Abstract
The principal biological role of mitochondria is to supply energy to cells; although intriguingly, evolution has bestowed another essential function upon these cellular organelles: under physiological stress, mitochondria become the cornerstone of apoptotic cell death. Specifically, mitochondrial outer membrane permeabilization (MOMP) allows cell death factors such as cytochrome c to be released into the cytoplasm, thus inducing caspase activation and the eventual destruction of essential cellular components. Proteins of the B-cell lymphoma 2 (BCL-2) family control the tightly regulated pathway that causes MOMP. The equilibrium between pro-survival and pro-apoptotic members of the BCL-2 family dictates the fate of cells, the homeostasis of organs and, by extension, the health of whole organisms. Dysregulation of this equilibrium is involved in a large number of diseases such as cancer, autoimmunity and neurodegenerative conditions. Modulating the activity of the BCL-2 family of proteins with small molecules or peptides is an attractive but challenging therapeutic goal. This review highlights the latest developments in this field and provides evidence that this strategy is likely to have a positive effect on the treatment of still poorly addressed medical conditions.
LINKED ARTICLES
This article is part of a themed issue on Mitochondrial Pharmacology: Energy, Injury & Beyond. To view the other articles in this issue visit http://dx.doi.org/10.1111/bph.2014.171.issue-8
Keywords: apoptosis, BCL-2 family, protein–protein interactions, cancer, neurodegenerative diseases
Introduction
Apoptosis is a form of genetically programmed cell death, which is both evolutionally conserved and tightly regulated at a molecular level. The process plays a key role in embryonic development and in the destruction of diseased, damaged or unwanted cells. From the first coinage of the term in 1972, a poetic adoption of a Greek word used to describe how leaves are dropped from a tree (Kerr et al., 1972), it was predicted that defects in the process might play a role in a wide range of disease states, in which normal regulation of cell number becomes perturbed, such as cancer or autoimmunity (survival of unwanted cells), or developmental and degenerative disorders (inappropriate killing of vital cells).
Cells may be triggered to undergo apoptosis via either an ‘extrinsic’ (death receptor) or ‘intrinsic’ (mitochondrial) pathway; the latter being regulated by proteins of the B-cell lymphoma 2 protein (BCL-2) family and proceeding via key steps that lead to mitochondrial outer membrane permeabilization (MOMP). Both pathways converge in the activation of a cascade of downstream caspases (cysteine-aspartic proteases), which catalyse the process of cellular demolition. This results in the phenotype characteristic of apoptotic cells: DNA laddering, cell shrinkage, apoptotic body formation, chromatin condensation and plasma membrane changes such as blebbing and externalization of phosphatidyl serine, which rapidly signals for the ultimate engulfment and digestion of the dying cell by macrophages. Understanding the key molecular interactions between BCL-2 family members that regulate the intrinsic apoptotic pathway leading to MOMP has paved the way for the development of new therapies that modulate apoptosis.
This review focuses on the intrinsic BCL-2 family-driven pathway to apoptosis. It summarizes the biological context to targeting these proteins and describes recent advances in therapeutic approaches with compounds directly interacting with BCL-2 family proteins, including an assessment of their clinical potential.
The BCL-2 family: members and interactions
The BCL-2 family of proteins comprises two functionally opposing subsets: the pro-survival proteins and pro-apoptotic proteins. The relative proportions of these two subsets control the fine balance between cell survival and death via the intrinsic apoptotic pathway. In mammals, pro-survival proteins, such as BCL-2, BCL-XL (B-cell lymphoma extra large protein), BCL-W, MCL-1 (myeloid cell leukaemia sequence 1 protein) and A1 (BCL-2 related protein A1), act as gatekeepers to block apoptosis by inhibiting their pro-apoptotic counterparts (Adams and Cory, 2007). The pro-apoptotic proteins are divided into two further subgroups: the so-called BH3-only proteins, including BIM (BCL-2 interacting mediator of cell death), tBID, BAD (BCL-2-associated death promoter protein), PUMA (p53 up-regulated modulator of apoptosis protein), NOXA, act as initiators and are invoked in response to sensing discrete cellular apoptotic stimuli (such as growth factor withdrawal, DNA damage, anoikis) and the multi-domain BAK (BCL-2 homologous antagonist/killer protein)/BAX (BCL-2-associated X protein) proteins, which directly facilitate MOMP (Figure 1A) (Youle and Strasser, 2008).
Figure 1.

The mitochondrial pathway to apoptosis. Apoptosis (programmed cell death) via the mitochondrial or ‘intrinsic’ pathway is regulated by finely balanced interactions between members of the BCL-2 family of proteins. (A) In healthy cells, pro-survival BCL-2 proteins act as guardians of mitochondrial integrity to restrain the effector molecules BAX and BAK, either by sequestering them in heterodimeric complexes or preventing their activation by certain initiator BH3-only proteins (A and B). In response to apoptotic stimuli, BH3-only proteins become up-regulated or activated and overwhelm the pro-survival proteins. This allows for BAX/BAK activation by certain BH3-only proteins and relieves BAX/BAK restraint by pro-survival proteins. Following a series of conformational changes in BAK/BAX, they oligomerize on the outer-mitochondrial membrane, leading to an irreversible step known as MOMP (C). When this occurs, cytochrome c is released into the cytosol (with other apoptotic factors) and together with Apaf-1 forms a structure known as the apoptosome that activates a cascade of proteolytic caspases which demolish the cell, leading to its death.
In response to stimuli, BH3-only protein activity can be up-regulated by increased expression, activation by proteolytic cleavage or post-translational modification. These BH3-only proteins then trigger apoptosis either by directly activating BAK/BAX (Letai et al., 2002; Cartron et al., 2004; Kuwana et al., 2005; Certo et al., 2006; Deng et al., 2007; Gavathiotis et al., 2008) or by disrupting complexes between pro-survival proteins and activated BAK/BAX (Oltvai et al., 1993; Willis et al., 2007). The BAK/BAX molecules go on to oligomerize on the outer mitochondrial membrane (Figure 1B,C). This oligomeric assembly triggers MOMP (although whether this occurs due to formation of a pore or by some other mechanism is yet to be determined), allowing the release of cytochrome c and other apoptosis-inducing factors, from the mitochondrial inter-membrane space. Cytosolic cytochrome c interacts with apoptotic protease-activating factor 1 (Apaf-1) to form a structure known as the apoptosome. The apoptosome activates caspase-9, thus initiating a caspase cascade which ultimately leads to demolition of the cell (Bratton and Salvesen, 2010). MOMP is likely to constitute an irreversible step in the pathway as the amplification of the caspase activation cascade (upstream caspases activating downstream caspases) is difficult to interrupt.
Elevated levels of one or more pro-survival proteins, as observed in many tumours, can block apoptosis. This block can occur through sequestration of activator BH3-only proteins, or capture and restraint of active forms of BAK/BAX, or both (Llambi et al., 2011). Pro-survival proteins have been shown to be capable of forming heterotypic interactions with the BH3 domain of both BH3-only proteins and BAK/BAX (Sattler et al., 1997; Liu et al., 2003; Czabotar et al., 2011). Moreover, as direct activation of BAK/BAX by BH3-only proteins has been proposed to occur via 4 ‘hit-and-run’ mechanism, structural elucidation of this mechanism remains challenging, although there have been notable recent insights into how this process might proceed (Gavathiotis et al., 2008; Czabotar et al., 2013; Moldoveanu et al., 2013). In either case, the development of agents able to selectively inhibit pro-survival proteins (or to modulate pro-apoptotic BAK/BAX activation/oligomerization) offers significant therapeutic potential, which has been greatly assisted by a detailed structural understanding of these interactions.
Structural features of the BCL-2 family of proteins
All proteins forming part of the BCL-2 family share one or more ‘BCL-2 homology’ (BH) domains, named by reference to the founding member BCL-2. The pro-survival proteins and BAK/BAX each share four BH domains (BH1-4; Kvansakul et al., 2008), whereas the BH3-only proteins share only their eponymous BH3 domain and are otherwise structurally diverse (Figure 2A; Hinds et al., 2007). It is worth noting that while early reports claimed that BAX and BAK possessed only BH domains 1–3 (Zha et al., 1996), BH4 signature motifs are evident from later sequence and structural analyses (Kvansakul et al., 2008).
Figure 2.
