Abstract
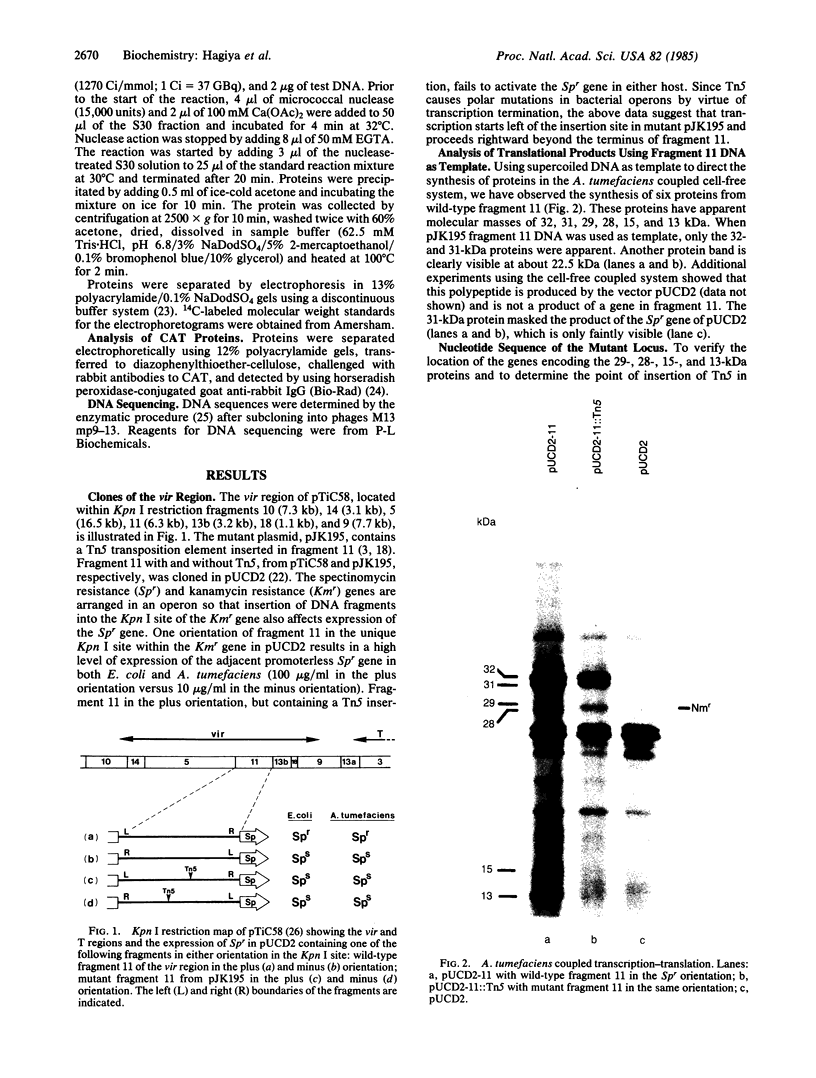
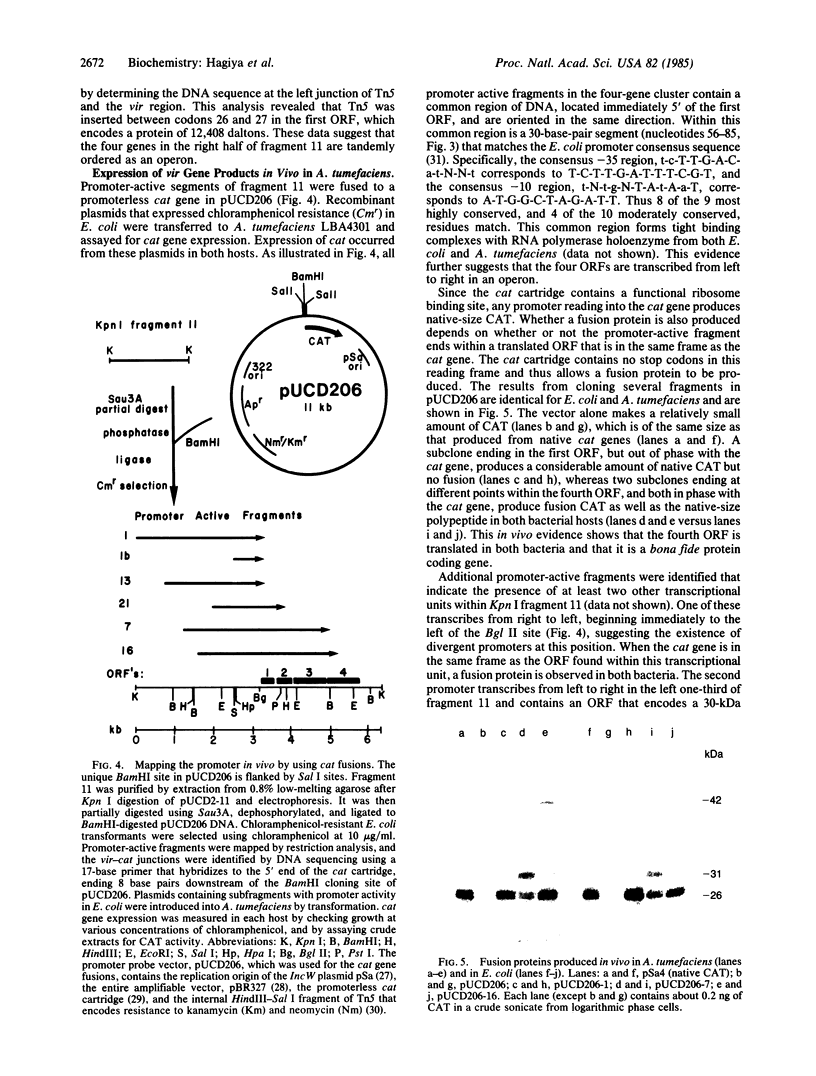
Analyses were made of the host-dependent-variation (hdv) locus of the virulence (vir) region of the pTiC58 plasmid of Agrobacterium tumefaciens. The hdv locus is comprised of at least four genes that encode polypeptides of 13, 15, 29, and 28 kDa. Insertion of transposon Tn5 in the first gene abolishes the expression of all four genes in vitro and in vivo. Nucleotide sequence analysis of the hdv locus revealed four open reading frames tandemly arranged with spacer sequences having no promoter-like sequences and lacking the ability to bind A. tumefaciens RNA polymerase. These studies suggest that the hdv locus is comprised of at least four genes arranged in an operon in the vir region. The protein products of these genes are likely to function in some aspect of the host-range determination of A. tumefaciens.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Close T. J., Rodriguez R. L. Construction and characterization of the chloramphenicol-resistance gene cartridge: a new approach to the transcriptional mapping of extrachromosomal elements. Gene. 1982 Dec;20(2):305–316. doi: 10.1016/0378-1119(82)90048-8. [DOI] [PubMed] [Google Scholar]
- Close T. J., Zaitlin D., Kado C. I. Design and development of amplifiable broad-host-range cloning vectors: analysis of the vir region of Agrobacterium tumefaciens plasmid pTiC58. Plasmid. 1984 Sep;12(2):111–118. doi: 10.1016/0147-619x(84)90057-x. [DOI] [PubMed] [Google Scholar]
- Depicker A., De Wilde M., De Vos G., De Vos R., Van Montagu M., Schell J. Molecular cloning of overlapping segments of the nopaline Ti-plasmid pTiC58 as a means to restriction endonuclease mapping. Plasmid. 1980 Mar;3(2):193–211. doi: 10.1016/0147-619x(80)90109-2. [DOI] [PubMed] [Google Scholar]
