Abstract
Background
Infants born prematurely are often treated with supplemental oxygen, which can increase their risk for airway hyperresponsiveness (AHR), asthma, reduced lung function, and altered responses to respiratory viral infections later in childhood. Likewise, exposure of newborn mice to hyperoxia alters baseline pulmonary mechanics and the host response to influenza A virus infection in adult mice. Here, we use this mouse model to test the hypothesis that neonatal hyperoxia also promotes AHR and exacerbated allergen-induced symptoms in adult mice.
Methods
Baseline lung mechanics and AHR measured by methacholine provocation were assessed in adult male and female mice exposed to room air or 100% oxygen (hyperoxia) between postnatal days 0 – 4. AHR and lung inflammation were evaluated after adult female mice were sensitized with ovalbumin (OVA) plus alum and challenged with aerosolized OVA. Results: Baseline lung compliance increased and resistance decreased in adult female, but not male, mice exposed to neonatal hyperoxia compared to siblings exposed to room air. Neonatal hyperoxia significantly enhanced methacholine-induced AHR in female mice, but did not affect allergen-induced AHR to methacholine or lung inflammation.
Conclusion
Increased incidence of AHR and asthma is reported in children born prematurely and exposed to supplemental oxygen. Our findings in adult female mice exposed to hyperoxia as neonates suggest that this AHR reported in children born prematurely may reflect non-atopic wheezing due to intrinsic structural changes in airway development.
Keywords: airway hyperresponsiveness, allergen, asthma, eosinophilia, lung inflammation, murine, neonatal hyperoxia
Introduction
Preterm infants, especially those with low birth weight, are often exposed to high oxygen, mechanical ventilation, antenatal steroids, and/or exogenous surfactant. While these treatments have markedly reduced infant mortality, survivors of prematurity have an increased risk for chronic lung disease, including airway hyperresponsiveness (AHR) and asthma (1-5). Asthma is characterized by wheezing, coughing, lung inflammation and AHR, affects more than 7% of the US population (6) and can by triggered by a variety of exposures including allergens, cold air and viral infections. In term born children, asthma and AHR have a strong association with allergy and pulmonary inflammation. However, this association is significantly weaker in children born preterm (4). Hence, the underlying cause of asthma and AHR in children born prematurely remains unclear.
Analogous to children born prematurely, animals exposed to high oxygen as newborns often exhibit long-term deficits in normal lung function, lung structure, and host response to respiratory pathogens. In neonates or immature animals, oxygen-dependent changes in AHR have been attributed in part to increased airway smooth muscle contractility, muscle thickness, and elastin production (7-9). Oxygen-dependent changes in AHR are specific for the developing lung because AHR is not observed in adult rats exposed to hyperoxia (10). A study in newborn mice suggested the oxygen-dependent changes in AHR persist into adulthood, and were mediated through inhibition of peroxisome proliferator-activated receptor-γ signaling (11). However, whether AHR persists into adulthood is unclear, with some studies showing persistence and other studies showing resolution (7, 11, 12). It would therefore be ideal to use an established animal model that exhibits persistence in other oxygen-dependent changes in lung disease to determine whether AHR persists into adulthood following neonatal oxygen exposure and whether it exacerbates allergen induced AHR and inflammation.
We previously demonstrated exposure of newborn mice to 100% oxygen between postnatal days 0-4 causes alveolar simplification and altered elastin deposition in young adult mice (13, 14). A concomitant increase in baseline pulmonary compliance was also observed. In addition, supplemental oxygen exposure increased the sensitivity of the adult mice to influenza virus infection with increased morbidity and an enhanced pulmonary inflammatory response, providing further evidence that this mouse model recapitulates childhood diseases attributed to prematurity and particularly early life oxygen exposure (15-18). Using this model, we tested the hypothesis that neonatal hyperoxia promotes AHR and/or exacerbates allergen-induced increases in AHR and lung inflammation in adult mice.
METHODS
Exposure of mice to hyperoxia
Within 12 hours of birth, newborn mice were exposed to either room air or 100% oxygen until postnatal day 4 (13) and studied between 54-77 days of age. All animal experiments conformed to NIH guidelines, and were reviewed and approved by University of Rochester and University of Minnesota Institutional Animal Care and Use Committees. C57Bl6/J mice (Jackson Laboratory, Bar Harbor, ME) were used in this study because their response to neonatal hyperoxia has been extensively characterized in our laboratory (13-16) and they have been widely used in the ovalbumin mouse model of asthma.
