Abstract
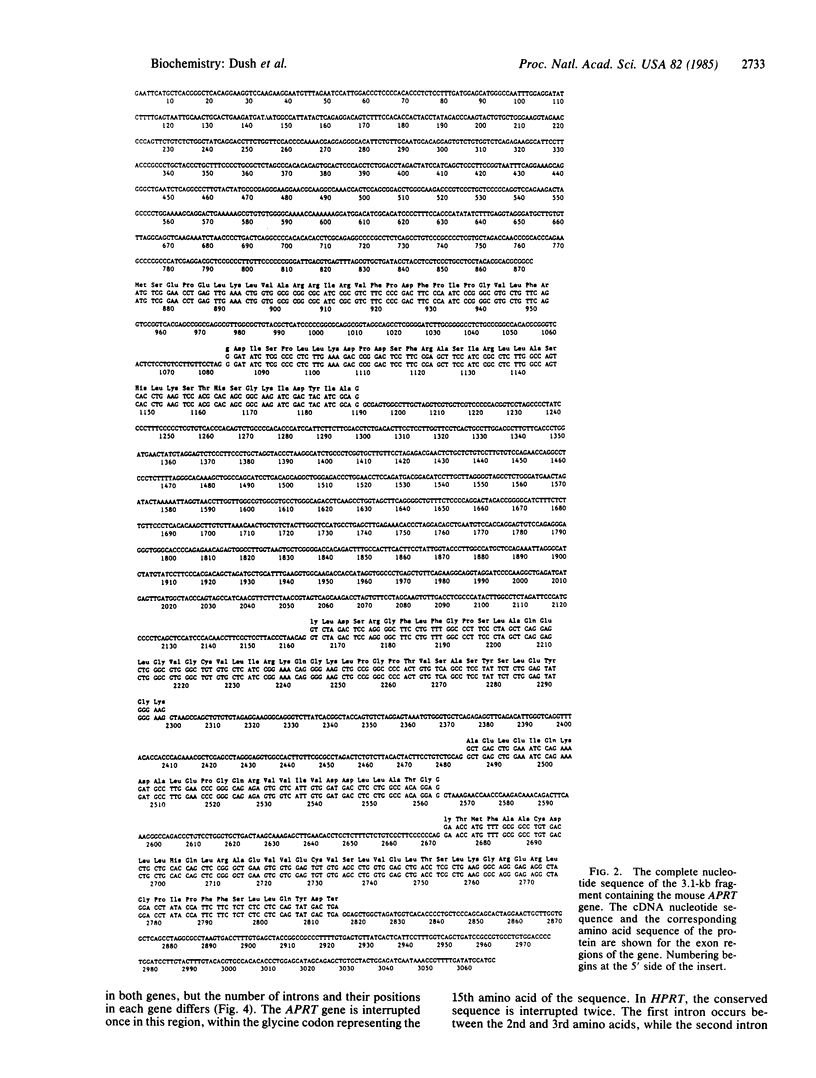
We have determined the nucleotide sequence of a functional mouse adenine phosphoribosyltransferase (APRT) gene and its cDNA. The amino acid sequence of the enzyme is deduced from an open reading frame in the cDNA and predicts a protein with a molecular weight of 19,560. The protein coding region of the gene is approximately 2 kilobases, and it is composed of five exons and four introns. While the body of the gene is 53% G + C, the 200 nucleotides upstream from the ATG translation start codon are 66% G + C and contain three copies of the sequence C-C-G-C-C-C. The mouse APRT enzyme shares a homologous 20-amino acid sequence with mouse, hamster, and human hypoxanthine phosphoribosyltransferases (HPRTs) and several bacterial phosphoribosyltransferases. This sequence has previously been shown to be a likely catalytic domain in human HPRT and Escherichia coli glutamine phosphoribosyltransferase. Because of the similarities in function of these proteins, both eukaryotic and prokaryotic, it is not unexpected that they should exhibit one or more regions of homology, particularly at the 5-phosphoribosyl-1-pyrophosphate and purine binding sites, especially if they are related via a common evolutionary lineage. This homologous sequence is interrupted by a single intron in the mouse APRT gene and by two introns in the mouse HPRT gene. Furthermore, the positions of both introns in the HPRT sequence are different from that of the single intron in the corresponding sequence of the APRT gene.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argos P., Hanei M., Wilson J. M., Kelley W. N. A possible nucleotide-binding domain in the tertiary fold of phosphoribosyltransferases. J Biol Chem. 1983 May 25;258(10):6450–6457. [PubMed] [Google Scholar]
- Blanchetot A., Wilson V., Wood D., Jeffreys A. J. The seal myoglobin gene: an unusually long globin gene. Nature. 1983 Feb 24;301(5902):732–734. doi: 10.1038/301732a0. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Brisson N., Verma D. P. Soybean leghemoglobin gene family: normal, pseudo, and truncated genes. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4055–4059. doi: 10.1073/pnas.79.13.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu F. K., Maley G. F., Maley F., Belfort M. Intervening sequence in the thymidylate synthase gene of bacteriophage T4. Proc Natl Acad Sci U S A. 1984 May;81(10):3049–3053. doi: 10.1073/pnas.81.10.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett R. D., Baty D., Chambon P. The repeated GC-rich motifs upstream from the TATA box are important elements of the SV40 early promoter. Nucleic Acids Res. 1983 Apr 25;11(8):2447–2464. doi: 10.1093/nar/11.8.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W. Why genes in pieces? Nature. 1978 Feb 9;271(5645):501–501. doi: 10.1038/271501a0. [DOI] [PubMed] [Google Scholar]
- Go M. Correlation of DNA exonic regions with protein structural units in haemoglobin. Nature. 1981 May 7;291(5810):90–92. doi: 10.1038/291090a0. [DOI] [PubMed] [Google Scholar]
- Hershey H. V., Taylor M. W. Purification of adenine phosphoribosyltransferase by affinity chromatography. Prep Biochem. 1978;8(6):453–462. doi: 10.1080/00327487808061662. [DOI] [PubMed] [Google Scholar]
