Abstract
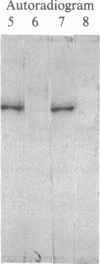
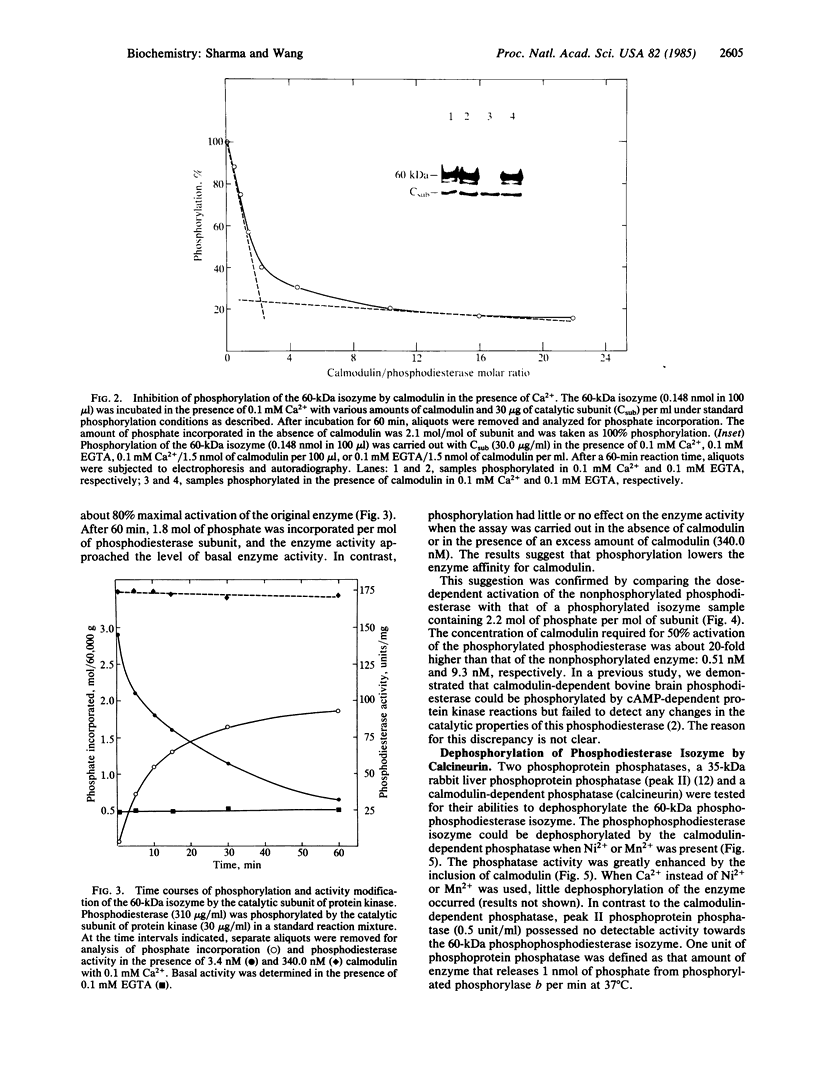
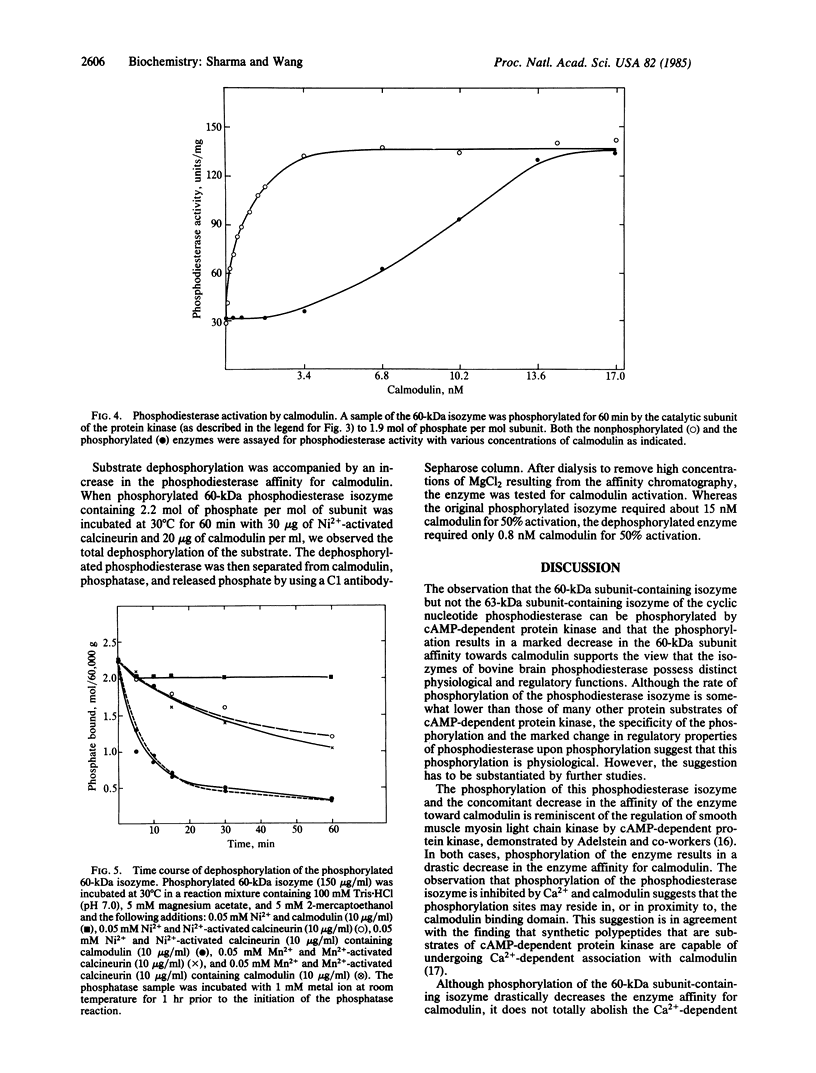
Purified bovine brain calmodulin-dependent cyclic nucleotide phosphodiesterase (3',5'-cyclic-nucleotide 5'-nucleotidohydrolase, EC 3.1.4.17) contains isozymes that are composed of two distinct subunits with molecular masses of 60,000 and 63,000 daltons. Analysis by NaDodSO4 gel electrophoresis and autoradiography of a phosphodiesterase sample phosphorylated in the presence of [32P]ATP and bovine heart cAMP-dependent protein kinase catalytic subunit revealed that only the 60-kDa subunit was phosphorylated. By using an isozyme preparation greatly enriched with the 60-kDa subunit, the following observations regarding the subunit phosphorylation were made. First, the phosphorylation resulted in the maximal incorporation of about 2 mol of phosphate per mol of subunit. Second, complete inhibition of 60-kDa subunit phosphorylation was approached at a saturating concentration of Ca2+ when a molar ratio of calmodulin to phosphodiesterase of 2:1 was used. No inhibition was observed in the presence of either Ca2+ or calmodulin alone. Third, the phosphorylation was accompanied by a decrease in the enzyme affinity for calmodulin; calmodulin concentrations required for 50% activation of nonphosphorylated and maximally phosphorylated phosphodiesterase isozyme samples were 0.51 and 9.3 nM, respectively. Fourth, the phosphodiesterase isozyme could be dephosphorylated by the calmodulin-dependent phosphatase (calcineurin) in the presence of Ni2+ or Mn2+, the dephosphorylation being associated with an increase in the enzyme affinity for calmodulin. Fifth, peak II rabbit liver phosphoprotein phosphatase catalytic unit did not catalyze the dephosphorylation of the phosphodiesterase isozyme.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Conti M. A., Adelstein R. S. The relationship between calmodulin binding and phosphorylation of smooth muscle myosin kinase by the catalytic subunit of 3':5' cAMP-dependent protein kinase. J Biol Chem. 1981 Apr 10;256(7):3178–3181. [PubMed] [Google Scholar]