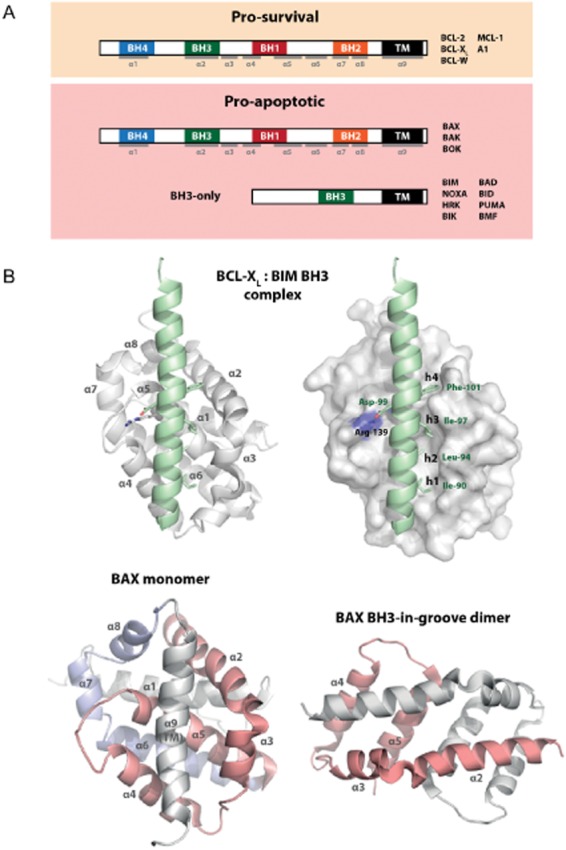
(A) Proteins of the BCL-2 family. The BCL-2 family is composed of proteins that share in common at least one of the so-called BCL-2 homology (BH) domains (BH1–BH4). They fall into two functional subclasses – the pro-survival proteins (e.g. BCL-2, BCL-XL), which act to restrain apoptosis, and the pro-apoptotics, which can be further subdivided into the multidomain effectors BAK, BAX (and possibly BOK) and the BH3-only proteins (e.g. BIM, BAD), that sense apoptotic stimuli to trigger apoptosis. This occurs either by displacing heterodimeric complexes of pro-survivals bound to active BAK/BAX or by BH3-only proteins saturating binding to pro-survivals, allowing release of sequestered ‘activator’ BH3-only proteins, which directly bind to and activate BAK/BAX, or a combination of both. The BH3 domain is essential for this killing function. (B) Structures and key interactions of BCL-2 family members. Both pro-survivals and BAK/BAX share domains BH1–BH4 as well as a similar overall fold. The X-ray crystal structure of the BH3 domain of BIM (green) bound to human BCL-XL (white) (PDB: 1PQ1, Liu et al., 2003), left figure in ribbon representation, right figure with BCL-XL in surface representation) revealed that the BH3 domain of BH3-only proteins can form an amphipathic α-helix and binds along a hydrophobic surface groove on BCL-XL (formed mostly from helices α2–α5). Key binding interactions include four hydrophobic residues (h1–h4) present in all BH3 domains, which bind into corresponding pockets (P1–P4) on the pro-survival protein and a salt bridge formed between a conserved Asp on the BH3 domain and an Arg residue on the pro-survival protein. The structure of inactive monomeric human BAX (PDB: 1F16, Suzuki et al., 2000) shows a very similar structure, albeit instead with the putative transmembrane (TM) domain α9 sequestered along the hydrophobic groove, consistent with its capacity to localize in the cytosol. Based on recent structural information (Czabotar et al., 2013), we have proposed the following model for BAX activation: on direct binding by an ‘activator’ BH3 into the groove of BAX (after insertion of α9 into the outer-mitochondrial membrane), a ‘latch’ domain of BAX (α6–α8, blue) becomes released from the ‘core’ domain (α2–α5, red) of the protein, allowing homodimerization with the core domain of another BAX molecule to form a symmetric BH3-in-groove dimer (PDB: 4BDU, Czabotar et al., 2013). This would form the starting point to nucleate further BAX oligomerization at another face, ultimately triggering MOMP leading to cell death.
Interestingly, despite their opposing function, both the pro-survival proteins and BAK/BAX share remarkably similar tertiary structures (Figure 2B). Structures of pro-survival proteins BCL-XL (Muchmore et al., 1996), BCL-2 (Petros et al., 2001), BCL-W (Denisov et al., 2003; Hinds et al., 2003), MCL-1 (Day et al., 2005), A1 (Herman et al., 2008) as well as BAK (Moldoveanu et al., 2006) and BAX (Suzuki et al., 2000) reveal in each case an 8 α-helical bundle, which folds to form a conserved hydrophobic surface groove (the ‘canonical groove’). The BH3-only protein BH3 interacting-domain death agonist (BID) is exceptional among the BH3-only proteins in that it also shares this fold, albeit with a shorter and shallower surface groove (Chou et al., 1999; McDonnell et al., 1999).
Numerous structural and functional studies have demonstrated that the canonical grooves on multi-domain BCL-2 family members are capable of binding to BH3 domains with varying specificity and affinity. The BH3 domains are essential for the killing activity of the BH3-only proteins: these BH3•groove interfaces represent key interactions that can be targeted with small molecules (Simonen et al., 1997; Chen et al., 2005; Certo et al., 2006).
Thus, the BH3-domain appears to be the endogenous ‘ligand’ for the native hydrophobic binding groove located on the surface of pro-survival proteins and BAX/BAK. In all BH3•pro-survival structures solved, the BH3 domain forms an amphipathic α-helix upon binding. Key interactions have been shown to be conserved: a set of four hydrophobic residues (h1–h4) project into corresponding binding pockets along the groove (p1–p4; Figure 2B, right-hand panel), and a salt bridge formed between an Asp conserved among BH3 domains and an Arg present on all pro-survival proteins. Despite these commonalities, subtle variations between the BH3 domain ligands and the hydrophobic binding grooves impart differences in binding selectivity between BH3 domains and pro-survival partners (Chen et al., 2005). While BIM, BID and PUMA display tight binding affinities for all pro-survival proteins, BAD and NOXA are more selective (the former binding to only BCL-2, BCL-XL and BCL-W and the latter to MCL-1 and A1). This diversity in binding profile accounts partly for the complex interactions between BCL-2 family proteins. From a drug discovery standpoint, this intrinsic selectivity offered the promise of developing small molecule ‘BH3 mimetics,’ which might selectively antagonize one or more pro-survival proteins in the same manner as a BH3 domain. As the binding groove is relatively shallow and hydrophobic, this endeavour has required a significant amount of ingenuity in medicinal chemistry. The recent successes of several drug discovery programmes, due to unexpected plasticity in the canonical hydrophobic groove (to be discussed later), have demonstrated the feasibility of this approach.
The BCL-2 family proteins exert their activity at the MOM
One further important structural feature of the majority of the BCL-2 proteins is their C-terminal hydrophobic extensions, often removed to solve these structures as they impair protein solubility. These C-terminal portions act as membrane-anchoring domains to direct sub-cellular localization; for example, in BAK, this region is important for its constitutive localization to the outer mitochondrial and endoplasmic reticulum membranes (Breckenridge et al., 2003). In the case of BCL-W and BAX, this C-terminal α-helix (α9) can bind intramolecularly into the canonical hydrophobic groove (Figure 2B) (Suzuki et al., 2000; Petros et al., 2004). This characteristic apparently allows for the normal localization of BAX as a cytosolic monomer, which is then translocated to the outer-mitochondrial membrane for apoptosis to occur (Hsu et al., 1997). However, the precise mechanisms by which BAX translocates to and inserts into the outer mitochondrial membrane and how BAX and BAK become activated and oligomerize to cause MOMP are still not fully understood (Westphal et al., 2011).
Structural studies in this area have been hampered by the membrane localization of these events and the difficulties of preparing full length active forms of BAX and BAK. However, biochemical trapping and cross-linking experiments of BAX or BAK at various points in the oligomerization process suggest that key conformational changes occur: exposure of the BH3 domain of BAK/BAX (in the α2 helix), exposure of an N-terminal segment and in the case of BAX additional exposure of the C-terminal α9 for membrane insertion. Additionally, the BH3 domain of BAX is known to be required for its homo-oligomerization (Wang et al., 1998). Initial activation and translocation of BAX has been proposed to be either a spontaneous process (Edlich et al., 2011; Schellenberg et al., 2013) or to be caused by direct and transient binding of an ‘activator’ BH3-only protein (such as BIM or BID) to BAK or BAX (Letai et al., 2002; Kuwana et al., 2005; Certo et al., 2006). For example, it has been suggested that α-helices of the BH3 domains of BIM or BAX stabilized through staples [stabilized α-helices of BCL-2 domains (SAHB)] can bind transiently to a trigger site located near the N-terminus (α1/α6) of BAX and cause its activation (Gavathiotis et al., 2008; 2010). This interaction may act to displace the BAX α9 helix from the canonical groove in order to initiate translocation to the outer mitochondrial membrane (Figures 1B and 2B, lower left panel). Once at the membrane, the canonical groove is free to interact with activator BH3 domains, which induce further conformational changes (Czabotar et al., 2013). BAK is primarily located at the outer mitochondrial membrane and similarly binds activator BH3 domains within its canonical hydrophobic groove (Leshchiner et al., 2013; Moldoveanu et al., 2013).
Recent advances have provided significant insights into the nature of the BAX and BAK oligomers that facilitate MOMP. The actual sizes of the oligomers remain unresolved, with some liposome studies suggesting that at least four BAX molecules are required to release cytochrome c (Saito et al., 2000) or 20 to release other important proteins (Lovell et al., 2008), but as many as a few hundred subunits have also been proposed (Zhou and Chang, 2008). Cross-linking studies suggest that activated BAK and BAX both initially form a symmetrical BH3•groove homodimer (Dewson et al., 2008; 2012; Oh et al., 2010) and that a second lower affinity interaction, on which the larger oligomer builds, occurs in the region of the α6 helix (Dewson et al., 2008; 2012) – indeed, protein analysis by native PAGE suggests BH3-in-groove dimers and α6:α6 disulfide linkage is sufficient to stabilize higher order BAK oligomers (Ma et al., 2013). Other models suggest an asymmetric ‘daisy chain’ (or nose-to-tail) arrangement of monomers, by which the BH3 domain binds either into a site involving the α6 region or a ‘rear pocket’ (Gavathiotis et al., 2010; Leber et al., 2010; Zhang et al., 2010). However, such models are inconsistent with a symmetric dimer within the larger oligomer that involves the BAX BH3 domain. The structure of such a symmetric BH3-in-groove dimer interface has recently been solved (Czabotar et al., 2013) and an elaborated model has been proposed for the conversion of BAX from its monomeric form to its active oligomeric state. In this model, binding of activator BH3-only proteins to the canonical groove of membrane-bound BAX initiates release of a ‘core’ domain of BAX (α2–α5 and possibly also α1) from a ‘latch’ domain (α6–α8) and leads to destabilization of α2 (the BH3 domain of BAX) (Czabotar et al., 2013). Once this event has occurred, two neighbouring BAX molecules with exposed BH3 domains can come together to form a BH3-in-groove symmetric dimer as the basic unit on which the larger oligomer builds (George et al., 2007; Dewson et al., 2012). The model is consistent with a previous study, which found that the α2–α5 of BAX is the minimal domain sufficient for oligomerization, and that when fused to the α9 region, this portion is able to cause MOMP (George et al., 2007).
