Abstract
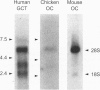
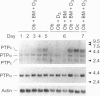
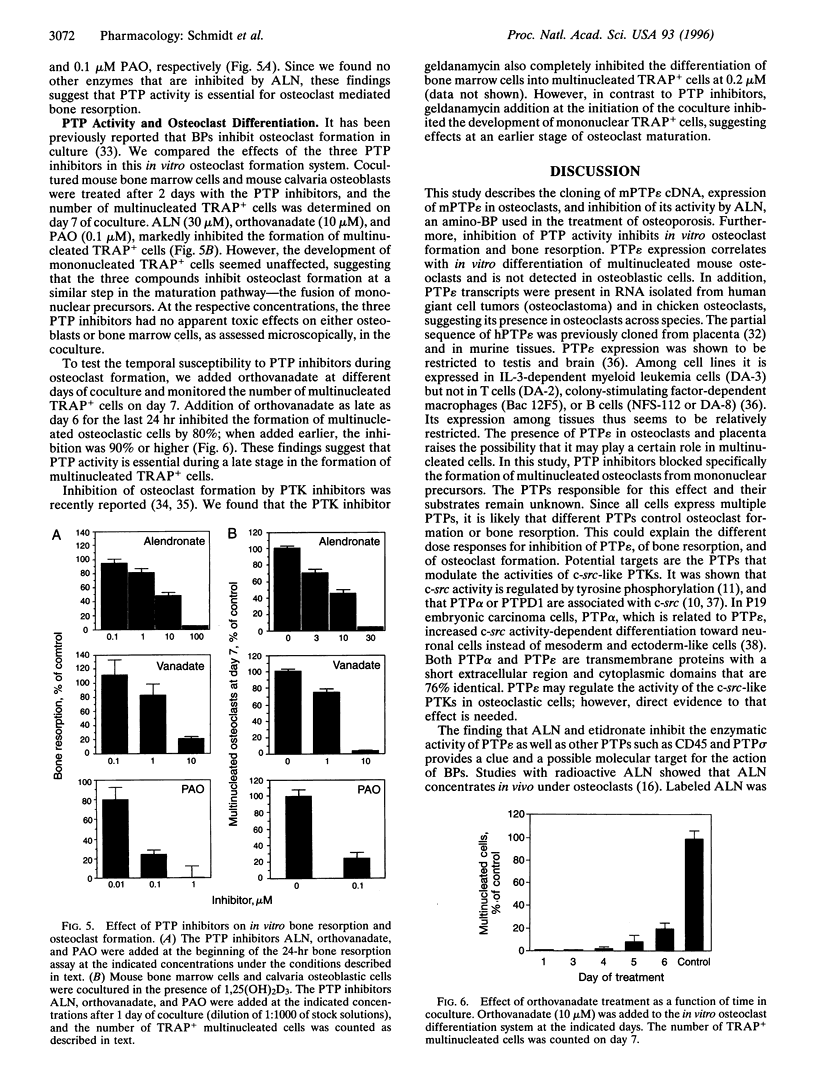
Alendronate (ALN), an aminobisphosphonate used in the treatment of osteoporosis, is a potent inhibitor of bone resorption. Its molecular target is still unknown. This study examines the effects of ALN on the activity of osteoclast protein-tyrosine phosphatase (PTP; protein-tyrosine-phosphate phosphohydrolase, EC 3.1.3.48), called PTPepsilon. Using osteoclast-like cells generated by coculturing mouse bone marrow cells with mouse calvaria osteoblasts, we found by molecular cloning and RNA blot hybridization that PTPepsilon is highly expressed in osteoclastic cells. A purified fusion protein of PTPepsilon expressed in bacteria was inhibited by ALN with an IC50 of 2 microM. Other PTP inhibitors--orthovanadate and phenylarsine oxide (PAO)-inhibited PTPepsilon with IC50 values of 0.3 microM and 18 microM, respectively. ALN and another bisphosphonate, etidronate, also inhibited the activities of other bacterially expressed PTPs such as PTPsigma and CD45 (also called leukocyte common antigen). The PTP inhibitors ALN, orthovanadate, and PAO suppressed in vitro formation of multinucleated osteoclasts from osteoclast precursors and in vitro bone resorption by isolated rat osteoclasts (pit formation) with estimated IC50 values of 10 microM, 3 microM, and 0.05 microM, respectively. These findings suggest that tyrosine phosphatase activity plays an important role in osteoclast formation and function and is a putative molecular target of bisphosphonate action.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akatsu T., Tamura T., Takahashi N., Udagawa N., Tanaka S., Sasaki T., Yamaguchi A., Nagata N., Suda T. Preparation and characterization of a mouse osteoclast-like multinucleated cell population. J Bone Miner Res. 1992 Nov;7(11):1297–1306. doi: 10.1002/jbmr.5650071109. [DOI] [PubMed] [Google Scholar]
- Alexander D. R., Cantrell D. A. Kinases and phosphatases in T-cell activation. Immunol Today. 1989 Jun;10(6):200–205. doi: 10.1016/0167-5699(89)90325-3. [DOI] [PubMed] [Google Scholar]
- Amin D., Cornell S. A., Gustafson S. K., Needle S. J., Ullrich J. W., Bilder G. E., Perrone M. H. Bisphosphonates used for the treatment of bone disorders inhibit squalene synthase and cholesterol biosynthesis. J Lipid Res. 1992 Nov;33(11):1657–1663. [PubMed] [Google Scholar]
- Andersson G. N., Ek-Rylander B., Hammarström L. E., Lindskog S., Toverud S. U. Immunocytochemical localization of a tartrate-resistant and vanadate-sensitive acid nucleotide tri- and diphosphatase. J Histochem Cytochem. 1986 Mar;34(3):293–298. doi: 10.1177/34.3.3005390. [DOI] [PubMed] [Google Scholar]
- Boyce B. F., Yoneda T., Lowe C., Soriano P., Mundy G. R. Requirement of pp60c-src expression for osteoclasts to form ruffled borders and resorb bone in mice. J Clin Invest. 1992 Oct;90(4):1622–1627. doi: 10.1172/JCI116032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Howell B. The when and how of Src regulation. Cell. 1993 Jun 18;73(6):1051–1054. doi: 10.1016/0092-8674(93)90634-3. [DOI] [PubMed] [Google Scholar]
- Fischer E. H., Charbonneau H., Tonks N. K. Protein tyrosine phosphatases: a diverse family of intracellular and transmembrane enzymes. Science. 1991 Jul 26;253(5018):401–406. doi: 10.1126/science.1650499. [DOI] [PubMed] [Google Scholar]
- Gautier J., Solomon M. J., Booher R. N., Bazan J. F., Kirschner M. W. cdc25 is a specific tyrosine phosphatase that directly activates p34cdc2. Cell. 1991 Oct 4;67(1):197–211. doi: 10.1016/0092-8674(91)90583-k. [DOI] [PubMed] [Google Scholar]
