. 310(5980):789–792. doi: 10.1038/310789a0. [DOI] [PubMed] [Google Scholar]
- Coppel R. L., Cowman A. F., Lingelbach K. R., Brown G. V., Saint R. B., Kemp D. J., Anders R. F. Isolate-specific S-antigen of Plasmodium falciparum contains a repeated sequence of eleven amino acids. Nature. 1983 Dec 22;306(5945):751–756. doi: 10.1038/306751a0. [DOI] [PubMed] [Google Scholar]
- Dame J. B., Williams J. L., McCutchan T. F., Weber J. L., Wirtz R. A., Hockmeyer W. T., Maloy W. L., Haynes J. D., Schneider I., Roberts D. Structure of the gene encoding the immunodominant surface antigen on the sporozoite of the human malaria parasite Plasmodium falciparum. Science. 1984 Aug 10;225(4662):593–599. doi: 10.1126/science.6204383. [DOI] [PubMed] [Google Scholar]
- Dubois P., Dedet J. P., Fandeur T., Roussilhon C., Jendoubi M., Pauillac S., Mercereau-Puijalon O., Pereira Da Silva L. Protective immunization of the squirrel monkey against asexual blood stages of Plasmodium falciparum by use of parasite protein fractions. Proc Natl Acad Sci U S A. 1984 Jan;81(1):229–232. doi: 10.1073/pnas.81.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enea V., Ellis J., Zavala F., Arnot D. E., Asavanich A., Masuda A., Quakyi I., Nussenzweig R. S. DNA cloning of Plasmodium falciparum circumsporozoite gene: amino acid sequence of repetitive epitope. Science. 1984 Aug 10;225(4662):628–630. doi: 10.1126/science.6204384. [DOI] [PubMed] [Google Scholar]
- Freeman R. R., Holder A. A. Surface antigens of malaria merozoites. A high molecular weight precursor is processed to an 83,000 mol wt form expressed on the surface of Plasmodium falciparum merozoites. J Exp Med. 1983 Nov 1;158(5):1647–1653. doi: 10.1084/jem.158.5.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritzmacher C. A., Reese R. T. Translation in vitro of RNA from the human malarial parasite Plasmodium falciparum. Biosci Rep. 1982 Sep;2(9):667–673. doi: 10.1007/BF01114828. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Helfman D. M., Feramisco J. R., Fiddes J. C., Thomas G. P., Hughes S. H. Identification of clones that encode chicken tropomyosin by direct immunological screening of a cDNA expression library. Proc Natl Acad Sci U S A. 1983 Jan;80(1):31–35. doi: 10.1073/pnas.80.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard R. F., Reese R. T. Synthesis of merozoite proteins and glycoproteins during the schizogony of Plasmodium falciparum. Mol Biochem Parasitol. 1984 Mar;10(3):319–334. doi: 10.1016/0166-6851(84)90030-6. [DOI] [PubMed] [Google Scholar]
- Hyde J. E., Goman M., Hall R., Osland A., Hope I. A., Langsley G., Zolg J. W., Scaife J. G. Characterisation and translation studies of messenger RNA from the human malaria parasite Plasmodium falciparum and construction of a cDNA library. Mol Biochem Parasitol. 1984 Mar;10(3):269–285. doi: 10.1016/0166-6851(84)90026-4. [DOI] [PubMed] [Google Scholar]
- Jungery M., Boyle D., Patel T., Pasvol G., Weatherall D. J. Lectin-like polypeptides of P. falciparum bind to red cell sialoglycoproteins. Nature. 1983 Feb 24;301(5902):704–705. doi: 10.1038/301704a0. [DOI] [PubMed] [Google Scholar]
- Kemp D. J., Coppel R. L., Cowman A. F., Saint R. B., Brown G. V., Anders R. F. Expression of Plasmodium falciparum blood-stage antigens in Escherichia coli: detection with antibodies from immune humans. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3787–3791. doi: 10.1073/pnas.80.12.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langreth S. G., Reese R. T. Antigenicity of the infected-erythrocyte and merozoite surfaces in Falciparum malaria. J Exp Med. 1979 Nov 1;150(5):1241–1254. doi: 10.1084/jem.150.5.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langreth S. G., Reese R. T., Motyl M. R., Trager W. Plasmodium falciparum: loss of knobs on the infected erythrocyte surface after long-term cultivation. Exp Parasitol. 1979 Oct;48(2):213–219. doi: 10.1016/0014-4894(79)90101-2. [DOI] [PubMed] [Google Scholar]