Therapeutic potential of BCL-2 family inhibition
Agents modulating apoptosis by targeting members of the BCL-2 family offer significant potential for the development of new therapies for diseases involving aberrant cell accumulation or cell loss. The best illustration of this concept is found in the development of selective inhibitors of pro-survival BCL-2 family proteins as novel cancer therapies. Such tantalizing prospects were initially proposed from the discovery that, due to a chromosomal translocation, elevated levels of BCL-2 alone prevented follicular lymphoma cells from undergoing apoptosis (Vaux et al., 1988). Many subsequent in vitro and in vivo studies have shown that elevated levels of pro-survival proteins are frequently observed in cancer (BCL-XL and MCL-1, Beroukhim et al., 2010) and can contribute to cancer phenotype (Sentman et al., 1991; Miyashita and Reed, 1993; Adams and Cory, 2007). Down-regulation of apoptosis is now considered a key step for the initiation and maintenance of cancer (Hanahan and Weinberg, 2000,2011). Moreover, BCL-XL overexpression, in particular, has been strongly correlated with resistance to traditional anti-cancer chemotherapies, which often rely on triggering death via the apoptotic response (Amundson et al., 2000). Small molecule BH3 mimetics, which functionally replicate the pro-apoptotic effect of BH3-only proteins and can therefore counterbalance the over-expression of pro-survival proteins, offer the possibility of significantly impairing cancer cell growth and combatting chemoresistance. Yet, compound-induced apoptosis may raise the spectre of a narrow therapeutic window, especially because of the importance of the pro-survival proteins in a wide range of normal biological processes (e.g. immune system, platelet life span, spermatogenesis, cardiac function; Youle and Strasser, 2008). Despite this, the examples of small molecules to be given in detail later, some of which are already in the clinic, demonstrate that this approach is viable. A possible explanation for the excellent efficacy and manageable toxicities observed so far in patients may well be the strong apoptotic pressure exerted on cancer cells, compared with normal cells (Certo et al., 2006). BH3 mimetics might therefore be reinstating a pathway already primed to be unravelled in cancer cells.
Impaired apoptosis has also been implicated in autoimmunity: for example, loss of BIM or the combined loss of BAK and BAX in the hematopoietic compartment leads to a failure to eliminate auto-reactive thymocytes (Bouillet et al., 2002; Mason et al., 2013). BH3 mimetics may thus also prove to be effective as immune modulatory agents in certain contexts such as pancreatic transplantation to treat diabetes (Carrington et al., 2010).
Inhibitors of apoptosis, which block the pro-apoptotic activity of BAK or BAX, may offer potential therapies for diseases characterized by excessive cell death. Up-regulated apoptosis has been implicated in ischaemia/reperfusion injury following stroke or myocardial infarction (Martinou et al., 1994), neurodegeneration (such as Alzheimer's, Parkinson's and Huntington's diseases; Galluzzi et al., 2009; Lukiw and Bazan, 2010), allograft rejection, osteoarthritis, certain inflammatory disorders or even HIV (due to depletion of T lymphocytes; Reed, 2002). Thorough therapeutic validation of this approach remains to be established especially considering that other cell death pathways are also involved. A number of compounds shown to block apoptosis have already been reported. However, it is unclear whether they target BAX or BAK directly or other proteins associated with mitochondrial apoptosis (Bombrun et al., 2003; Polster et al., 2003; Rodrigues et al., 2003; Hetz et al., 2005; Peixoto et al., 2009). Well-validated, small molecule inhibitors of BAX and/or BAK will provide much needed tools to explore this strategy.
Pharmacological inhibition of the BCL-2 family of proteins
Traditionally considered difficult targets (Wells and Mcclendon, 2007), the interfaces between BCL-2 proteins are characterized by large, shallow and mainly hydrophobic areas that generally lack anchorage points for productive interactions. The development of small molecules targeting these protein–protein interfaces has therefore been extremely difficult. Conventional drug discovery methods have, in some rare cases, delivered validated BH3 mimetics. Most often, however, the success of such programmes has relied on structure-guided drug discovery, NMR fragment screening and peptido-mimetic approaches.
α/β foldamers
As pointed out earlier, the BH3 domain is essential for the binding of BH3-only proteins to their pro-survival targets and indeed isolated 26-mer BH3 peptides retain most of the binding affinity of the full-length protein. Peptides themselves seldom represent good drug candidates as they suffer from significant pharmacological liabilities (stability, cell membrane penetration). Therefore, strategies that can increase the binding affinities of short peptides (by artificially enhancing helicity) and improve their proteolytic stability have attracted significant efforts.
Incorporating β-amino acids is one approach that confers significant resistance to enzymatic degradation and enhances helicity (Johnson et al., 2012). BH3-mimetic α/β-peptides utilize combinations of α-and β-amino acids to replicate the binding interactions between endogenous BH3 domains and their pro-survival targets. Importantly for target recognition, the geometry of the backbone is maintained through ‘sequence-based design’ with only partial modifications so as to still reproduce the side-chain projection pattern of an α-helix. This approach has been successfully applied to the design and preparation of BIM and PUMA foldamers (Lee et al., 2011; Boersma et al., 2012; Smith et al., 2013). Initially, an α/β-peptide 21-mer comprising the PUMA BH3 domain demonstrated a strong affinity for BCL-XL, but not MCL-1 (Lee et al., 2011). Previous work had demonstrated that the ααβαααβ pattern exploiting the heptad repeat of an α-helix maintains the helicity of the construct while improving proteolytic stability (Boersma et al., 2012). Importantly, the α/β-peptides maintained all of the non-covalent interactions shown to be necessary for recognition of the PUMA-BH3 by BCL-XL (Lee et al., 2011). Interestingly, the IC50 values against BCL-2, BCL-W and BCL-XL for the best peptide prepared in this study were comparable with the PUMA-BH3 peptide, albeit alongside a 50-fold decrease in binding affinity for MCL-1. The difference in binding profile between the PUMA-BH3 domain and the α/β-peptide 21-mer was suggested to be due to steric clashes involving solvent-exposed residues on MCL-1. Mouse embryonic fibroblast (MEF) cells have been shown to undergo BAK-mediated apoptosis if both BCL-XL and MCL-1 are neutralized, providing a useful system for in vitro validation (Willis et al., 2005). Thus, consistent with its binding profile, in particular, weak MCL-1 affinity, the α/β-peptide 21-mer was found to be inactive in wild-type MEF cells but active in Mcl-1−/− MEFs. Further manipulations using structure-guided rational design were recently shown to achieve improved affinity for MCL-1, resulting in a series of novel PUMA-BH3-based foldamers characterized by their high affinity for both MCL-1 and BCL-XL (Smith et al., 2013).
Stapled peptides
Stapled peptides or SAHBs are modified peptides incorporating covalent constraints between two amino-acid residues located on the same face on an α-helix (Walensky et al., 2004). Although the term ‘stapling’ refers chiefly to constraints installed via the ring-closing olefin cross-metathesis reaction, the general strategy has also been applied using amide or ‘click’ triazole linkages (Skelton et al., 2001; Yang et al., 2004; Cantel et al., 2008; Kawamoto et al., 2012). Stapled peptides are reported to have improved pharmacokinetics through increased cell permeability and reduced enzymatic degradation (Walensky et al., 2004; Bird et al., 2008). More recent reports have found that a stapled BIM-BH3 peptide affects the viability of a number of haematological cancer cell lines (Labelle et al., 2012). Stapled BH3 peptides have also been used to study the direct activation model, whereby activator BH3-only proteins such as BID or BIM directly interact with pro-apoptotic proteins BAX/BAK (Walensky et al., 2006; Leshchiner et al., 2013). Finally, MCL-1-derived constructs have also been developed and used as molecular probes for selective MCL-1 inhibition (Stewart et al., 2010).
Recent studies have highlighted that the staple, far from being just a constraining element, can affect binding affinity in both positive and negative ways (Stewart et al., 2010; Brown et al., 2012; Okamoto et al., 2013). With these observations, it is becoming clear that the design of novel stapled peptides must take into account the staple when surveying structure–activity relationships.
Obatoclax and gossypol derivatives
Obatoclax (Figure 3A, GX-15–070, Gemin X Biotechnologies, Montreal, QC, Canada), derived from the natural product progidiosin, and (−)-gossypol (AT-101, Ascenta Therapeutics, Malvern, PA, USA, Figure 3B) are small molecules displaying low-to sub-micromolar affinities for multiple pro-survival proteins (as such, they are often referred to as pan-inhibitors; Zhai et al., 2006). Their discovery, biochemical and biological characterization have been reviewed previously (Lessene et al., 2008; Czabotar and Lessene, 2010). Although a number of studies have shown that their cell-killing activity is the result of modulation of the BCL-2-driven pathway, their mode of action is still a matter of debate. For example, recent studies suggest that obatoclax is also involved in programmed necrosis (Basit et al., 2013). Currently, obatoclax is in multiple phase I/II clinical trials as a single agent against haematological malignancies and in combination therapy (Schimmer et al., 2008; Chiappori et al., 2012). (−)-Gossypol demonstrated only moderate efficacy in clinical trials as single-agent against castration-resistant prostate cancer (Liu et al., 2009) and lacked efficacy as a single agent and in combination against small cell lung carcinoma (SCLC; Heist et al., 2010; Ready et al., 2011).
Figure 3.
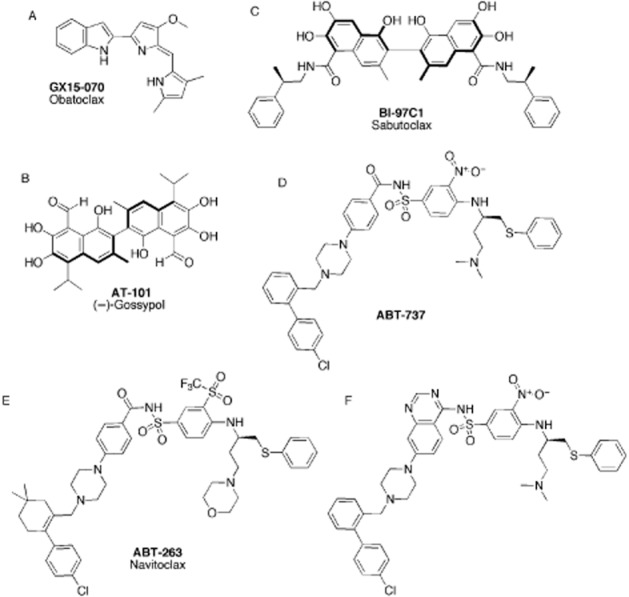
BH3 mimetics (1). (A) Pan-selective BCL-2 inhibitor GX15-070/obatoclax; (B) pan-selective BCL-2 inhibitor AT-101/(−)-Gossypol; (C) pan-selective BCL-2 inhibitor BI-97C1/sabutoclax; (D) BCL-XL, BCL-2 and BCL-W inhibitor ABT-737; (E) BCL-XL, BCL-2 and BCL-W inhibitor ABT-263/navitoclax; (F) BCL-XL and BCL-2 inhibitor derived from ABT-737.