- Demaille J. G., Peters K. A., Fischer E. H. Isolation and properties of the rabbit skeletal muscle protein inhibitor of adenosine 3',5'-monophosphate dependent protein kinases. Biochemistry. 1977 Jul 12;16(14):3080–3086. doi: 10.1021/bi00633a006. [DOI] [PubMed] [Google Scholar]
- Gopalakrishna R., Anderson W. B. Ca2+-induced hydrophobic site on calmodulin: application for purification of calmodulin by phenyl-Sepharose affinity chromatography. Biochem Biophys Res Commun. 1982 Jan 29;104(2):830–836. doi: 10.1016/0006-291x(82)90712-4. [DOI] [PubMed] [Google Scholar]
- Grand R. J., Perry S. V. Calmodulin-binding proteins from brain and other tissues. Biochem J. 1979 Nov 1;183(2):285–295. doi: 10.1042/bj1830285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen R. S., Beavo J. A. Purification of two calcium/calmodulin-dependent forms of cyclic nucleotide phosphodiesterase by using conformation-specific monoclonal antibody chromatography. Proc Natl Acad Sci U S A. 1982 May;79(9):2788–2792. doi: 10.1073/pnas.79.9.2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. Y., Chau V., Chock P. B., Wang J. H., Sharma R. K. Mechanism of activation of cyclic nucleotide phosphodiesterase: requirement of the binding of four Ca2+ to calmodulin for activation. Proc Natl Acad Sci U S A. 1981 Feb;78(2):871–874. doi: 10.1073/pnas.78.2.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandelwal R. L., Vandenheede J. R., Krebs E. G. Purification, properties, and substrate specificities of phosphoprotein phosphatase(s) from rabbit liver. J Biol Chem. 1976 Aug 25;251(16):4850–4858. [PubMed] [Google Scholar]
- Kincaid R. L., Manganiello V. C., Odya C. E., Osborne J. C., Jr, Stith-Coleman I. E., Danello M. A., Vaughan M. Purification and properties of calmodulin-stimulated phosphodiesterase from mammalian brain. J Biol Chem. 1984 Apr 25;259(8):5158–5166. [PubMed] [Google Scholar]
- Kincaid R. L., Vaughan M. Affinity chromatography of brain cyclic nucleotide phosphodiesterase using 3-(2-pyridyldithio)propionyl-substituted calmodulin linked to thiol-sepharose. Biochemistry. 1983 Feb 15;22(4):826–830. doi: 10.1021/bi00273a018. [DOI] [PubMed] [Google Scholar]