- Gordon J. A. Use of vanadate as protein-phosphotyrosine phosphatase inhibitor. Methods Enzymol. 1991;201:477–482. doi: 10.1016/0076-6879(91)01043-2. [DOI] [PubMed] [Google Scholar]
- Hagenaars C. E., Kawilarang-de Haas E. W., van der Kraan A. A., Spooncer E., Dexter T. M., Nijweide P. J. Interleukin-3-dependent hematopoietic stem cell lines capable of osteoclast formation in vitro. J Bone Miner Res. 1991 Sep;6(9):947–954. doi: 10.1002/jbmr.5650060908. [DOI] [PubMed] [Google Scholar]
- Hall T. J., Schaeublin M., Missbach M. Evidence that c-src is involved in the process of osteoclastic bone resorption. Biochem Biophys Res Commun. 1994 Mar 30;199(3):1237–1244. doi: 10.1006/bbrc.1994.1363. [DOI] [PubMed] [Google Scholar]
- Hughes D. E., MacDonald B. R., Russell R. G., Gowen M. Inhibition of osteoclast-like cell formation by bisphosphonates in long-term cultures of human bone marrow. J Clin Invest. 1989 Jun;83(6):1930–1935. doi: 10.1172/JCI114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. Protein-tyrosine phosphatases: the other side of the coin. Cell. 1989 Sep 22;58(6):1013–1016. doi: 10.1016/0092-8674(89)90496-0. [DOI] [PubMed] [Google Scholar]
- Kaplan R., Morse B., Huebner K., Croce C., Howk R., Ravera M., Ricca G., Jaye M., Schlessinger J. Cloning of three human tyrosine phosphatases reveals a multigene family of receptor-linked protein-tyrosine-phosphatases expressed in brain. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7000–7004. doi: 10.1073/pnas.87.18.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger N. S., Tashjian A. H., Jr Inhibition of stimulated bone resorption by vanadate. Endocrinology. 1983 Jul;113(1):324–328. doi: 10.1210/endo-113-1-324. [DOI] [PubMed] [Google Scholar]
- Krueger N. X., Streuli M., Saito H. Structural diversity and evolution of human receptor-like protein tyrosine phosphatases. EMBO J. 1990 Oct;9(10):3241–3252. doi: 10.1002/j.1460-2075.1990.tb07523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau K. H., Tanimoto H., Baylink D. J. Vanadate stimulates bone cell proliferation and bone collagen synthesis in vitro. Endocrinology. 1988 Dec;123(6):2858–2867. doi: 10.1210/endo-123-6-2858. [DOI] [PubMed] [Google Scholar]
- Matthews R. J., Cahir E. D., Thomas M. L. Identification of an additional member of the protein-tyrosine-phosphatase family: evidence for alternative splicing in the tyrosine phosphatase domain. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4444–4448. doi: 10.1073/pnas.87.12.4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrills R. J., Stein L. S., Fey C. P., Dempster D. W. The effects of parathyroid hormone (PTH) and PTH-related peptide on osteoclast resorption of bone slices in vitro: an analysis of pit size and the resorption focus. Endocrinology. 1990 Dec;127(6):2648–2653. doi: 10.1210/endo-127-6-2648. [DOI] [PubMed] [Google Scholar]
- Møller N. P., Møller K. B., Lammers R., Kharitonenkov A., Sures I., Ullrich A. Src kinase associates with a member of a distinct subfamily of protein-tyrosine phosphatases containing an ezrin-like domain. Proc Natl Acad Sci U S A. 1994 Aug 2;91(16):7477–7481. doi: 10.1073/pnas.91.16.7477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth G. G., Heydemann A., Bolander M. E. Isolation and analysis of ribonucleic acids from skeletal tissues. Anal Biochem. 1989 Dec;183(2):301–304. doi: 10.1016/0003-2697(89)90483-1. [DOI] [PubMed] [Google Scholar]