A recent derivative of the gossypol chemical class, sabutoclax (BI-97C1, Figure 3C), binds to MCL-1, BCL-2 and BCL-XL with IC50 values of 0.20, 0.32 and 0.31 μM respectively (Wei et al., 2010). Sabutoclax induced cell death in a BAX/BAK-dependent manner and displayed efficacy both in vitro and in vivo in a xenograft model using prostate cancer cell lines (Wei et al., 2009a; 2009b). In contrast to the BH3-mimetic ABT-737, which binds to BCL-2, BCL-W and BCL-XL with high affinity, sabutoclax was able to sensitize these malignant cells to the mdl-7/IL24 (Dash et al., 2011) due to its MCL-1 targeting. In separate studies, sabutoclax caused regression of castration-resistant prostate cancer cells (Jackson et al., 2012).
Acyl sulfonamides series
The AbbVie team (formerly Abbott) pioneered drug discovery targeting of BH3 mimetics. This team was indeed the first to translate the fundamental discoveries around the BCL-2 family of proteins to well-validated small molecule inhibitors of pro-survival proteins. This effort culminated in the disclosure of ABT-737 (Figure 3D), a potent inhibitor of BCL-2, BCL-XL and BCL-W (Oltersdorf et al., 2005), which has become a widely utilized chemical biology probe. The development of ABT-737 and of its orally available analogue ABT-263 (navitoclax, Figure 3E) has been reviewed extensively elsewhere (Lessene et al., 2008; Czabotar and Lessene, 2010; Juin et al., 2013). Notably, the mechanism of action of this class of compounds has been thoroughly studied and there is now little doubt that their potent cell-killing ability is mediated by direct interaction with pro-survival BCL-2 proteins (Konopleva et al., 2006; van Delft et al., 2006; Del Gaizo Moore et al., 2007; Tse et al., 2008). Their binding mode has also been disclosed and shows that these large hydrophobic molecules bind in two out of four key hydrophobic pockets on the surface of their pro-survival protein targets (p2 and p4, Figure 2B; Lee et al., 2007; Souers et al., 2013). Interestingly, these compounds induce an important remodelling of the p2 pocket.
ABT-263 is now in clinical trial for the treatment of haematological tumours and SCLC (Gandhi et al., 2011; Roberts et al., 2012; Rudin et al., 2012). As predicted from preclinical study results, this compound displays particularly good efficacy in patients suffering from chronic lymphocytic leukaemia (CLL) as a single agent and in combination (Campas et al., 2006; Del Gaizo Moore et al., 2007; Mason et al., 2009; Wilson et al., 2010; Roberts et al., 2012). Conversely, the recently published phase II clinical trial data for single-agent ABT-263 indicate its low efficacy in patients affected by recurrent metastatic SCLC (Rudin et al., 2012). ABT-263 induces sharp but temporary drop in circulating platelets (Roberts et al., 2012). The origin of this phenomenon lies in the exquisite sensitivity of platelets to BCL-XL inhibition (Mason et al., 2007; Zhang et al., 2007), and, as such, these blood cells represent excellent biomarkers of on-target BCL-XL inhibition.
ABT-737-derived BH3 mimetics
The acyl sulfonamide moiety in the compounds developed by AbbVie may represent a potential metabolic and chemical liability (Figure 3D,E). Efforts aimed at removing this group led to quinazoline derivatives (Figure 3F), which maintained low nanomolar binding affinity against BCL-2 (IC50 = 9 nM) and BCL-XL (IC50 = 7 nM; Sleebs et al., 2011). Intriguingly, this series of isosteric analogues of ABT-737 have significantly weaker affinity for BCL-W (IC50 = 440 nM). X-ray crystallographic studies show that the quinazoline derivative and ABT-737 interact with BCL-XL with similar binding modes. However, an additional polar interaction was observed between a quinazoline ring nitrogen and BCL-XL Tyr101 (Sleebs et al., 2011). Mechanism-based cellular activity of the quinazoline compounds was also consistent with a potent dual inhibitor of BCL-XL and BCL-2 (potent killing of MEF cells lacking MCL-1 and no effect on wild-type MEF). These quinazoline derivatives were also shown to have submicromolar activity against a panel of SCLC cell lines.
More recently, a Novartis team reported a similar approach exploring the isosteric replacement of the acyl sulfonamide with heterocyclic rings (Toure et al., 2013). Initial replacement of the acyl sulfonamide with a naphthyl ring led to a complete loss of binding affinity, a result consistent with the essential role of the acidic acyl sulfonamide NH. Moving towards analogues containing a more acidic sulfonamide allowed the development of the piperidyl pyrimidine ring system. The best analogue (Figure 4A) has good binding affinity for BCL-2 (KD = 7 nM, IC50 = 19 nM) and BCL-XL (IC50 = 24 nM) translating into cell-killing activity in the BCL-2-driven Toledo cell lines (LD50 = 0.298 μM).
Figure 4.
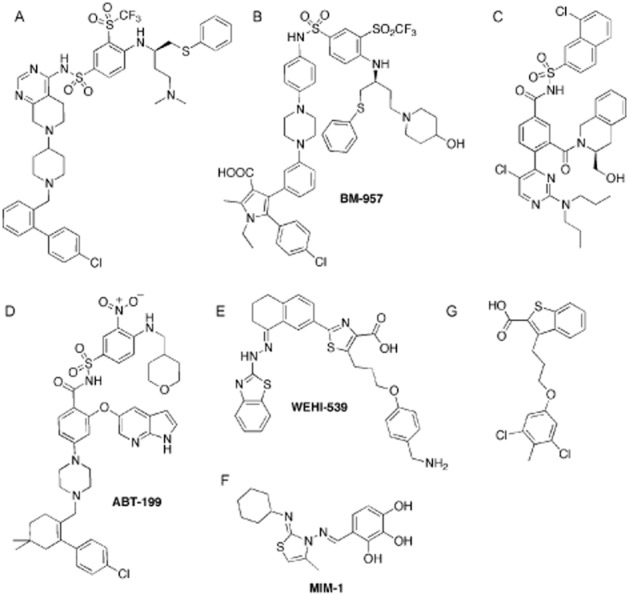
BH3 mimetics (2). (A) BCL-XL and BCL-2 inhibitor derived from ABT-737; (B) BCL-XL and BCL-2 inhibitor BM-957; (C) BCL-XL and BCL-2 inhibitor; (D) selective BCL-2 inhibitor ABT-199; (E) selective BCL-XL inhibitor WEHI-539; (F) selective MCL-1 inhibitor MIM-1; (G) selective MCL-1 inhibitor.
Using a three-dimensional pharmacophore template defined by key interactions between the BAD-BH3 peptide and BCL-XL, the Food and Drug Administration-approved drugs lipitor and celecoxib were selected as starting points towards the design of new BCL-2 inhibitors (Zhou et al., 2012a,b2012b). Modifications of the two drugs were guided by structure-based design that led to the 3,4-diphenyl-1H-pyrrole-2-carboxamide scaffolds linked to the nitrobenzene sulfonamide half of ABT-737 (Chen et al., 2012; Aguilar et al., 2013). This effort produced BM-957, which displays Ki values below 1 nM for both BCL-2 and BCL-XL (Chen et al., 2012). Cell growth inhibition in cancer cell lines was observed with IC50 values around 20 nM against the H1147 and H146 SCLC cell lines. BM-957 in vivo experiment in the H146 xenograft tumour model achieved rapid and complete tumour regression (Chen et al., 2012).
Combining previous work from Celltech (Porter et al., 2009 a,b) and the AbbVie acyl sulfonamide series, a collaborative team between Bristol–Myers–Squibbs and Nerviano has developed novel and potent dual BCL-2/BCL-XL inhibitors (Perez et al., 2012; Schroeder et al., 2012). The lead compound from this series has single-digit nanomolar activity against the two pro-survival proteins.
BCL-2-selective BH3 mimetics
As the dose-limiting toxicity in the ABT-263 clinical trials was thrombocytopenia induced by potent BCL-XL inhibition, it was thought that BCL-2-selective compounds would considerably improve the therapeutic window of such BH3 mimetics. It had also been shown that ABT-737 and ABT-263's main target in transformed lymphoid cells is BCL-2 (Merino et al., 2012; Rooswinkel et al., 2012). Using fortuitous structural insights on analogues of ABT-263, AbbVie designed and developed highly selective inhibitors of BCL-2 culminating in ABT-199 (Ki < 0.010 nM for BCL-2 and 48 nM for BCL-XL, Figure 4D; Souers et al., 2013). This compound engages the same binding pockets as ABT-263 (p2 and p4) but does so without a ‘bend-back’ π-stacking arrangement observed for ABT-737 and ABT-263. Instead, the azaindole linked directly to the molecule core forms a π-stacking arrangement with the nitroaryl moiety. The structural information reported for a close analogue of ABT-199 suggests that hydrogen bonding between the azaindole group and BCL-2 Asp103 is responsible for the enhanced selectivity (Souers et al., 2013). As expected, ABT-199 had negligible effects on platelets (in vitro and in vivo; Vandenberg and Cory, 2013). ABT-199 is currently in phase 1 clinical trials and early reports have demonstrated that this molecule did not induce thrombocytopenia, while retaining potent efficacy against CLL (Souers et al., 2013). Interestingly, ABT-199 may find application beyond that of treatment of blood-related tumours. In particular, there is evidence that ABT-199 may be effective against oestrogen receptor-positive breast cancers, in which BCL-2 is over-expressed (Vaillant et al., 2013).