- LaPorte D. C., Toscano W. A., Jr, Storm D. R. Cross-linking of iodine-125-labeled, calcium-dependent regulatory protein to the Ca2+-sensitive phosphodiesterase purified from bovine heart. Biochemistry. 1979 Jun 26;18(13):2820–2825. doi: 10.1021/bi00580a021. [DOI] [PubMed] [Google Scholar]
- Malencik D. A., Anderson S. R. Binding of hormones and neuropeptides by calmodulin. Biochemistry. 1983 Apr 12;22(8):1995–2001. doi: 10.1021/bi00277a040. [DOI] [PubMed] [Google Scholar]
- Morrill M. E., Thompson S. T., Stellwagen E. Purification of a cyclic nucleotide phosphodiesterase from bovine brain using blue dextran-Sepharose chromatography. J Biol Chem. 1979 Jun 10;254(11):4371–4374. [PubMed] [Google Scholar]
- Pallen C. J., Wang J. H. Regulation of calcineurin by metal ions. Mechanism of activation by Ni2+ and an enhanced response to Ca2+/calmodulin. J Biol Chem. 1984 May 25;259(10):6134–6141. [PubMed] [Google Scholar]
- Reimann E. M., Walsh D. A., Krebs E. G. Purification and properties of rabbit skeletal muscle adenosine 3',5'-monophosphate-dependent protein kinases. J Biol Chem. 1971 Apr 10;246(7):1986–1995. [PubMed] [Google Scholar]
- Sharma R. K., Adachi A. M., Adachi K., Wang J. H. Demonstration of bovine brain calmodulin-dependent cyclic nucleotide phosphodiesterase isozymes by monoclonal antibodies. J Biol Chem. 1984 Jul 25;259(14):9248–9254. [PubMed] [Google Scholar]
- Sharma R. K., Taylor W. A., Wang J. H. Use of calmodulin affinity chromatography for purification of specific calmodulin-dependent enzymes. Methods Enzymol. 1983;102:210–219. doi: 10.1016/s0076-6879(83)02022-4. [DOI] [PubMed] [Google Scholar]
- Sharma R. K., Wang T. H., Wirch E., Wang J. H. Purification and properties of bovine brain calmodulin-dependent cyclic nucleotide phosphodiesterase. J Biol Chem. 1980 Jun 25;255(12):5916–5923. [PubMed] [Google Scholar]
- Wang J. H., Sharma R. K., Huang C. Y., Chau V., Chock P. B. On the mechanism of activation of cyclic mucleotide phosphodiesterase by calmodulin. Ann N Y Acad Sci. 1980;356:190–204. doi: 10.1111/j.1749-6632.1980.tb29611.x. [DOI] [PubMed] [Google Scholar]