Abstract
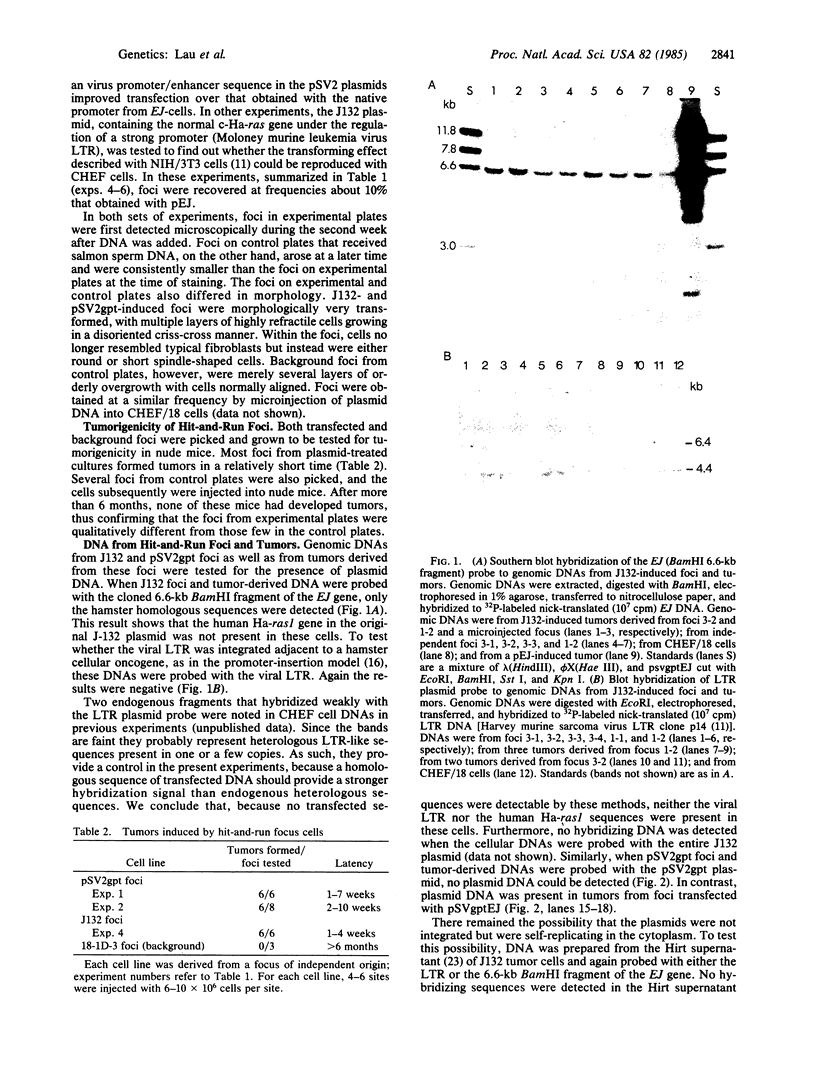
The Chinese hamster embryo fibroblast cell line CHEF/18 is readily transfected by plasmid DNA. In the present transfection studies with CHEF/18 cells, focus formation induced by plasmids containing the mutant human c-Ha-ras gene EJ was compared with that of control plasmids without the EJ insert. The focus-forming activity of the transfected plasmid J132, a recombinant of the Harvey murine sarcoma virus LTR and the normal human c-Ha-ras1 in pBR322, also was assessed. Foci were recovered after transfection with either pSV2gpt or pSV2neo at about 10% the frequency obtained with the EJ-containing plasmids, and J132 gave a similar frequency, all well above background obtained with salmon sperm DNA. Whereas foci from transfection with EJ-containing plasmids contained the EJ DNA, no plasmid DNA was detected in either tumorigenic or tumor-derived cells from foci transfected with pSVgpt, pSVneo, or J132. Evidence that genomic changes were induced by plasmid transfection is based on finding chromosomal aberrations in all expanded foci and tumor-derived cells examined. The results suggest the occurrence of "hit-and-run" tumorigenesis induced by transient plasmid transfection.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop J. M. Cellular oncogenes and retroviruses. Annu Rev Biochem. 1983;52:301–354. doi: 10.1146/annurev.bi.52.070183.001505. [DOI] [PubMed] [Google Scholar]
- Chang E. H., Furth M. E., Scolnick E. M., Lowy D. R. Tumorigenic transformation of mammalian cells induced by a normal human gene homologous to the oncogene of Harvey murine sarcoma virus. Nature. 1982 Jun 10;297(5866):479–483. doi: 10.1038/297479a0. [DOI] [PubMed] [Google Scholar]
- Craig R. W., Sager R. Suppression of tumorigenicity in hybrids of normal and oncogene-transformed CHEF cells. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2062–2066. doi: 10.1073/pnas.82.7.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadi I. K., Harrison J. J., Sager R. Genetic analysis of tumorigenesis: XVI. Chromosome changes in azacytidine- and insulin-induced tumorigenesis. Somat Cell Mol Genet. 1984 Sep;10(5):521–529. doi: 10.1007/BF01534856. [DOI] [PubMed] [Google Scholar]
- Galloway D. A., McDougall J. K. The oncogenic potential of herpes simplex viruses: evidence for a 'hit-and-run' mechanism. Nature. 1983 Mar 3;302(5903):21–24. doi: 10.1038/302021a0. [DOI] [PubMed] [Google Scholar]
- Galloway D. A., Nelson J. A., McDougall J. K. Small fragments of herpesvirus DNA with transforming activity contain insertion sequence-like structures. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4736–4740. doi: 10.1073/pnas.81.15.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graessmann A., Graessmann M., Mueller C. Microinjection of early SV40 DNA fragments and T antigen. Methods Enzymol. 1980;65(1):816–825. doi: 10.1016/s0076-6879(80)65076-9. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Harrison J. J., Anisowicz A., Gadi I. K., Raffeld M., Sager R. Azacytidine-induced tumorigenesis of CHEF/18 cells: correlated DNA methylation and chromosome changes. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6606–6610. doi: 10.1073/pnas.80.21.6606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward W. S., Neel B. G., Astrin S. M. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature. 1981 Apr 9;290(5806):475–480. doi: 10.1038/290475a0. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Kitchin R. M., Gadi I. K., Smith B. L., Sager R. Genetic analysis of tumorigenesis: X. Chromosome studies of transformed mutants and tumor-derived CHEF/18 cells. Somatic Cell Genet. 1982 Sep;8(5):677–689. doi: 10.1007/BF01542860. [DOI] [PubMed] [Google Scholar]