Measurement of lung mechanics and AHR
Mice were anesthetized with ketamine/xylazine/acepromazine, trachea and external jugular vein cannulated, paralyzed with decamethonium and ventilated at 150 breaths per min (tidal volume 10 ml/kg) using a computer-controlled SciReq FlexiVent (Flexivent 5.1 software, Montreal, Canada). Initially, a step-wise volume driven inflation/deflation PV loop (PVs-V) was used to determine static compliance, followed by a Snapshot 150 maneuver that applied a sinusoidal signal (2.5 Hz for 2 sec) and a linear single compartment model to calculate initial dynamic resistance (R) and compliance (C). Central airway resistance (Rn), tissue damping (G) and tissue elastance (H) in these groups of animals was also determined using a forced oscillation technique (Quick Prime 3) prior to determination of methacholine responsiveness. For determination of AHR, methacholine doses of 0.03 - 3 mg/kg were delivered successively by bolus injection into external jugular vein. Maximal change in each parameter to each methacholine dose was recorded. To standardize volume history, initial pulmonary maneuvers as well as each dose of methacholine were preceded by two consecutive inflations of lung to total lung capacity. The change in resistance (maximum/initial value) and compliance (minimum/initial value) was expressed as a ratio.
Allergic sensitization of mice
Mice were sensitized twice at 5-6 wk of age by intraperitoneal administration of 5 mg/kg ovalbumin (OVA; Sigma Chemical Co., St. Louis, MO) or saline in alum (Pierce Chemical Co, St. Louis, MO). All animals were then challenged on 3 consecutive days with 1% OVA aerosol for 30 min. Airway responsiveness to methacholine and lung inflammation was determined 20-24 hours after the last OVA exposure. After assessment of methacholine responsiveness, animals were euthanized, lungs lavaged in situ twice with 0.5 ml of phosphate buffered saline, bled by cardiac puncture, protein in bronchoalveolar lavage (BAL) supernatant determined and BAL cells identified and counted as previously described (19). After lavage, the left caudal lung lobe was homogenized and processed for measurement of eosinophil peroxidase (EPO, total OD/min) and the right caudal lung lobe for myeloperoxidase (MPO, total units of enzyme activity), respectively, as previously described (19). EPO and MPO values were normalized with g dry weight of lung to approximate density of a particular cell type in the lung after removal of cells in airspace by lavage. OVA specific IgG1 in serum was determined by a standard method using OVA coated ELISA plates and IgG1 monoclonal antibody to OVA as standard (Thermo Scientific, Rockford, IL, HYB 094-06-02).
Statistical analysis
All data were log transformed for statistical analysis. Figures show geometric mean + 1 standard error (SE) with 7- 9 animals per treatment group. Statistical significance was defined as p<0.05 with one-sided or two-sided comparisons as appropriate. ANOVA analysis and repeated measures ANOVA were conducted using JMP and SAS software (SAS Institute Inc., Cary NC).
Results
Neonatal hyperoxia alters baseline lung function and AHR in adult mice
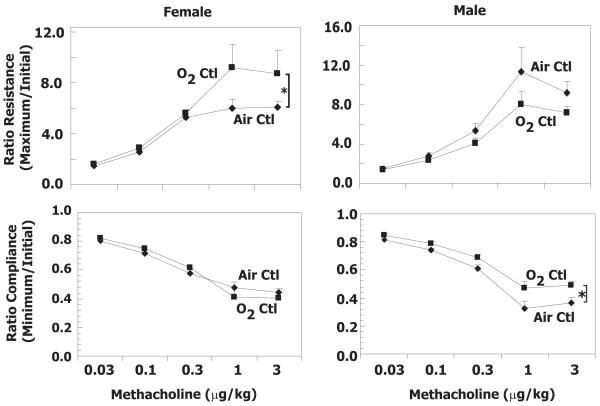
Baseline resistance and compliance were evaluated in adult mice exposed to room air or hyperoxia (100% oxygen) between postnatal days 0-4. Neonatal hyperoxia significantly decreased resistance and increased compliance in adult female but not male mice (Fig 1). Tissue damping and elastance were also significantly decreased in females exposed to oxygen as neonates, whereas the decrease in central airway resistance did not reach statistical significance (Table 1). In contrast, the same effects of hyperoxia on lung mechanics were not evident in male mice. Static compliance was calculated from the PV loop and paralleled changes in compliance (p<0.05) in oxygen-exposed male and female animals when compared to room air exposure (data not shown).