- MacKay P., Pasek M., Magazin M., Kovacic R. T., Allet B., Stahl S., Gilbert W., Schaller H., Bruce S. A., Murray K. Production of immunologically active surface antigens of hepatitis B virus by Escherichia coli. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4510–4514. doi: 10.1073/pnas.78.7.4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- McGarvey M. J., Sheybani E., Loche M. P., Perrin L., Mach B. Identification and expression in Escherichia coli of merozoite stage-specific genes of the human malarial parasite Plasmodium falciparum. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3690–3694. doi: 10.1073/pnas.81.12.3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold C. I. Intraerythrocytic development and antigenicity of asexual malaria parasites. Mol Biochem Parasitol. 1984 Apr;11:1–22. doi: 10.1016/0166-6851(84)90051-3. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Perrin L. H., Dayal R. Immunity to asexual erythrocytic stages of Plasmodium falciparum: role of defined antigens in the humoral response. Immunol Rev. 1982;61:245–269. doi: 10.1111/j.1600-065x.1982.tb00379.x. [DOI] [PubMed] [Google Scholar]
- Perrin L. H., Dayal R., Rieder H. Characterization of antigens from erythrocytic stages of Plasmodium falciparum reacting with human immune sera. Trans R Soc Trop Med Hyg. 1981;75(1):163–165. doi: 10.1016/0035-9203(81)90055-9. [DOI] [PubMed] [Google Scholar]
- Perrin L. H., Merkli B., Loche M., Chizzolini C., Smart J., Richle R. Antimalarial immunity in Saimiri monkeys. Immunization with surface components of asexual blood stages. J Exp Med. 1984 Aug 1;160(2):441–451. doi: 10.1084/jem.160.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese R. T., Langreth S. G., Trager W. Isolation of stages of the human parasite Plasmodium falciparum from culture and from animal blood. Bull World Health Organ. 1979;57 (Suppl 1):53–61. [PMC free article] [PubMed] [Google Scholar]
- Reese R. T., Motyl M. R., Hofer-Warbinek R. Reaction of immune sera with components of the human malarial parasite, Plasmodium falciparum. Am J Trop Med Hyg. 1981 Nov;30(6):1168–1178. doi: 10.4269/ajtmh.1981.30.1168. [DOI] [PubMed] [Google Scholar]
- Reese R. T., Motyl M. R. Inhibition of the in vitro growth of Plasmodium falciparum. I. The effects of immune serum and purified immunoglobulin from owl monkeys. J Immunol. 1979 Oct;123(4):1894–1899. [PubMed] [Google Scholar]
- Reese R. T., Trager W., Jensen J. B., Miller D. A., Tantravahi R. Immunization against malaria with antigen from Plasmodium falciparum cultivated in vitro. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5665–5668. doi: 10.1073/pnas.75.11.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saul A., Myler P., Schofield L., Kidson C. A high molecular weight antigen in Plasmodium falciparum recognized by inhibitory monoclonal antibodies. Parasite Immunol. 1984 Jan;6(1):39–50. doi: 10.1111/j.1365-3024.1984.tb00780.x. [DOI] [PubMed] [Google Scholar]
- Siddiqui W. A. An effective immunization of experimental monkeys against a human malaria parasite, Plasmodium falciparum. Science. 1977 Jul 22;197(4301):388–389. doi: 10.1126/science.406671. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stanley H. A., Langreth S. G., Reese R. T., Trager W. Plasmodium falciparum merozoites: isolation by density gradient centrifugation using Percoll and antigenic analysis. J Parasitol. 1982 Dec;68(6):1059–1067. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]