- Kitchin R. M., Sager R. Genetic analysis of tumorigenesis: V. Chromosomal analysis of tumorigenic and nontumorigenic diploid chinese hamster cell lines. Somatic Cell Genet. 1980 Jan;6(1):75–87. doi: 10.1007/BF01538697. [DOI] [PubMed] [Google Scholar]
- Kuhlmann I., Achten S., Rudolph R., Doerfler W. Tumor induction by human adenovirus type 12 in hamsters: loss of the viral genome from adenovirus type 12-induced tumor cells is compatible with tumor formation. EMBO J. 1982;1(1):79–86. doi: 10.1002/j.1460-2075.1982.tb01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebkowski J. S., DuBridge R. B., Antell E. A., Greisen K. S., Calos M. P. Transfected DNA is mutated in monkey, mouse, and human cells. Mol Cell Biol. 1984 Oct;4(10):1951–1960. doi: 10.1128/mcb.4.10.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Expression of a bacterial gene in mammalian cells. Science. 1980 Sep 19;209(4463):1422–1427. doi: 10.1126/science.6251549. [DOI] [PubMed] [Google Scholar]
- Razzaque A., Mizusawa H., Seidman M. M. Rearrangement and mutagenesis of a shuttle vector plasmid after passage in mammalian cells. Proc Natl Acad Sci U S A. 1983 May;80(10):3010–3014. doi: 10.1073/pnas.80.10.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager R. Genetic suppression of tumor formation. Adv Cancer Res. 1985;44:43–68. doi: 10.1016/s0065-230x(08)60025-1. [DOI] [PubMed] [Google Scholar]
- Sager R., Kovac P. E. Genetic analysis of tumorigenesis: I. Expression of tumor-forming ability in hamster hybrid cell lines. Somatic Cell Genet. 1978 May;4(3):375–392. doi: 10.1007/BF01542849. [DOI] [PubMed] [Google Scholar]
- Sager R., Tanaka K., Lau C. C., Ebina Y., Anisowicz A. Resistance of human cells to tumorigenesis induced by cloned transforming genes. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7601–7605. doi: 10.1073/pnas.80.24.7601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider E. L., Stanbridge E. J., Epstein C. J. Incorporation of 3H-uridine and 3H-uracil into RNA: a simple technique for the detection of mycoplasma contamination of cultured cells. Exp Cell Res. 1974 Mar 15;84(1):311–318. doi: 10.1016/0014-4827(74)90411-x. [DOI] [PubMed] [Google Scholar]
- Shih C., Weinberg R. A. Isolation of a transforming sequence from a human bladder carcinoma cell line. Cell. 1982 May;29(1):161–169. doi: 10.1016/0092-8674(82)90100-3. [DOI] [PubMed] [Google Scholar]
- Smith B. L., Anisowicz A., Chodosh L. A., Sager R. DNA transfer of focus- and tumor-forming ability into nontumorigenic CHEF cells. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1964–1968. doi: 10.1073/pnas.79.6.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. L., Sager R. Multistep origin of tumor-forming ability in Chinese hamster embryo fibroblast cells. Cancer Res. 1982 Feb;42(2):389–396. [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Temin H. M. Malignant transformation of cells by viruses. Perspect Biol Med. 1970 Autumn;14(1):11–26. doi: 10.1353/pbm.1970.0006. [DOI] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R., Urlaub G., Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]