- Drummond M. H., Chilton M. D. Tumor-inducing (Ti) plasmids of Agrobacterium share extensive regions of DNA homology. J Bacteriol. 1978 Dec;136(3):1178–1183. doi: 10.1128/jb.136.3.1178-1183.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler G., Depicker A., Maenhaut R., Villarroel R., Van Montagu M., Schell J. Physical mapping of DNA base sequence homologies between an octopine and a nopaline Ti plasmid of Agrobacterium tumefaciens. J Mol Biol. 1981 Oct 25;152(2):183–208. doi: 10.1016/0022-2836(81)90239-4. [DOI] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille J., Klasen I., Schilperoort R. Construction and application of R prime plasmids, carrying different segments of an octopine Ti plasmid from Agrobacterium tumefaciens, for complementation of vir genes. Plasmid. 1982 Mar;7(2):107–118. doi: 10.1016/0147-619x(82)90071-3. [DOI] [PubMed] [Google Scholar]
- Hille J., van Kan J., Schilperoort R. trans-Acting virulence functions of the octopine Ti plasmid from Agrobacterium tumefaciens. J Bacteriol. 1984 May;158(2):754–756. doi: 10.1128/jb.158.2.754-756.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooykaas P. J., Hofker M., den Dulk-Ras H., Schilperoort R. A. A comparison of virulence determinants in an octopine Ti plasmid, a nopaline Ti plasmid, and an Ri plasmid by complementation analysis of Agrobacterium tumefaciens mutants. Plasmid. 1984 May;11(3):195–205. doi: 10.1016/0147-619x(84)90026-x. [DOI] [PubMed] [Google Scholar]
- Iyer V. N., Klee H. J., Nester E. W. Units of genetic expression in the virulence region of a plant tumor-inducing plasmid of Agrobacterium tumefaciens. Mol Gen Genet. 1982;188(3):418–424. doi: 10.1007/BF00330043. [DOI] [PubMed] [Google Scholar]
- Kao J. C., Perry K. L., Kado C. I. Indoleacetic acid complementation and its relation to host range specifying genes on the Ti plasmid of Agrobacterium tumefaciens. Mol Gen Genet. 1982;188(3):425–432. doi: 10.1007/BF00330044. [DOI] [PubMed] [Google Scholar]