Figure 1.
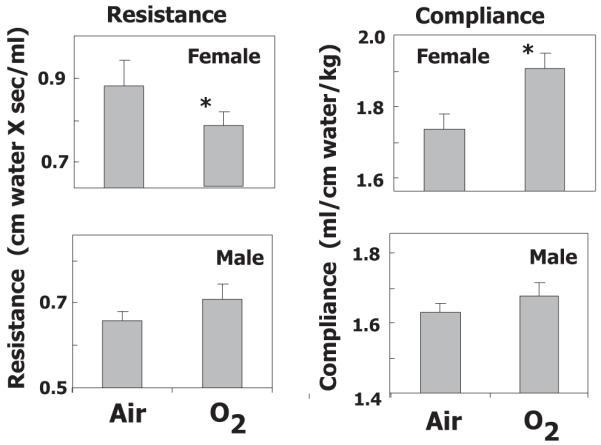
Neonatal oxygen exposure alters baseline lung mechanics in adult mice. Lung mechanics were evaluated in adult female and male mice exposed to room air (Air) or 100% oxygen (O2) between postnatal days 0-4. Values represent the geometric mean + SE for 7-9 animals per group. * p<0.05 relative to Air.
Table 1.
Baseline values for airway resistance (Rn), tissue damping (G), tissue elastance (H)
| Female |
Male |
|||
|---|---|---|---|---|
| Air | O2 | Air | O2 | |
| Rn (cm H2OXsec/ml) |
0.47±0.06 | 0.43±0.02 | 0.36±0.02 | 0.37±0.03 |
| G (cm H2O/ml) |
5.27±0.31 | 4.63±0.11* | 4.16±0.11 | 4.51±0.18 |
| H (cm H2O/ml) |
31.3±0.95 | 28.32±1.26* | 25.73±0.68 | 26.14±0.96 |
p<0.05, Air vs O2
Airway responsiveness was measured by determining increased resistance and decreased compliance in response to methacholine in male and female adult mice exposed to hyperoxia or room air as neonates. Female mice exposed to hyperoxia as neonates had significantly increased resistance to methacholine challenge (Fig 2). This increased airway responsiveness following neonatal oxygen exposure was only evident in resistance changes induced by methacholine but not compliance changes. Males exposed to oxygen as neonates had a slight reduction in methacholine responsiveness compared to air exposed neonates with the difference in compliance being significant (Fig 2).
Figure 2.
Neonatal oxygen exposure increases AHR to methacholine in adult mice. Airway resistance and lung compliance were evaluated in adult female and male mice exposed as neonates to room air (Air Ctl) or 100% oxygen (O2 Ctl ). Values represent the geometric mean + SE for 7-9 animals administered increasing amounts of methacholine intravenously (0.03 – 3.0 μg/kg). * p<0.05 by repeated measures ANOVA.
