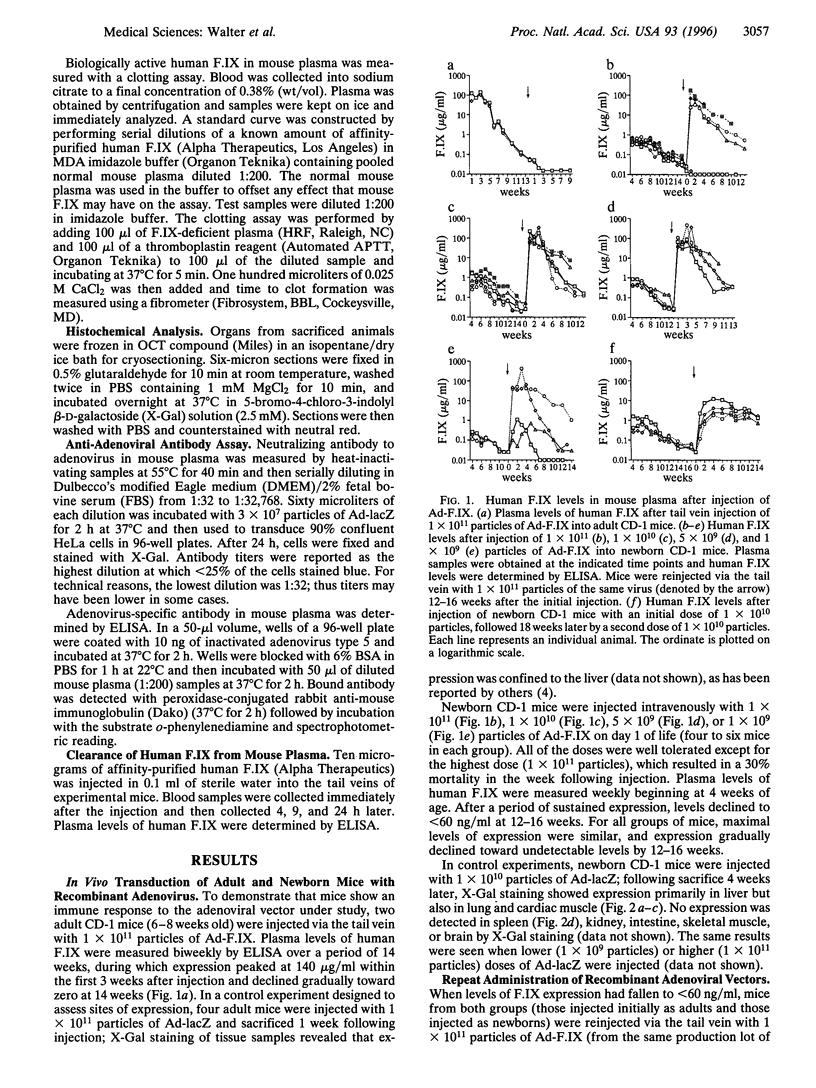
Abstract
Adenoviral vectors can direct high-level expression of a transgene, but, due to a host immune response to adenoviral antigens, expression is of limited duration, and repetitive administration has generally been unsuccessful. Exposure to foreign proteins beginning in the neonatal period may alter or ablate the immune response. We injected adult and neonatal (immunocompetent) CD-1 mice intravenously with an adenoviral vector expressing human blood coagulation factor IX. In both groups of mice, expression of human factor IX persisted for 12-16 weeks. However, in mice initially injected as adults, repeat administration of the vector resulted in no detectable expression of the transgene, whereas in mice initially injected in the neonatal period, repeat administration resulted in high-level expression of human factor IX. We show that animals that fail to express the transgene on repeat administration have developed high-titer neutralizing antibodies to adenovirus, whereas those that do express factor IX have not. This experimental model suggests that newborn mice can be tolerized to adenoviral vectors and demonstrates that at least one repeat injection of the adenoviral vector is possible; the model will be useful in elucidating the immunologic mechanisms underlying successful repeat administration of adenoviral vectors.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BILLINGHAM R. E., BRENT L., MEDAWAR P. B. Actively acquired tolerance of foreign cells. Nature. 1953 Oct 3;172(4379):603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- Barr D., Tubb J., Ferguson D., Scaria A., Lieber A., Wilson C., Perkins J., Kay M. A. Strain related variations in adenovirally mediated transgene expression from mouse hepatocytes in vivo: comparisons between immunocompetent and immunodeficient inbred strains. Gene Ther. 1995 Mar;2(2):151–155. [PubMed] [Google Scholar]
- Dai Y., Roman M., Naviaux R. K., Verma I. M. Gene therapy via primary myoblasts: long-term expression of factor IX protein following transplantation in vivo. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10892–10895. doi: 10.1073/pnas.89.22.10892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y., Schwarz E. M., Gu D., Zhang W. W., Sarvetnick N., Verma I. M. Cellular and humoral immune responses to adenoviral vectors containing factor IX gene: tolerization of factor IX and vector antigens allows for long-term expression. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1401–1405. doi: 10.1073/pnas.92.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzierzak E., Medvinsky A. Mouse embryonic hematopoiesis. Trends Genet. 1995 Sep;11(9):359–366. doi: 10.1016/s0168-9525(00)89107-6. [DOI] [PubMed] [Google Scholar]
- Engelhardt J. F., Ye X., Doranz B., Wilson J. M. Ablation of E2A in recombinant adenoviruses improves transgene persistence and decreases inflammatory response in mouse liver. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):6196–6200. doi: 10.1073/pnas.91.13.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furie B., Limentani S. A., Rosenfield C. G. A practical guide to the evaluation and treatment of hemophilia. Blood. 1994 Jul 1;84(1):3–9. [PubMed] [Google Scholar]
- Kaufman R. J., Wasley L. C., Furie B. C., Furie B., Shoemaker C. B. Expression, purification, and characterization of recombinant gamma-carboxylated factor IX synthesized in Chinese hamster ovary cells. J Biol Chem. 1986 Jul 25;261(21):9622–9628. [PubMed] [Google Scholar]