BCL-XL-selective BH3 mimetics
Potent inhibition of BCL-2 has also been linked to potential toxicities such as reversible neutropenia (Roberts et al., 2012). As a large number of solid tumours rely on BCL-XL for survival (Beroukhim et al., 2010), a compound selectively inhibiting this pro-survival protein could have applications in this context. Recently, we, in collaboration with colleagues at Genentech, have reported a potent and highly selective inhibitor of BCL-XL (WEHI-539, Figure 4E; Lessene et al., 2013). Interestingly, this novel series is the first to arise in this field from high-throughput screening. A combination of classical medicinal chemistry and structure-guided design was key to the success of this work (Lessene et al., 2013; Sleebs et al., 2013). WEHI-539 binds tightly to BCL-XL with IC50 and KD values close to or below 1 nM and demonstrated high selectivity for BCL-XL over other pro-survival BCL-2 family members (at least 400-fold). Structural information showed that, like ABT-737, WEHI-539 induces a significant conformational change around p2 to accommodate its benzothiazole moiety. It was suggested that WEHI-539's selectivity for BCL-XL may be due to an array of hydrogen bonds taking place in this enlarged p2 pocket between the benzothiazole hydrazone group and backbone NH and carbonyls. Careful analysis of WEHI-539's mechanism of action supports direct engagement of BCL-XL: in contrast to ABT-737, WEHI-539 induces primarily BAK-mediated cell death, consistent with BCL-XL's key role alongside MCL-1 in BAK inhibition (Willis et al., 2005). This compound also efficiently induced apoptosis in isolated mice and human platelets.
MCL-1-selective BH3 mimetics
Using a stapled MCL-1 peptide, a high-throughput screen led to the discovery of MIM-1, a selective inhibitor of MCL-1 (Figure 4F; Cohen et al., 2012). Despite its modest binding affinity (IC50 = 4.8 μM), MIM-1 is active against leukaemic cell lines and synergized with ABT-737 (Cohen et al., 2012). In contrast, a recent comparison of MCL-1 inhibitors showed that MIM-1 induced apoptosis only weakly at very high concentration (Varadarajan et al., 2013), suggesting that other pathways may be involved in its activity in other cell lines.
More recently, a team at Vanderbilt University reported a novel MCL-1 inhibitor discovered through NMR-based fragment screening (Figure 4G; Friberg et al., 2013). Stephen Fesik, a member of this team, was one of the inventors of the ‘SAR by NMR’ technique that led to the discovery of ABT-737 (Petros et al., 2006). The chemical class identified through this screen resembles the MCL-1-selective compounds reported by AbbVie (Bruncko et al., 2008; Elmore et al., 2008) and suggests a privileged scaffold binding to MCL-1. The lead compound obtained in this series displays a Ki of 0.055 μM for MCL-1, and weaker binding affinity for BCL-XL and BCL-2. Interestingly, this compound induced the same changes around p2 to fit the phenoxyalkyl extension. As discussed above, induced structural changes to this pocket are also observed in the complexes of ABT-737 and WEHI-539 bound to BCL-XL and ABT-199 bound to BCL-2. Notably, these different compounds derive from diverse chemical starting points. This highlights the plasticity of this pocket across the pro-survival protein subgroup and indicates that it represents a hotspot for the discovery of potent and, in some cases, highly selective inhibitors of pro-survival BCL-2 family proteins.
Targeting the pro-apoptotic BCL-2 family proteins BAX and BAK
The development of agents targeting BAX and BAK has lagged significantly behind the development of agents targeting their pro-survival relatives. This is, in part, due to a scarcity of structural information on the nature of the interactions between BAX and BAK and other family members, or on the larger BAX and BAK homoligomers, the formation of which initiates MOMP. However, recent advances in this area are providing new insights and opportunities for targeting these proteins. In particular, the structural details for interactions between BAX and BAK and activating BH3-only proteins (Czabotar et al., 2013; Moldoveanu et al., 2013) and of an interface within the larger BAX oligomer (Czabotar et al., 2013) highlight the canonical BH3 binding groove of these proteins as a potential target for therapeutic agents.
The BAX and BAK canonical grooves share many structural similarities with their pro-survival relatives, and thus, it seems likely that compounds targeting this interface on the pro-apoptotic members can also be developed. Nonetheless, there is a range of subtle differences in the nature of these interactions, indicating that it may be possible to develop compounds specifically targeting the BAX or BAK groove. For example, interactions between BH3 domains and BAX rely on contacts at the N-terminal region of the bound BH3 domain (Czabotar et al., 2013); such residues do not appear to be critical for interactions with pro-survival proteins.
While the nature of the interactions between BH3 domains and the canonical grooves of the two BCL-2 family subgroups is similar, in a structural sense, the consequence of these interactions varies wildly. In the case of the pro-survival proteins, a stable complex is formed (Figure 2). In the case of BAX, and probably also for BAK, interactions at this interface instead are transient and initiate widespread conformational changes that lead to homo-oligomerization (Czabotar et al., 2013). Consequently, there exists the possibility of developing therapeutic agents targeting the canonical groove of BAX, and probably for BAK, with opposing cell death activities. For example, agents that mimic the BH3-peptide-mediated initiating event could promote apoptosis through induction of BAX and/or BAK conformational change. Such agents may be useful in cancer settings in a similar manner to the pro-apoptotic drugs being developed against pro-survival proteins. Alternatively, agents that bind the groove without initiating conformational change, but which would inhibit BH3-domain interactions, could instead inhibit apoptosis. The canonical groove is also involved in BAX homo-oligomerization (Dewson et al., 2008; 2012; Czabotar et al., 2013), and thus, agents with inhibiting interactions at this interface could additionally block the critical step of BAX and BAK oligomerization. Such anti-apoptotic agents might hold promise for the treatment of conditions where excessive apoptosis leads to pathology such as in neurodegenerative disorders or cardiovascular disease, as described earlier.
A second potential therapeutic target for the development of BAX modulators is a proposed ‘rear site’ on this pro-apoptotic protein (Gavathiotis et al., 2008). The relationship between this novel interaction site and the canonical groove remains unclear. As discussed above, it is possible that this rear site represents a triggering interface for initiating BAX translocation from the cytosol to the mitochondria. Alternatively, this might be an alternative site for triggering BAX conformational change. In any case, the rear site has been the subject of computational screening studies for small molecule ligands and compounds with pro-apoptotic activity have been reported (Gavathiotis et al., 2012). However, mutation to a reportedly key residue on this interaction surface suggests that engagement of the site is not essential for apoptosis to proceed (Okamoto et al., 2013; Peng et al., 2013). This holds important implications for the development of therapeutic agents aimed at inhibiting BAX activity as it suggests that such agents would be ineffective in inhibiting apoptosis. Nonetheless, it may be possible to target this pocket with agents aimed at accelerating apoptosis and thus be of interest for the development of anti-cancer agents.
Conclusion
Through their association with BCL-2 family proteins, the mitochondria play key roles in apoptosis induction. The function of apoptosis in normal cell physiology as well as in many diseases makes it a compelling pathway to target pharmacologically. The development of small molecules targeting the BCL-2 family of proteins has, however, proved extremely challenging and only a handful have reached the clinic. Among the preclinical and clinical compounds reported, few have been carefully characterized. The use of appropriate biochemical and biological tools (e.g. cell lines engineered to depend on particular BCL2 proteins for survival) reported in the literature is limited. The increasing number of genuine BCL-2 inhibitors, coupled with the development of robust assays, should positively influence future developments in this field.
We will conclude by highlighting some of the future important directions in chemical and drug discovery, targeting the BCL-2 family of proteins. The development of BH3 mimetics to treat tumours and possibly autoimmune diseases has now reached a mature stage. Nonetheless, the development of compounds selectively targeting MCL-1 or A1, which would complement the current set of available molecules, has lagged. Such agents would not only help elucidate the role of these two key pro-survival proteins but would also provide tools to establish the therapeutic relevance of, and safety associated with, MCL-1 or A1 inhibition. The lack of characterized inhibitors of apoptosis is also hindering major progress towards definitively establishing the link between up-regulated apoptosis and diseases such as neurodegeneration. Recent progress linking structural and biological studies on the sequence of events leading to activation of the pro-apoptotic proteins BAX and BAK will almost certainly accelerate the discovery of compounds modulating their activity.
Acknowledgments
We thank our colleagues from the Structural Biology, Chemical Biology and Molecular Genetic of Cancer Divisions, in particular, Profs Adams, Colman, Huang and Strasser, Drs Fairlie, Lee and Segal for many insightful discussions. Work in the laboratories of G. L. and P. E. C. is supported by funding from the NHRMC (Project Grants No GNT1025138 and GNT1023055) and the ARC (Future Fellowship FT0992105). M. J. R. is supported by an Australian Postrgraduate Award (APA) from the Australian Government. We also thank Life Arts for providing support in preparing Figure 1.
Glossary
- A1 or BCL-2 A1
BCL-2 related protein A1
- Apaf-1
apoptotic protease-activating factor 1
- BAD
BCL-2-associated death promoter protein
- BAK
BCL-2 homologous antagonist/killer protein
- BAX
BCL-2-associated X protein
- BCL-2
B-cell lymphoma 2 protein
- BCL-XL
B-cell lymphoma-extra large protein
- BH
BCL-2 homology domain
- BID
BH3 interacting-domain death agonist
- BIM
BCL-2 interacting mediator of cell death
- CLL
chronic lymphocytic leukaemia
- MCL-1
myeloid cell leukaemia sequence 1 protein
- MEF
mouse embryonic fibroblast
- MOMP
mitochondrial outer membrane permeabilization
- PUMA
p53 up-regulated modulator of apoptosis protein
- SAHB
stabilized α-helices of BCL-2 domains
- SCLC
small cell lung carcinoma
Conflict of interest
G.L. and P.E.C. are employees of WEHI, which received commercial income and research funding from Genentech, Inc., for part of their work.