- Klee H. J., Gordon M. P., Nester E. W. Complementation analysis of Agrobacterium tumefaciens Ti plasmid mutations affecting oncogenicity. J Bacteriol. 1982 Apr;150(1):327–331. doi: 10.1128/jb.150.1.327-331.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee H. J., White F. F., Iyer V. N., Gordon M. P., Nester E. W. Mutational analysis of the virulence region of an Agrobacterium tumefaciens Ti plasmid. J Bacteriol. 1983 Feb;153(2):878–883. doi: 10.1128/jb.153.2.878-883.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu S. T., Perry K. L., Schardl C. L., Kado C. I. Agrobacterium Ti plasmid indoleacetic acid gene is required for crown gall oncogenesis. Proc Natl Acad Sci U S A. 1982 May;79(9):2812–2816. doi: 10.1073/pnas.79.9.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundquist R. C., Close T. J., Kado C. I. Genetic complementation of Agrobacterium tumefaciens Ti plasmid mutants in the virulence region. Mol Gen Genet. 1984;193(1):1–7. doi: 10.1007/BF00327406. [DOI] [PubMed] [Google Scholar]
- Renart J., Reiser J., Stark G. R. Transfer of proteins from gels to diazobenzyloxymethyl-paper and detection with antisera: a method for studying antibody specificity and antigen structure. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3116–3120. doi: 10.1073/pnas.76.7.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risuleo G., Battistoni P., Costantino P. Regions of homology between tumorigenic plasmids from Agrobacterium rhizogenes and Agrobacterium tumefaciens. Plasmid. 1982 Jan;7(1):45–51. doi: 10.1016/0147-619x(82)90025-7. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soberon X., Covarrubias L., Bolivar F. Construction and characterization of new cloning vehicles. IV. Deletion derivatives of pBR322 and pBR325. Gene. 1980 May;9(3-4):287–305. doi: 10.1016/0378-1119(90)90328-o. [DOI] [PubMed] [Google Scholar]
- Tait R. C., Close T. J., Rodriguez R. L., Kado C. I. Isolation of the origin of replication of the IncW-group plasmid pSa. Gene. 1982 Nov;20(1):39–49. doi: 10.1016/0378-1119(82)90085-3. [DOI] [PubMed] [Google Scholar]
- White F. F., Nester E. W. Relationship of plasmids responsible for hairy root and crown gall tumorigenicity. J Bacteriol. 1980 Nov;144(2):710–720. doi: 10.1128/jb.144.2.710-720.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]