Neonatal oxygen exposure does not exacerbate allergen-induced AHR and lung inflammation
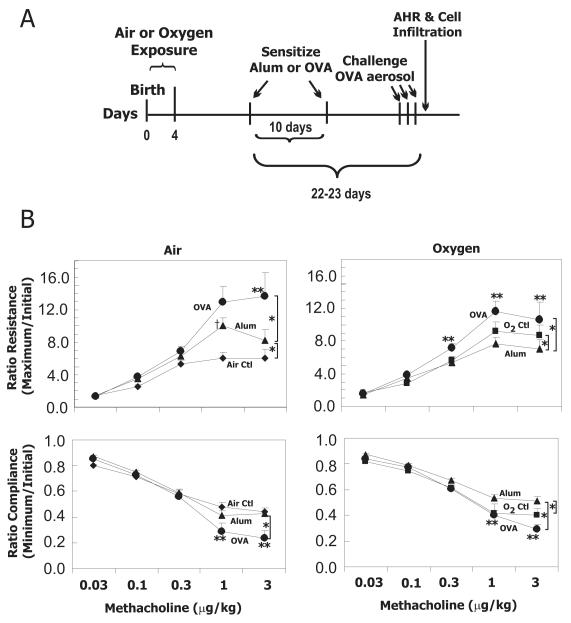
Since neonatal exposure to supplemental oxygen increased methacholine responsiveness in adult female mice, we examined its effect on allergen-induced AHR and lung inflammation in a separate group of adult females. Adult female mice exposed to room air or hyperoxia as neonates were sensitized with alum or OVA and challenged with OVA aerosol on three consecutive days (Fig 3A). In this group, body weights at time of sensitization were also different: Air 16.3 ± 0.2 g vs Oxygen 14.7 ± 0.2 g. Airway responsiveness to methacholine of unsensitized animals (Air Ctl, O2 Ctl) is duplicated in Fig 3B for comparison and reporting of statistical analysis. As expected, OVA sensitized mice had greater airway reactivity to methacholine than animals treated with alum, regardless of whether they were exposed to room air or hyperoxia as neonates. While increased methacholine reactivity was observed at 3μg/kg in mice exposed to room air as neonates, increased methacholine reactivity was observed at lower doses of methacholine (0.3-3μg/kg) in mice exposed to hyperoxia as neonates. However, the maximum methacholine-induced increase in resistance was not significantly different between allergen challenged mice exposed to room air or hyperoxia as neonates. Likewise, methacholine-induced decrease in lung compliance was more severe following OVA challenge, but the change was not affected by early oxygen exposure. Alum treatment alone significantly enhanced methacholine-induced increase in resistance in air-exposed mice, but significantly impaired methacholine-induced decrease in compliance in oxygen-exposed mice.
Figure 3.
Neonatal oxygen exposure does not alter allergen-induced AHR in adult mice. a) Timeline for allergic sensitization of adult mice exposed to room air or hyperoxia as neonates. AHR was assessed in adult animals between 54-77 days of age. b) Airway resistance and lung compliance were evaluated in adult female mice exposed to room air (Air) or 100% oxygen (O2) as neonates. Animals were not sensitized controls (Ctl), or sensitized with Alum plus saline (Alum) or Alum plus ovalbumin (OVA). All mice were challenged with OVA aerosol. Values represent the geometric mean + SE of 7-9 animals administered increasing amounts of methacholine intravenously (0.03 – 3.0 μg/kg). * p<0.05 by repeated measures ANOVA with individual contrasts showed a significant overall effect comparing Ctl versus Alum or Alum versus OVA. **p<0.05, OVA versus alum at individual methacholine doses. † p<0.05, Ctl versus Alum at individual methacholine doses.
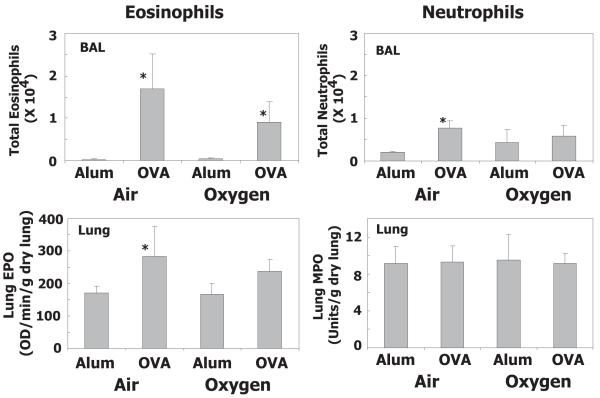
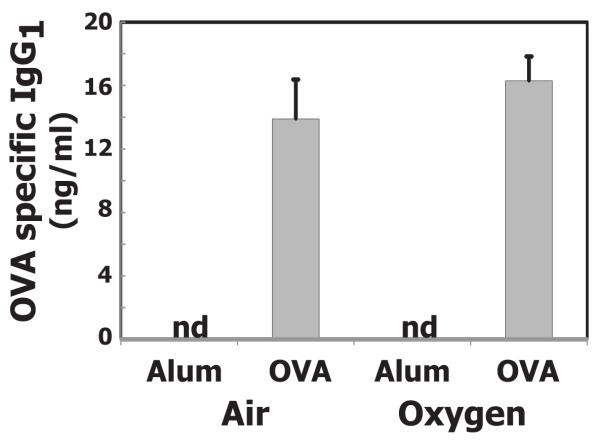
As expected, OVA sensitization and challenge significantly increased eosinophil numbers in bronchoalveolar lavage (BAL) fluid of mice exposed to room air or hyperoxia as neonates (Fig 4). Although total number of eosinophils in BAL was less in mice exposed to hyperoxia, it was not significantly different when compared to mice exposed to room air. After lavage, the relative number of eosinophils and neutrophils remaining in tissue were estimated by determining EPO and MPO activity, respectively, in lung homogenates. Increased EPO activity was detected in both groups of mice, but significant increases were only observed in mice exposed to room air. Allergen challenge weakly stimulated neutrophil recruitment into airspace of mice exposed to room air, but this was not reflected in increased neutrophils in lung tissue (Fig 4). OVA also increased BAL protein content compared to alum, but the increase did not differ whether animals were exposed neonatally to oxygen or room air (data not shown). OVA-specific IgG1 did not differ between OVA sensitized and challenged groups, whether air or oxygen exposed as neonates (Fig 5). At the time examined, OVA specific IgE was not detectable in OVA sensitized and challenged animals.