References
- Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–1337. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar A, Zhou H, Chen J, Liu L, Bai L, McEachern D, et al. A potent and highly efficacious Bcl-2/Bcl-xL inhibitor. J Med Chem. 2013;56:3048–3067. doi: 10.1021/jm4001105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amundson SA, Myers TG, Scudiero D, Kitada S, Reed JC, Fornace AJ., Jr An informatics approach identifying markers of chemosensitivity in human cancer cell lines. Cancer Res. 2000;60:6101–6110. [PubMed] [Google Scholar]
- Basit F, Cristofanon S, Fulda S. Obatoclax (GX15-070) triggers necroptosis by promoting the assembly of the necrosome on autophagosomal membranes. Cell Death Differ. 2013;20:1161–1173. doi: 10.1038/cdd.2013.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, Donovan J, et al. The landscape of somatic copy-number alteration across human cancers. Nature. 2010;463:899–905. doi: 10.1038/nature08822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird GH, Bernal F, Pitter K, Walensky LD. Synthesis and biophysical characterization of stabilized alpha-helices of BCL-2 domains. Methods Enzymol. 2008;446:369–386. doi: 10.1016/S0076-6879(08)01622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boersma MD, Haase HS, Peterson-Kaufman KJ, Lee EF, Clarke OB, Colman PM, et al. Evaluation of diverse alpha/beta-backbone patterns for functional alpha-helix mimicry: analogues of the Bim BH3 domain. J Am Chem Soc. 2012;134:315–323. doi: 10.1021/ja207148m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bombrun A, Gerber P, Casi G, Terradillos O, Antonsson B, Halazy S. 3,6-dibromocarbazole piperazine derivatives of 2-propanol as first inhibitors of cytochrome c release via Bax channel modulation. J Med Chem. 2003;46:4365–4368. doi: 10.1021/jm034107j. [DOI] [PubMed] [Google Scholar]
- Bouillet P, Purton JF, Godfrey DI, Zhang LC, Coultas L, Puthalakath H, et al. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 2002;415:922–926. doi: 10.1038/415922a. [DOI] [PubMed] [Google Scholar]
- Bratton SB, Salvesen GS. Regulation of the Apaf-1-caspase-9 apoptosome. J Cell Sci. 2010;123:3209–3214. doi: 10.1242/jcs.073643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breckenridge DG, Germain M, Mathai JP, Nguyen M, Shore GC. Regulation of apoptosis by endoplasmic reticulum pathways. Oncogene. 2003;22:8608–8618. doi: 10.1038/sj.onc.1207108. [DOI] [PubMed] [Google Scholar]
- Brown CJ, Quah ST, Jong J, Goh AM, Chiam PC, Khoo KH, et al. Stapled peptides with improved potency and specificity that activate p53. ACS Chem Biol. 2012;8:506–512. doi: 10.1021/cb3005148. [DOI] [PubMed] [Google Scholar]
- Bruncko M, Song X, Ding H, Tao Z-F, Kunzer AR. 2008. Nonsubstituted indoles as Mcl-1 inhibitors and their preparation WO-0130970-A1.
- Campas C, Cosialls AM, Barragan M, Iglesias-Serret D, Santidrian AF, Coll-Mulet L, et al. Bcl-2 inhibitors induce apoptosis in chronic lymphocytic leukemia cells. Exp Hematol. 2006;34:1663–1669. doi: 10.1016/j.exphem.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Cantel S, Isaad Ale C, Scrima M, Levy JJ, DiMarchi RD, Rovero P, et al. Synthesis and conformational analysis of a cyclic peptide obtained via i to i+4 intramolecular side-chain to side-chain azide-alkyne 1,3-dipolar cycloaddition. J Org Chem. 2008;73:5663–5674. doi: 10.1021/jo800142s. [DOI] [PubMed] [Google Scholar]
- Carrington EM, Vikstrom IB, Light A, Sutherland RM, Londrigan SL, Mason KD, et al. BH3 mimetics antagonizing restricted prosurvival Bcl-2 proteins represent another class of selective immune modulatory drugs. Proc Natl Acad Sci USA. 2010;107:10967–10971. doi: 10.1073/pnas.1005256107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartron PF, Gallenne T, Bougras G, Gautier F, Manero F, Vusio P, et al. The first alpha helix of Bax plays a necessary role in its ligand-induced activation by the BH3-only proteins Bid and PUMA. Mol Cell. 2004;16:807–818. doi: 10.1016/j.molcel.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA, et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9:351–365. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhou H, Aguilar A, Liu L, Bai L, McEachern D, et al. Structure-based discovery of BM-957 as a potent small-molecule inhibitor of Bcl-2 and Bcl-xL capable of achieving complete tumor regression. J Med Chem. 2012;55:8502–8514. doi: 10.1021/jm3010306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Chiappori AA, Schreeder MT, Moezi MM, Stephenson JJ, Blakely J, Salgia R, et al. A phase I trial of pan-Bcl-2 antagonist obatoclax administered as a 3-h or a 24-h infusion in combination with carboplatin and etoposide in patients with extensive-stage small cell lung cancer. Br J Cancer. 2012;106:839–845. doi: 10.1038/bjc.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou JJ, Li H, Salvesen GS, Yuan J, Wagner G. Solution structure of BID, an intracellular amplifier of apoptotic signaling. Cell. 1999;96:615–624. doi: 10.1016/s0092-8674(00)80572-3. [DOI] [PubMed] [Google Scholar]
- Cohen NA, Stewart ML, Gavathiotis E, Tepper JL, Bruekner SR, Koss B, et al. A competitive stapled peptide screen identifies a selective small molecule that overcomes MCL-1-dependent leukemia cell survival. Chem Biol. 2012;19:1175–1186. doi: 10.1016/j.chembiol.2012.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czabotar PE, Lessene G. Bcl-2 family proteins as therapeutic targets. Curr Pharm Des. 2010;16:3132–3148. doi: 10.2174/138161210793292429. [DOI] [PubMed] [Google Scholar]
- Czabotar PE, Lee EF, Thompson GV, Wardak AZ, Fairlie WD, Colman PM. Mutations to Bax beyond the BH3 domain disrupts interactions with pro-survival proteins and promotes apoptosis. J Biol Chem. 2011;286:7123–7131. doi: 10.1074/jbc.M110.161281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czabotar PE, Westphal D, Dewson G, Ma S, Hockings C, Fairlie WD, et al. Bax crystal structures reveal how BH3 domains activate Bax and nucleate its oligomerization to induce apoptosis. Cell. 2013;152:519–531. doi: 10.1016/j.cell.2012.12.031. [DOI] [PubMed] [Google Scholar]
- Dash R, Azab B, Quinn BA, Shen X, Wang X-Y, Das SK, et al. Apogossypol derivative BI-97C1 (sabutoclax) targeting Mcl-1 sensitizes prostate cancer cells to mda-7/IL-24-mediated toxicity. Proc Natl Acad Sci USA. 2011;108:8785–8790. doi: 10.1073/pnas.1100769108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day CL, Chen L, Richardson SJ, Harrison PJ, Huang DC, Hinds MG. Solution structure of prosurvival Mcl-1 and characterization of its binding by proapoptotic BH3-only ligands. J Biol Chem. 2005;280:4738–4744. doi: 10.1074/jbc.M411434200. [DOI] [PubMed] [Google Scholar]
- Del Gaizo Moore V, Brown JR, Certo M, Love TM, Novina CD, Letai A. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. J Clin Invest. 2007;117:112–121. doi: 10.1172/JCI28281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–399. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J, Carlson N, Takeyama K, Dal Cin P, Shipp M, Letai A. BH3 profiling identifies three distinct classes of apoptotic blocks to predict response to ABT-737 and conventional chemotherapeutic agents. Cancer Cell. 2007;12:171–185. doi: 10.1016/j.ccr.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Denisov AY, Madiraju MS, Chen G, Khadir A, Beauparlant P, Attardo G, et al. Solution structure of human BCL-w: modulation of ligand binding by the C-terminal helix. J Biol Chem. 2003;278:21124–21128. doi: 10.1074/jbc.M301798200. [DOI] [PubMed] [Google Scholar]
- Dewson G, Kratina T, Sim HW, Puthalakath H, Adams JM, Colman PM, et al. To trigger apoptosis, Bak exposes its BH3 domain and homodimerizes via BH3:groove interactions. Mol Cell. 2008;30:369–380. doi: 10.1016/j.molcel.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Dewson G, Ma S, Frederick P, Hockings C, Tan I, Kratina T, et al. Bax dimerizes via a symmetric BH3:groove interface during apoptosis. Cell Death Differ. 2012;19:661–670. doi: 10.1038/cdd.2011.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlich F, Banerjee S, Suzuki M, Cleland MM, Arnoult D, Wang C, et al. Bcl-x(L) retrotranslocates Bax from the mitochondria into the cytosol. Cell. 2011;145:104–116. doi: 10.1016/j.cell.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore SW, Souers AJ, Bruncko M, Song X, Wang X, Hasvold LA, et al. 2008. 7-substituted indoles as Mcl-1 protein inhibitors and their preparation WO-0131000-A2.