- Hobart P. M., Fogliano M., O'Connor B. A., Schaefer I. M., Chirgwin J. M. Human renin gene: structure and sequence analysis. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5026–5030. doi: 10.1073/pnas.81.16.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden J. A., Meredith G. S., Kelley W. N. Human adenine phosphoribosyltransferase. Affinity purification, subunit structure, amino acid composition, and peptide mapping. J Biol Chem. 1979 Aug 10;254(15):6951–6955. [PubMed] [Google Scholar]
- Jolly D. J., Okayama H., Berg P., Esty A. C., Filpula D., Bohlen P., Johnson G. G., Shively J. E., Hunkapillar T., Friedmann T. Isolation and characterization of a full-length expressible cDNA for human hypoxanthine phosphoribosyl transferase. Proc Natl Acad Sci U S A. 1983 Jan;80(2):477–481. doi: 10.1073/pnas.80.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaine B. P., Gupta R., Woese C. R. Putative introns in tRNA genes of prokaryotes. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3309–3312. doi: 10.1073/pnas.80.11.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konecki D. S., Brennand J., Fuscoe J. C., Caskey C. T., Chinault A. C. Hypoxanthine-guanine phosphoribosyltransferase genes of mouse and Chinese hamster: construction and sequence analysis of cDNA recombinants. Nucleic Acids Res. 1982 Nov 11;10(21):6763–6775. doi: 10.1093/nar/10.21.6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano T., Long C., Green H. A new reduced human-mouse somatic cell hybrid containing the human gene for adenine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1971 Jan;68(1):82–86. doi: 10.1073/pnas.68.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leicht M., Long G. L., Chandra T., Kurachi K., Kidd V. J., Mace M., Jr, Davie E. W., Woo S. L. Sequence homology and structural comparison between the chromosomal human alpha 1-antitrypsin and chicken ovalbumin genes. Nature. 1982 Jun 24;297(5868):655–659. doi: 10.1038/297655a0. [DOI] [PubMed] [Google Scholar]
- Makaroff C. A., Zalkin H., Switzer R. L., Vollmer S. J. Cloning of the Bacillus subtilis glutamine phosphoribosylpyrophosphate amidotransferase gene in Escherichia coli. Nucleotide sequence determination and properties of the plasmid-encoded enzyme. J Biol Chem. 1983 Sep 10;258(17):10586–10593. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight S. L., Kingsbury R. Transcriptional control signals of a eukaryotic protein-coding gene. Science. 1982 Jul 23;217(4557):316–324. doi: 10.1126/science.6283634. [DOI] [PubMed] [Google Scholar]
- Melton D. W., Konecki D. S., Brennand J., Caskey C. T. Structure, expression, and mutation of the hypoxanthine phosphoribosyltransferase gene. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2147–2151. doi: 10.1073/pnas.81.7.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgard M. V., Tocci M. J., Monahan J. J. On the cloning of eukaryotic total poly(A)-RNA populations in Escherichia coli. J Biol Chem. 1980 Aug 25;255(16):7665–7672. [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piszkiewicz D., Tilley B. E., Rand-Meir T., Parsons S. M. Amino acid sequence of ATP phosphoribosyltransferase of Salmonella typhimurium. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1589–1592. doi: 10.1073/pnas.76.4.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen P., Jensen K. F., Valentin-Hansen P., Carlsson P., Lundberg L. G. Nucleotide sequence of the Escherichia coli pyrE gene and of the DNA in front of the protein-coding region. Eur J Biochem. 1983 Sep 15;135(2):223–229. doi: 10.1111/j.1432-1033.1983.tb07641.x. [DOI] [PubMed] [Google Scholar]