Figure 4.
Neonatal oxygen does not alter allergen-induced lung inflammation. Adult mice exposed to room air (Air) or 100% oxygen (O2) as neonates were sensitized with Alum plus saline (Alum) or Alum plus ovalbumin (OVA). Values represent the geometric mean + SE of 7-9 animals. ANOVA showed a significant OVA effect for both air and oxygen exposed animals (p<0.05) but the interaction with air or oxygen treatment was not significant. * p<0.05 for the individual contrast of OVA versus alum in either air or oxygen exposed animals. BAL, bronchoalveolar lavage; EPO, eosinophil peroxidase; MPO, myeloperoxidase.
Figure 5.
Neonatal oxygen does not alter allergen-induced OVA specific IgG1 production. Adult mice exposed to room air (Air) or 100% oxygen (O2) as neonates were sensitized with Alum plus saline (Alum) or Alum plus ovalbumin (OVA). All mice were challenged with OVA aerosol. Serum was collected and levels of IgG1 quantified by ELISA. Values represent the geometric mean + SE of 6-9 animals; nd indicates no detectable OVA specific IgG1. OVA specific IgG1 production did not differ in Alum versus OVA animals.
Discussion
Using a well-established adult mouse model of neonatal oxygen exposure that causes persistent changes in lung structure, lung mechanics and the host response to influenza A virus infection (13, 15-18), we found neonatal hyperoxia alters baseline pulmonary mechanics, increases AHR, but does not alter the response to a model antigen. Specifically, administration of supplemental oxygen during the neonatal period of alveolar development resulted in increased lung compliance and decreased airway resistance. Although neonatal hyperoxia increased AHR measured by methacholine provocation, it did not exacerbate AHR or pulmonary inflammation triggered by sensitization and challenge with OVA. Such findings were readily observed in female but not male mice. Our findings suggest that asthma diagnosed in children born preterm may reflect changes in airway structure and differ from the asthma phenotype elicited by allergen and pharmacologically managed.
In our study, increased compliance at baseline in adult mice exposed to high oxygen at birth likely reflects alveolar simplification and changes in elastogenic properties (20). This alveolar simplification and increased compliance is similar to changes seen in emphysema and chronic lung disease after premature birth (3). A decrease in baseline resistance is noted in our mouse model but is not statistically significant in all cohorts (13, 21). In general, decreased resistance is not a prominent finding in lung disease because normal lung resistance is very small. However, when challenged with a bronchoconstrictor such as methacholine, resistance readily increases and compliance decreases due to smooth muscle contraction. One force limiting bronchoconstriction in normal lung is alveolar tethering of airway walls. With alveolar simplification, this tethering is reduced and may result in a greater bronchoconstrictor response to methacholine. In our study, the increased response to methacholine in adult female mice exposed to oxygen at birth is potentially due to decreased alveolar tethering with alveolar simplification. Altered elastin deposition seen in oxygen-exposed mice is also consistent with elevated elastin present in asthmatics with AHR (22, 23).