- Friberg A, Vigil D, Zhao B, Daniels RN, Burke JP, Garcia-Barrantes PM, et al. Discovery of potent myeloid cell leukemia 1 (Mcl-1) inhibitors using fragment-based methods and structure-based design. J Med Chem. 2013;56:15–30. doi: 10.1021/jm301448p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Morselli E, Kepp O, Kroemer G. Targeting post-mitochondrial effectors of apoptosis for neuroprotection. Biochim Biophys Acta. 2009;1787:402–413. doi: 10.1016/j.bbabio.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Gandhi L, Camidge DR, Ribeiro de Oliveira M, Bonomi P, Gandara D, Khaira D, et al. Phase I study of navitoclax (ABT-263), a novel Bcl-2 family inhibitor, in patients with small-cell lung cancer and other solid tumors. J Clin Oncol. 2011;29:909–916. doi: 10.1200/JCO.2010.31.6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavathiotis E, Suzuki M, Davis ML, Pitter K, Bird GH, Katz SG, et al. BAX activation is initiated at a novel interaction site. Nature. 2008;455:1076–1081. doi: 10.1038/nature07396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavathiotis E, Reyna DE, Davis ML, Bird GH, Walensky LD. BH3-triggered structural reorganization drives the activation of proapoptotic BAX. Mol Cell. 2010;40:481–492. doi: 10.1016/j.molcel.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavathiotis E, Reyna DE, Bellairs JA, Leshchiner ES, Walensky LD. Direct and selective small-molecule activation of proapoptotic BAX. Nat Chem Biol. 2012;8:639–645. doi: 10.1038/nchembio.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George NM, Evans JJ, Luo X. A three-helix homo-oligomerization domain containing BH3 and BH1 is responsible for the apoptotic activity of Bax. Genes Dev. 2007;21:1937–1948. doi: 10.1101/gad.1553607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Heist RS, Fain J, Chinnasami B, Khan W, Molina JR, Sequist LV, et al. Phase I/II study of AT-101 with topotecan in relapsed and refractory small cell lung cancer. J Thorac Oncol. 2010;5:1637–1643. doi: 10.1097/JTO.0b013e3181e8f4dc. [DOI] [PubMed] [Google Scholar]
- Herman MD, Nyman T, Welin M, Lehtio L, Flodin S, Tresaugues L, et al. Completing the family portrait of the anti-apoptotic Bcl-2 proteins: crystal structure of human Bfl-1 in complex with Bim. FEBS Lett. 2008;582:3590–3594. doi: 10.1016/j.febslet.2008.09.028. [DOI] [PubMed] [Google Scholar]
- Hetz C, Vitte PA, Bombrun A, Rostovtseva TK, Montessuit S, Hiver A, et al. Bax channel inhibitors prevent mitochondrion-mediated apoptosis and protect neurons in a model of global brain ischemia. J Biol Chem. 2005;280:42960–42970. doi: 10.1074/jbc.M505843200. [DOI] [PubMed] [Google Scholar]
- Hinds MG, Lackmann M, Skea GL, Harrison PJ, Huang DCS, Day CL. The structure of Bcl-w reveals a role for the C-terminal residues in modulating biological activity. EMBO J. 2003;22:1497–1507. doi: 10.1093/emboj/cdg144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds MG, Smits C, Fredericks-Short R, Risk JM, Bailey M, Huang DC, et al. Bim, Bad and Bmf: intrinsically unstructured BH3-only proteins that undergo a localized conformational change upon binding to prosurvival Bcl-2 targets. Cell Death Differ. 2007;14:128–136. doi: 10.1038/sj.cdd.4401934. [DOI] [PubMed] [Google Scholar]
- Hsu YT, Wolter KG, Youle RJ. Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during apoptosis. Proc Natl Acad Sci USA. 1997;94:3668–3672. doi: 10.1073/pnas.94.8.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson RS, Placzek W, Fernandez A, Ziaee S, Chu C-Y, Wei J, et al. Sabutoclax, a Mcl-1 antagonist, inhibits tumorigenesis in transgenic mouse and human xenograft models of prostate cancer. Neoplasia. 2012;14:656–665. doi: 10.1593/neo.12640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LM, Mortenson DE, Yun HG, Horne WS, Ketas TJ, Lu M, et al. Enhancement of alpha-helix mimicry by an alpha/beta-peptide foldamer via incorporation of a dense ionic side-chain array. J Am Chem Soc. 2012;134:7317–7320. doi: 10.1021/ja302428d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juin P, Geneste O, Gautier F, Depil S, Campone M. Decoding and unlocking the BCL-2 dependency of cancer cells. Nat Rev Cancer. 2013;13:455–465. doi: 10.1038/nrc3538. [DOI] [PubMed] [Google Scholar]
- Kawamoto SA, Coleska A, Ran X, Yi H, Yang CY, Wang S. Design of triazole-stapled BCL9 alpha-helical peptides to target the beta-catenin/B-cell CLL/lymphoma 9 (BCL9) protein-protein interaction. J Med Chem. 2012;55:1137–1146. doi: 10.1021/jm201125d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopleva M, Contractor R, Tsao T, Samudio I, Ruvalo PP, Kitada S, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10:375–388. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, et al. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell. 2005;17:525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Kvansakul M, Yang H, Fairlie WD, Czabotar PE, Fischer SF, Perugini MA, et al. Vaccinia virus anti-apoptotic F1L is a novel Bcl-2-like domain-swapped dimer that binds a highly selective subset of BH3-containing death ligands. Cell Death Differ. 2008;15:1564–1571. doi: 10.1038/cdd.2008.83. [DOI] [PubMed] [Google Scholar]
- Labelle JL, Katz SG, Bird GH, Gavathiotis E, Stewart ML, Lawrence C, et al. A stapled BIM peptide overcomes apoptotic resistance in hematologic cancers. J Clin Invest. 2012;122:2018–2031. doi: 10.1172/JCI46231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leber B, Lin J, Andrews DW. Still embedded together binding to membranes regulates Bcl-2 protein interactions. Oncogene. 2010;29:5221–5230. doi: 10.1038/onc.2010.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EF, Czabotar PE, Smith BJ, Deshayes K, Zobel K, Colman PM, et al. Crystal structure of ABT-737 complexed with Bcl-x(L): implications for selectivity of antagonists of the Bcl-2 family. Cell Death Differ. 2007;14:1711–1713. doi: 10.1038/sj.cdd.4402178. [DOI] [PubMed] [Google Scholar]
- Lee EF, Smith BJ, Horne WS, Mayer KN, Evangelista M, Colman PM, et al. Structural basis of Bcl-x(L) recognition by a BH3-mimetic α/β-peptide generated by sequence-based design. ChemBioChem. 2011;12:2025–2032. doi: 10.1002/cbic.201100314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshchiner ES, Braun CR, Bird GH, Walensky LD. Direct activation of full-length proapoptotic BAK. Proc Natl Acad Sci USA. 2013;110:E986–E995. doi: 10.1073/pnas.1214313110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessene G, Czabotar PE, Colman PM. BCL-2 family antagonists for cancer therapy. Nat Rev Drug Discov. 2008;7:989–1000. doi: 10.1038/nrd2658. [DOI] [PubMed] [Google Scholar]
- Lessene G, Czabotar PE, Sleebs BE, Zobel K, Lowes KN, Adams JM, et al. Structure-guided design of a selective BCL-XL inhibitor. Nat Chem Biol. 2013;9:390–397. doi: 10.1038/nchembio.1246. [DOI] [PubMed] [Google Scholar]
- Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–192. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- Liu G, Kelly WK, Wilding G, Leopold L, Brill K, Somer B. An open-label, multicenter, phase I/II study of single-agent AT-101 in men with castrate-resistant prostate cancer. Clin Cancer Res. 2009;15:3172–3176. doi: 10.1158/1078-0432.CCR-08-2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Dai S, Zhu Y, Marrack P, Kappler JW. The structure of a Bcl-xL/Bim fragment complex: implications for Bim function. Immunity. 2003;19:341–352. doi: 10.1016/s1074-7613(03)00234-6. [DOI] [PubMed] [Google Scholar]
- Llambi F, Moldoveanu T, Tait SW, Bouchier-Hayes L, Temirov J, McCormick LL, et al. A unified model of mammalian BCL-2 protein family interactions at the mitochondria. Mol Cell. 2011;44:517–531. doi: 10.1016/j.molcel.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell JF, Billen LP, Bindner S, Shamas-Din A, Fradin C, Leber B, et al. Membrane binding by tBid initiates an ordered series of events culminating in membrane permeabilization by Bax. Cell. 2008;135:1074–1084. doi: 10.1016/j.cell.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Lukiw WJ, Bazan NG. Inflammatory, apoptotic, and survival gene signaling in Alzheimer's disease. Mol Neurobiol. 2010;42:10–16. doi: 10.1007/s12035-010-8126-4. [DOI] [PubMed] [Google Scholar]
- Ma S, Hockings C, Anwari K, Kratina T, Fennell S, Lazarou M, et al. Assembly of the Bak apoptotic pore: a critical role for the Bak alpha6 helix in the multimerization of homodimers during apoptosis. J Biol Chem. 2013;288:26027–26038. doi: 10.1074/jbc.M113.490094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell JM, Fushman D, Milliman CL, Korsmeyer SJ, Cowburn D. Solution structure of the proapoptotic molecule BID: a structural basis for apoptotic agonists and antagonists. Cell. 1999;96:625–634. doi: 10.1016/s0092-8674(00)80573-5. [DOI] [PubMed] [Google Scholar]
- Martinou JC, Dubois-Dauphin M, Staple JK, Rodriguez I, Frankowski H, Missotten M, et al. Overexpression of BCL-2 in transgenic mice protects neurons from naturally occurring cell death and experimental ischemia. Neuron. 1994;13:1017–1030. doi: 10.1016/0896-6273(94)90266-6. [DOI] [PubMed] [Google Scholar]
- Mason KD, Carpinelli MR, Fletcher JI, Collinge JE, Hilton AA, Ellis S, et al. Programmed anuclear cell death delimits platelet life span. Cell. 2007;128:1173–1186. doi: 10.1016/j.cell.2007.01.037. [DOI] [PubMed] [Google Scholar]
- Mason KD, Khaw SL, Rayeroux KC, Chew E, Lee EF, Fairlie WD, et al. The BH3 mimetic compound, ABT-737, synergizes with a range of cytotoxic chemotherapy agents in chronic lymphocytic leukemia. Leukemia. 2009;23:2034–2041. doi: 10.1038/leu.2009.151. [DOI] [PubMed] [Google Scholar]