- Kay M. A., Holterman A. X., Meuse L., Gown A., Ochs H. D., Linsley P. S., Wilson C. B. Long-term hepatic adenovirus-mediated gene expression in mice following CTLA4Ig administration. Nat Genet. 1995 Oct;11(2):191–197. doi: 10.1038/ng1095-191. [DOI] [PubMed] [Google Scholar]
- Kay M. A., Landen C. N., Rothenberg S. R., Taylor L. A., Leland F., Wiehle S., Fang B., Bellinger D., Finegold M., Thompson A. R. In vivo hepatic gene therapy: complete albeit transient correction of factor IX deficiency in hemophilia B dogs. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2353–2357. doi: 10.1073/pnas.91.6.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay M. A., Rothenberg S., Landen C. N., Bellinger D. A., Leland F., Toman C., Finegold M., Thompson A. R., Read M. S., Brinkhous K. M. In vivo gene therapy of hemophilia B: sustained partial correction in factor IX-deficient dogs. Science. 1993 Oct 1;262(5130):117–119. doi: 10.1126/science.8211118. [DOI] [PubMed] [Google Scholar]
- Lu C. Y., Calamai E. G., Unanue E. R. A defect in the antigen-presenting function of macrophages from neonatal mice. Nature. 1979 Nov 15;282(5736):327–329. doi: 10.1038/282327a0. [DOI] [PubMed] [Google Scholar]
- Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- Smith T. A., Mehaffey M. G., Kayda D. B., Saunders J. M., Yei S., Trapnell B. C., McClelland A., Kaleko M. Adenovirus mediated expression of therapeutic plasma levels of human factor IX in mice. Nat Genet. 1993 Dec;5(4):397–402. doi: 10.1038/ng1293-397. [DOI] [PubMed] [Google Scholar]
- Stevenson S. C., Marshall-Neff J., Teng B., Lee C. B., Roy S., McClelland A. Phenotypic correction of hypercholesterolemia in apoE-deficient mice by adenovirus-mediated in vivo gene transfer. Arterioscler Thromb Vasc Biol. 1995 Apr;15(4):479–484. doi: 10.1161/01.atv.15.4.479. [DOI] [PubMed] [Google Scholar]
- Stratford-Perricaudet L. D., Levrero M., Chasse J. F., Perricaudet M., Briand P. Evaluation of the transfer and expression in mice of an enzyme-encoding gene using a human adenovirus vector. Hum Gene Ther. 1990 Fall;1(3):241–256. doi: 10.1089/hum.1990.1.3-241. [DOI] [PubMed] [Google Scholar]
- Stratford-Perricaudet L. D., Makeh I., Perricaudet M., Briand P. Widespread long-term gene transfer to mouse skeletal muscles and heart. J Clin Invest. 1992 Aug;90(2):626–630. doi: 10.1172/JCI115902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A. R., Forrey A. W., Gentry P. A., Smith K. J., Harker L. A. Human factor IX in animals: kinetics from isolated, radiolabelled protein and platelet destruction following crude concentrate infusions. Br J Haematol. 1980 Jun;45(2):329–342. doi: 10.1111/j.1365-2141.1980.tb07152.x. [DOI] [PubMed] [Google Scholar]
- Tripathy S. K., Goldwasser E., Lu M. M., Barr E., Leiden J. M. Stable delivery of physiologic levels of recombinant erythropoietin to the systemic circulation by intramuscular injection of replication-defective adenovirus. Proc Natl Acad Sci U S A. 1994 Nov 22;91(24):11557–11561. doi: 10.1073/pnas.91.24.11557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Nunes F. A., Berencsi K., Furth E. E., Gönczöl E., Wilson J. M. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zauber N. P., Levin J. Factor IX levels in patients with hemophilia B (Christmas disease) following transfusion with concentrates of factor IX or fresh frozen plasma (FFP). Medicine (Baltimore) 1977 May;56(3):213–224. doi: 10.1097/00005792-197705000-00003. [DOI] [PubMed] [Google Scholar]