- Pratt D., Subramani S. Nucleotide sequence of the Escherichia coli xanthine-guanine phosphoribosyl transferase gene. Nucleic Acids Res. 1983 Dec 20;11(24):8817–8823. doi: 10.1093/nar/11.24.8817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Reynolds G. A., Basu S. K., Osborne T. F., Chin D. J., Gil G., Brown M. S., Goldstein J. L., Luskey K. L. HMG CoA reductase: a negatively regulated gene with unusual promoter and 5' untranslated regions. Cell. 1984 Aug;38(1):275–285. doi: 10.1016/0092-8674(84)90549-x. [DOI] [PubMed] [Google Scholar]
- Richardson K. K., Fostel J., Skopek T. R. Nucleotide sequence of the xanthine guanine phosphoribosyl transferase gene of E. coli. Nucleic Acids Res. 1983 Dec 20;11(24):8809–8816. doi: 10.1093/nar/11.24.8809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakano H., Rogers J. H., Hüppi K., Brack C., Traunecker A., Maki R., Wall R., Tonegawa S. Domains and the hinge region of an immunoglobulin heavy chain are encoded in separate DNA segments. Nature. 1979 Feb 22;277(5698):627–633. doi: 10.1038/277627a0. [DOI] [PubMed] [Google Scholar]
- Sikela J. M., Khan S. A., Feliciano E., Trill J., Tischfield J. A., Stambrook P. J. Cloning and expression of a mouse adenine phosphoribosyltransferase gene. Gene. 1983 May-Jun;22(2-3):219–228. doi: 10.1016/0378-1119(83)90106-3. [DOI] [PubMed] [Google Scholar]
- Stambrook P. J., Dush M. K., Trill J. J., Tischfield J. A. Cloning of a functional human adenine phosphoribosyltransferase (APRT) gene: identification of a restriction fragment length polymorphism and preliminary analysis of DNAs from APRT-deficient families and cell mutants. Somat Cell Mol Genet. 1984 Jul;10(4):359–367. doi: 10.1007/BF01535631. [DOI] [PubMed] [Google Scholar]
- Stein J. P., Catterall J. F., Kristo P., Means A. R., O'Malley B. W. Ovomucoid intervening sequences specify functional domains and generate protein polymorphism. Cell. 1980 Oct;21(3):681–687. doi: 10.1016/0092-8674(80)90431-6. [DOI] [PubMed] [Google Scholar]
- Tischfield J. A., Ruddle F. H. Assignment of the gene for adenine phosphoribosyltransferase to human chromosome 16 by mouse-human somatic cell hybridization. Proc Natl Acad Sci U S A. 1974 Jan;71(1):45–49. doi: 10.1073/pnas.71.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso J. Y., Zalkin H., van Cleemput M., Yanofsky C., Smith J. M. Nucleotide sequence of Escherichia coli purF and deduced amino acid sequence of glutamine phosphoribosylpyrophosphate amidotransferase. J Biol Chem. 1982 Apr 10;257(7):3525–3531. [PubMed] [Google Scholar]
- Verde P., Stoppelli M. P., Galeffi P., Di Nocera P., Blasi F. Identification and primary sequence of an unspliced human urokinase poly(A)+ RNA. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4727–4731. doi: 10.1073/pnas.81.15.4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiborg O., Hyldig-Nielsen J. J., Jensen E. O., Paludan K., Marcker K. A. The nucleotide sequences of two leghemoglobin genes from soybean. Nucleic Acids Res. 1982 Jun 11;10(11):3487–3494. doi: 10.1093/nar/10.11.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. K., Masters J. N., Attardi G. Human dihydrofolate reductase gene organization. Extensive conservation of the G + C-rich 5' non-coding sequence and strong intron size divergence from homologous mammalian genes. J Mol Biol. 1984 Jun 25;176(2):169–187. doi: 10.1016/0022-2836(84)90419-4. [DOI] [PubMed] [Google Scholar]