- ROTMAN B., ZDERIC J. A., EDELSTEIN M. Fluorogenic substrates for beta-D-galactosidases and phosphatases derived from flurescein (3,6-dihydroxyfluoran) and its monomethylether. Proc Natl Acad Sci U S A. 1963 Jul;50:1–6. doi: 10.1073/pnas.50.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodan G. A., Balena R. Bisphosphonates in the treatment of metabolic bone diseases. Ann Med. 1993 Aug;25(4):373–378. doi: 10.3109/07853899309147299. [DOI] [PubMed] [Google Scholar]
- Rossini M., Gatti D., Zamberlan N., Braga V., Dorizzi R., Adami S. Long-term effects of a treatment course with oral alendronate of postmenopausal osteoporosis. J Bone Miner Res. 1994 Nov;9(11):1833–1837. doi: 10.1002/jbmr.5650091121. [DOI] [PubMed] [Google Scholar]
- Sahni M., Guenther H. L., Fleisch H., Collin P., Martin T. J. Bisphosphonates act on rat bone resorption through the mediation of osteoblasts. J Clin Invest. 1993 May;91(5):2004–2011. doi: 10.1172/JCI116422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sap J., D'Eustachio P., Givol D., Schlessinger J. Cloning and expression of a widely expressed receptor tyrosine phosphatase. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6112–6116. doi: 10.1073/pnas.87.16.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M., Grasser W., Endo N., Akins R., Simmons H., Thompson D. D., Golub E., Rodan G. A. Bisphosphonate action. Alendronate localization in rat bone and effects on osteoclast ultrastructure. J Clin Invest. 1991 Dec;88(6):2095–2105. doi: 10.1172/JCI115539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P., Montgomery C., Geske R., Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991 Feb 22;64(4):693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- Suda T., Takahashi N., Martin T. J. Modulation of osteoclast differentiation. Endocr Rev. 1992 Feb;13(1):66–80. doi: 10.1210/edrv-13-1-66. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Akatsu T., Udagawa N., Sasaki T., Yamaguchi A., Moseley J. M., Martin T. J., Suda T. Osteoblastic cells are involved in osteoclast formation. Endocrinology. 1988 Nov;123(5):2600–2602. doi: 10.1210/endo-123-5-2600. [DOI] [PubMed] [Google Scholar]
- Tonks N. K., Charbonneau H. Protein tyrosine dephosphorylation and signal transduction. Trends Biochem Sci. 1989 Dec;14(12):497–500. doi: 10.1016/0968-0004(89)90184-9. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Valkema R., Vismans F. J., Papapoulos S. E., Pauwels E. K., Bijvoet O. L. Maintained improvement in calcium balance and bone mineral content in patients with osteoporosis treated with the bisphosphonate APD. Bone Miner. 1989 Jan;5(2):183–192. doi: 10.1016/0169-6009(89)90095-0. [DOI] [PubMed] [Google Scholar]
- Yi T., Cleveland J. L., Ihle J. N. Identification of novel protein tyrosine phosphatases of hematopoietic cells by polymerase chain reaction amplification. Blood. 1991 Nov 1;78(9):2222–2228. [PubMed] [Google Scholar]
- Yoneda T., Lowe C., Lee C. H., Gutierrez G., Niewolna M., Williams P. J., Izbicka E., Uehara Y., Mundy G. R. Herbimycin A, a pp60c-src tyrosine kinase inhibitor, inhibits osteoclastic bone resorption in vitro and hypercalcemia in vivo. J Clin Invest. 1993 Jun;91(6):2791–2795. doi: 10.1172/JCI116521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X. M., Wang Y., Pallen C. J. Cell transformation and activation of pp60c-src by overexpression of a protein tyrosine phosphatase. Nature. 1992 Sep 24;359(6393):336–339. doi: 10.1038/359336a0. [DOI] [PubMed] [Google Scholar]
- den Hertog J., Pals C. E., Peppelenbosch M. P., Tertoolen L. G., de Laat S. W., Kruijer W. Receptor protein tyrosine phosphatase alpha activates pp60c-src and is involved in neuronal differentiation. EMBO J. 1993 Oct;12(10):3789–3798. doi: 10.1002/j.1460-2075.1993.tb06057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]