A higher incidence of asthma is noted in people born prematurely, and AHR is more severe in individuals born preterm, treated with supplemental oxygen and diagnosed with BPD (4). Thus, we hypothesized that allergen-induced AHR in adult mice exposed neonatally to high oxygen would be exacerbated. Our study was designed with a suboptimal sensitization and challenge regimen to increase the likelihood that an exacerbation of airway inflammation and AHR could be detected. Since structural differences in lung could affect aerosol delivery of allergen or methacholine, we used the ip route for OVA sensitization and the iv route for methacholine challenge. However, OVA challenge was done by aerosol so the allergen dose delivered to elicit the lung inflammation and AHR might differ between air and oxygen exposed animals with structurally different lungs. Neonatal oxygen exposure did not alter production of OVA specific antibody or allergen-induced inflammation and AHR in adult female mice. Overall, our results do not reflect the increased risk of asthma noted in adults with BPD as infants.
A clear limitation of our study is the modeling of only one asthma phenotype: allergic asthma. Atopy is a major risk factor for asthma, but not all asthma is triggered by allergens. Recent evidence demonstrates a reduced incidence of atopy in adults born preterm with or without BPD (4, 24, 25). Others found a weak association between allergy/airway inflammation and asthma/AHR subsequent to premature birth indicating that asthma and AHR in a premature population differed from typical allergic childhood asthma (4). Our data indicate that hyperoxia in infancy does not exacerbate asthma-like symptoms triggered by allergen in the adult female mouse. Our study only reflects the ramifications of neonatal oxygen exposure on allergic asthma and does not address the possibility that other factors including low birth weight or intrauterine growth restriction may be more important in increased asthma incidence. Also, our study reflects the response of oxygen exposure in a normal weight full term mouse whose airways are more likely to be developed than low birth weight preterm human infant.
In addition to examining only one asthma phenotype, our studies are limited by examination of only one model allergen, OVA. Occupational OVA exposure causes asthma in humans (26) and the mouse OVA model of asthma has been extensively characterized. However, studies also clearly indicate allergic mechanisms differ depending on allergen (19). Mouse asthma models using more common allergens such as house dust mite have been developed and result in a similar phenotype (27). Given the lack of effect of oxygen exposure on the intensity of the allergic inflammation and AHR induced by OVA, evaluating the response to house dust mite is not currently warranted.
Our study also found that oxygen-dependent changes in baseline lung mechanics were evident in female but not male C57Bl/6J mice. As adults, severe asthma occurs predominantly in human females (6), and female mice have more severe allergic inflammation than male mice in models of allergic asthma (27). Considering oxygen toxicity, male mice tend to be more sensitive than females (28). Studies examining persistent respiratory disease in former preterm humans generally examine low birth weight infants, with clinically defined BPD being a subset of the population under consideration. BPD comprises about 35% of very low birth weight infants (3) and when considering disease incidence, males are more prone to develop BPD than females (29). However, when considering respiratory symptoms in adults born preterm, females are more likely to report respiratory symptoms (30) and there is a higher incidence of asthma, wheeze and shortness of breath. The sex specific effect on AHR in our mouse model is consistent with the increased respiratory symptoms in females born preterm compared to males (30). Thus, this mouse model may provide a means to mechanistically explore how sex differences influence health of people born prematurely.
In summary, our studies reveal neonatal hyperoxia provokes AHR but does not exacerbate responses to allergen sensitization or inflammation in adult mice. Such knowledge suggests future studies should investigate how neonatal hyperoxia reprograms development of the mouse airway. For children born prematurely who present with asthma, our findings imply that diagnosis should carefully differentiate the asthma phenotype from chronic lung disease of the premature infant, and physicians should use caution when prescribing asthma medications if not indicated by the evidence.
Acknowledgements
We are grateful to Min Yee, Brad Buczynski, Kyle Martin and Steve Bauer, University of Rochester, for assistance with the animal treatment and some of the assays, and to Dr. Ronald R. Regal, Department of Mathematics and Statistics, University of Minnesota Duluth for assistance in statistical analysis of the data.
Source of funding This work was funded, in part by National Institutes of Health (NIH) Grants HL-091968 (M. A. O’Reilly), HL-097141 (M. A. O’Reilly and B. P. Lawrence), ES-018750 (B. P. Lawrence), and University of Minnesota Grant-in-Aid (J.F. Regal). NIH Training Grants ES-07026 and HL-66988 supported B. W. Buczynski and NIH R25 GM086669 supported S. Lojovich. NIH Center Grant ES-01247 supported the animal inhalation facility at the University of Rochester.
Footnotes
conflict of interest: The authors do not have any conflicts to disclose.
REFERENCES
- 1.Baraldi E, Carraro S, Filippone M. Bronchopulmonary dysplasia: definitions and long-term respiratory outcome. Early human development. 2009;85:S1–3. doi: 10.1016/j.earlhumdev.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Lum S, Kirkby J, Welsh L, Marlow N, Hennessy E, Stocks J. Nature and severity of lung function abnormalities in extremely pre-term children at 11 years of age. Eur Respir J. 2011;37:1199–207. doi: 10.1183/09031936.00071110. [DOI] [PubMed] [Google Scholar]
- 3.Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med. 2007;357:1946–55. doi: 10.1056/NEJMra067279. [DOI] [PubMed] [Google Scholar]
- 4.Halvorsen T, Skadberg BT, Eide GE, Roksund O, Aksnes L, Oymar K. Characteristics of asthma and airway hyper-responsiveness after premature birth. Pediatr Allergy Immunol. 2005;16:487–94. doi: 10.1111/j.1399-3038.2005.00314.x. [DOI] [PubMed] [Google Scholar]
- 5.Fawke J, Lum S, Kirkby J, et al. Lung function and respiratory symptoms at 11 years in children born extremely preterm: the EPICure study. Am J Respir Crit Care Med. 2010;182:237–45. doi: 10.1164/rccm.200912-1806OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.NHLBI . National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR 3): Guidelines for the Diagnosis and Management of Asthma. National Heart Lung and Blood Institute. National Institutes of Health; Bethesda: 2007. [Google Scholar]
- 7.Schulman SR, Canada AT, Fryer AD, Winsett DW, Costa DL. Airway hyperreactivity produced by short-term exposure to hyperoxia in neonatal guinea pigs. The American journal of physiology. 1997;272:L1211–6. doi: 10.1152/ajplung.1997.272.6.L1211. [DOI] [PubMed] [Google Scholar]
- 8.Hershenson MB, Aghili S, Punjabi N, et al. Hyperoxia-induced airway hyperresponsiveness and remodeling in immature rats. The American journal of physiology. 1992;262:L263–9. doi: 10.1152/ajplung.1992.262.3.L263. [DOI] [PubMed] [Google Scholar]
- 9.Hershenson MB, Garland A, Kelleher MD, Zimmermann A, Hernandez C, Solway J. Hyperoxia-induced airway remodeling in immature rats. Correlation with airway responsiveness. The American review of respiratory disease. 1992;146:1294–300. doi: 10.1164/ajrccm/146.5_Pt_1.1294. [DOI] [PubMed] [Google Scholar]
- 10.Denis D, Fayon MJ, Berger P, et al. Prolonged moderate hyperoxia induces hyperresponsiveness and airway inflammation in newborn rats. Pediatr Res. 2001;50:515–9. doi: 10.1203/00006450-200110000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Takeda K, Okamoto M, de Langhe S, et al. Peroxisome proliferator-activated receptor-g agonist treatment increases septation and angiogenesis and decreases airway hyperresponsiveness in a model of experimental neonatal chronic lung disease. Anat Rec (Hoboken) 2009;292:1045–61. doi: 10.1002/ar.20921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hershenson MB, Abe MK, Kelleher MD, et al. Recovery of airway structure and function after hyperoxic exposure in immature rats. Am J Respir Crit Care Med. 1994;149:1663–9. doi: 10.1164/ajrccm.149.6.8004327. [DOI] [PubMed] [Google Scholar]
- 13.Yee M, Chess PR, McGrath-Morrow SA, et al. Neonatal oxygen adversely affects lung function in adult mice without altering surfactant composition or activity. Am J Physiol Lung Cell Mol Physiol. 2009;297:L641–9. doi: 10.1152/ajplung.00023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yee M, Buczynski BW, O’Reilly MA. Neonatal Hyperoxia Stimulates the Expansion of Alveolar Epithelial Type II Cells. American journal of respiratory cell and molecular biology. 2013 doi: 10.1165/rcmb.2013-0207OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Reilly MA, Marr SH, Yee M, McGrath-Morrow SA, Lawrence BP. Neonatal hyperoxia enhances the inflammatory response in adult mice infected with influenza A virus. Am J Respir Crit Care Med. 2008;177:1103–10. doi: 10.1164/rccm.200712-1839OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Reilly MA, Yee M, Buczynski BW, et al. Neonatal oxygen increases sensitivity to influenza A virus infection in adult mice by suppressing epithelial expression of Ear1. Am J Pathol. 2012;181:441–51. doi: 10.1016/j.ajpath.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buczynski BW, Yee M, Paige Lawrence B, O’Reilly MA. Lung development and the host response to influenza A virus are altered by different doses of neonatal oxygen in mice. Am J Physiol Lung Cell Mol Physiol. 2012;302:L1078–87. doi: 10.1152/ajplung.00026.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giannandrea M, Yee M, O’Reilly MA, Lawrence BP. Memory CD8+ T cells are sufficient to alleviate impaired host resistance to influenza A virus infection caused by neonatal oxygen supplementation. Clinical and vaccine immunology : CVI. 2012;19:1432–41. doi: 10.1128/CVI.00265-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greene AL, Rutherford MS, Regal RR, et al. Arginase activity differs with allergen in the effector phase of ovalbumin- versus trimellitic anhydride-induced asthma. Toxicol Sci. 2005;88:420–33. doi: 10.1093/toxsci/kfi311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yee M, White RJ, Awad HA, Bates WA, McGrath-Morrow SA, O’Reilly MA. Neonatal hyperoxia causes pulmonary vascular disease and shortens life span in aging mice. Am J Pathol. 2011;178:2601–10. doi: 10.1016/j.ajpath.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yee M, Vitiello PF, Roper JM, et al. Type II epithelial cells are critical target for hyperoxia-mediated impairment of postnatal lung development. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1101–11. doi: 10.1152/ajplung.00126.2006. [DOI] [PubMed] [Google Scholar]
- 22.Slats AM, Janssen K, van Schadewijk A, et al. Expression of smooth muscle and extracellular matrix proteins in relation to airway function in asthma. J Allergy Clin Immunol. 2008;121:1196–202. doi: 10.1016/j.jaci.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 23.Bai TR, Knight DA. Structural changes in the airways in asthma: observations and consequences. Clin Sci (Lond) 2005;108:463–77. doi: 10.1042/CS20040342. [DOI] [PubMed] [Google Scholar]
- 24.Crump C, Sundquist K, Sundquist J, Winkleby MA. Gestational age at birth and risk of allergic rhinitis in young adulthood. J Allergy Clin Immunol. 2011;127:1173–9. doi: 10.1016/j.jaci.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siltanen M, Wehkalampi K, Hovi P, et al. Preterm birth reduces the incidence of atopy in adulthood. J Allergy Clin Immunol. 2011;127:935–42. doi: 10.1016/j.jaci.2010.12.1107. [DOI] [PubMed] [Google Scholar]
- 26.Boeniger MF, Lummus ZL, Biagini RE, et al. Exposure to protein aeroallergens in egg processing facilities. Applied occupational and environmental hygiene. 2001;16:660–70. doi: 10.1080/10473220118319. [DOI] [PubMed] [Google Scholar]
- 27.Blacquiere MJ, Hylkema MN, Postma DS, Geerlings M, Timens W, Melgert BN. Airway inflammation and remodeling in two mouse models of asthma: comparison of males and females. Int Arch Allergy Immunol. 2010;153:173–81. doi: 10.1159/000312635. [DOI] [PubMed] [Google Scholar]
- 28.Lingappan K, Jiang W, Wang L, Couroucli XI, Barrios R, Moorthy B. Sex-specific differences in hyperoxic lung injury in mice: implications for acute and chronic lung disease in humans. Toxicol Appl Pharmacol. 2013;272:281–90. doi: 10.1016/j.taap.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laughon MM, Langer JC, Bose CL, et al. Prediction of bronchopulmonary dysplasia by postnatal age in extremely premature infants. Am J Respir Crit Care Med. 2011;183:1715–22. doi: 10.1164/rccm.201101-0055OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vrijlandt EJ, Gerritsen J, Boezen HM, Duiverman EJ. Gender differences in respiratory symptoms in 19-year-old adults born preterm. Respiratory research. 2005;6:117. doi: 10.1186/1465-9921-6-117. [DOI] [PMC free article] [PubMed] [Google Scholar]