- Mason KD, Lin A, Robb L, Josefsson EC, Henley KJ, Gray DH, et al. Proapoptotic Bak and Bax guard against fatal systemic and organ-specific autoimmune disease. Proc Natl Acad Sci USA. 2013;110:2599–2604. doi: 10.1073/pnas.1215097110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino D, Khaw SL, Glaser SP, Anderson DJ, Belmont LD, Wong C, et al. Bcl-2, Bcl-xL, and Bcl-w are not equivalent targets of ABT-737 and navitoclax (ABT-263) in lymphoid and leukemic cells. Blood. 2012;119:5807–5816. doi: 10.1182/blood-2011-12-400929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita T, Reed JC. Bcl-2 oncoprotein blocks chemotherapy-induced apoptosis in a human leukemia cell line. Blood. 1993;81:151–157. [PubMed] [Google Scholar]
- Moldoveanu T, Liu Q, Tocilj A, Watson M, Shore G, Gehring K. The X-ray structure of a BAK homodimer reveals an inhibitory zinc binding site. Mol Cell. 2006;24:677–688. doi: 10.1016/j.molcel.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Moldoveanu T, Grace CR, Llambi F, Nourse A, Fitzgerald P, Gehring K, et al. BID-induced structural changes in BAK promote apoptosis. Nat Struct Mol Biol. 2013;20:589–597. doi: 10.1038/nsmb.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchmore SW, Sattler M, Liang H, Meadows RP, Harlan JE, Yoon HS, et al. X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death. Nature. 1996;381:335–341. doi: 10.1038/381335a0. [DOI] [PubMed] [Google Scholar]
- Oh KJ, Singh P, Lee K, Foss K, Lee S, Park M, et al. Conformational changes in BAK, a pore-forming proapoptotic Bcl-2 family member, upon membrane insertion and direct evidence for the existence of BH3-BH3 contact interface in BAK homo-oligomers. J Biol Chem. 2010;285:28924–28937. doi: 10.1074/jbc.M110.135293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto T, Zobel K, Fedorova A, Quan C, Yang H, Fairbrother WJ, et al. Stabilizing the pro-apoptotic BimBH3 helix (BimSAHB) does not necessarily enhance affinity or biological activity. ACS Chem Biol. 2013;8:297–302. doi: 10.1021/cb3005403. [DOI] [PubMed] [Google Scholar]
- Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- Peixoto PM, Ryu SY, Bombrun A, Antonsson B, Kinnally KW. MAC inhibitors suppress mitochondrial apoptosis. Biochem J. 2009;423:381–387. doi: 10.1042/BJ20090664. [DOI] [PubMed] [Google Scholar]
- Peng Z, Xue B, Kurgan L, Uversky VN. Resilience of death: intrinsic disorder in proteins involved in the programmed cell death. Cell Death Differ. 2013;20:1257–1267. doi: 10.1038/cdd.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez HL, Banfi P, Bertrand J, Cai Z-W, Grebinski JW, Kim K, et al. Identification of a phenylacylsulfonamide series of dual Bcl-2/Bcl-xL antagonists. Bioorg Med Chem Lett. 2012;22:3946–3950. doi: 10.1016/j.bmcl.2012.04.103. [DOI] [PubMed] [Google Scholar]
- Petros AM, Medek A, Nettesheim DG, Kim DH, Yoon HS, Swift K, et al. Solution structure of the antiapoptotic protein bcl-2. Proc Natl Acad Sci USA. 2001;98:3012–3037. doi: 10.1073/pnas.041619798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petros AM, Olejniczak ET, Fesik SW. Structural biology of the Bcl-2 family of proteins. Biochim Biophys Acta. 2004;1644:83–94. doi: 10.1016/j.bbamcr.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Petros AM, Dinges J, Augeri DJ, Baumeister SA, Betebenner DA, Bures MG, et al. Discovery of a potent inhibitor of the antiapoptotic protein Bcl-x(L) from NMR and parallel synthesis. J Med Chem. 2006;49:656–663. doi: 10.1021/jm0507532. [DOI] [PubMed] [Google Scholar]
- Polster BM, Basanez G, Young M, Suzuki M, Fiskum G. Inhibition of Bax-induced cytochrome c release from neural cell and brain mitochondria by dibucaine and propranolol. J Neurosci. 2003;23:2735–2743. doi: 10.1523/JNEUROSCI.23-07-02735.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter J, Payne A, De Candole B, Ford D, Hutchinson B, Trevitt G, et al. Tetrahydroisoquinoline amide substituted phenyl pyrazoles as selective Bcl-2 inhibitors. Bioorg Med Chem Lett. 2009a;19:230–233. doi: 10.1016/j.bmcl.2008.10.113. [DOI] [PubMed] [Google Scholar]
- Porter J, Payne A, Whitcombe I, De Candole B, Ford D, Garlish R, et al. Atropisomeric small molecule Bcl-2 ligands: determination of bioactive conformation. Bioorg Med Chem Lett. 2009b;19:1767–1772. doi: 10.1016/j.bmcl.2009.01.071. [DOI] [PubMed] [Google Scholar]
- Ready N, Karaseva NA, Orlov SV, Luft AV, Popovych O, Holmlund JT, et al. Double-blind, placebo-controlled, randomized phase 2 study of the proapoptotic agent AT-101 plus docetaxel, in second-line non-small cell lung cancer. J Thorac Oncol. 2011;6:781–785. doi: 10.1097/JTO.0b013e31820a0ea6. [DOI] [PubMed] [Google Scholar]
- Reed JC. Apoptosis-based therapies. Nat Rev Drug Discov. 2002;1:111–121. doi: 10.1038/nrd726. [DOI] [PubMed] [Google Scholar]
- Roberts AW, Seymour JF, Brown JR, Wierda WG, Kipps TJ, Khaw SL, et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J Clin Oncol. 2012;30:488–496. doi: 10.1200/JCO.2011.34.7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues CM, Sola S, Sharpe JC, Moura JJ, Steer CJ. Tauroursodeoxycholic acid prevents Bax-induced membrane perturbation and cytochrome C release in isolated mitochondria. Biochemistry. 2003;42:3070–3080. doi: 10.1021/bi026979d. [DOI] [PubMed] [Google Scholar]
- Rooswinkel RW, van de Kooij B, Verheij M, Borst J. Bcl-2 is a better ABT-737 target than Bcl-xL or Bcl-w and only Noxa overcomes resistance mediated by Mcl-1: Bfl-1, or Bcl-B. Cell Death Dis. 2012;3:e366. doi: 10.1038/cddis.2012.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudin CM, Hann CL, Garon EB, Ribeiro de Oliveira M, Bonomi PD, Camidge DR, et al. Phase II study of single-agent navitoclax (ABT-263) and biomarker correlates in patients with relapsed small cell lung cancer. Clin Cancer Res. 2012;18:3163–3169. doi: 10.1158/1078-0432.CCR-11-3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Korsmeyer SJ, Schlesinger PH. BAX-dependent transport of cytochrome c reconstituted in pure liposomes. Nat Cell Biol. 2000;2:553–555. doi: 10.1038/35019596. [DOI] [PubMed] [Google Scholar]
- Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, Eberstadt M, et al. Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science. 1997;275:983–986. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- Schellenberg B, Wang P, Keeble JA, Rodriguez-Enriquez R, Walker S, Owens TW, et al. Bax exists in a dynamic equilibrium between the cytosol and mitochondria to control apoptotic priming. Mol Cell. 2013;49:959–971. doi: 10.1016/j.molcel.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmer AD, O'Brien S, Kantarjian H, Brandwein J, Cheson BD, Minden MD, et al. A phase I study of the pan bcl-2 family inhibitor obatoclax mesylate in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14:8295–8301. doi: 10.1158/1078-0432.CCR-08-0999. [DOI] [PubMed] [Google Scholar]
- Schroeder GM, Wei D, Banfi P, Cai Z-W, Lippy J, Menichincheri M, et al. Pyrazole and pyrimidine phenylacylsulfonamides as dual Bcl-2/Bcl-xL antagonists. Bioorg Med Chem Lett. 2012;22:3951–3956. doi: 10.1016/j.bmcl.2012.04.106. [DOI] [PubMed] [Google Scholar]
- Sentman CL, Shutter JR, Hockenbery D, Kanagawa O, Korsmeyer SJ. bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell. 1991;67:879–888. doi: 10.1016/0092-8674(91)90361-2. [DOI] [PubMed] [Google Scholar]
- Simonen M, Keller H, Heim J. The BH3 domain of Bax is sufficient for interaction of Bax with itself and with other family members and it is required for induction of apoptosis. Eur J Biochem. 1997;249:85–91. doi: 10.1111/j.1432-1033.1997.t01-1-00085.x. [DOI] [PubMed] [Google Scholar]
- Skelton NJ, Chen YM, Dubree N, Quan C, Jackson DY, Cochran A, et al. Structure-function analysis of a phage display-derived peptide that binds to insulin-like growth factor binding protein 1. Biochemistry. 2001;40:8487–8498. doi: 10.1021/bi0103866. [DOI] [PubMed] [Google Scholar]
- Sleebs BE, Czabotar PE, Fairbrother WJ, Fairlie WD, Flygare JA, Huang DC, et al. Quinazoline sulfonamides as dual binders of the proteins B-cell lymphoma 2 and B-cell lymphoma extra long with potent proapoptotic cell-based activity. J Med Chem. 2011;54:1914–1926. doi: 10.1021/jm101596e. [DOI] [PubMed] [Google Scholar]
- Sleebs BE, Kersten WJ, Kulasegaram S, Nikolakopoulos G, Hatzis E, Moss RM, et al. Discovery of potent and selective benzothiazole hydrazone inhibitors of Bcl-XL. J Med Chem. 2013;56:5514–5540. doi: 10.1021/jm400556w. [DOI] [PubMed] [Google Scholar]
- Smith BJ, Lee EF, Checco JW, Evangelista M, Gellman SH, Fairlie WD. Structure-guided rational design of α/β-peptide foldamers with high affinity for BCL-2 family prosurvival proteins. ChemBioChem. 2013;14:1564–1572. doi: 10.1002/cbic.201300351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19:202–208. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- Stewart ML, Fire E, Keating AE, Walensky LD. The MCL-1 BH3 helix is an exclusive MCL-1 inhibitor and apoptosis sensitizer. Nat Chem Biol. 2010;6:595–601. doi: 10.1038/nchembio.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Youle RJ, Tjandra N. Structure of Bax: coregulation of dimer formation and intracellular localization. Cell. 2000;103:645–654. doi: 10.1016/s0092-8674(00)00167-7. [DOI] [PubMed] [Google Scholar]
- Toure BB, Miller-Moslin K, Yusuff N, Perez L, Dore M, Joud C, et al. The role of the acidity of N-heteroaryl sulfonamides as inhibitors of Bcl-2 family protein–protein interactions. ACS Med Chem Lett. 2013;4:186–190. doi: 10.1021/ml300321d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- Vaillant F, Mérino D, Lee L, Breslin K, Pal B, Ritchie ME, et al. Targeting BCL-2 with the BH3 mimetic ABT-199 in estrogen receptor-positive breast cancer. Cancer Cell. 2013;24:120–129. doi: 10.1016/j.ccr.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Vandenberg CJ, Cory S. ABT-199, a new Bcl-2-specific BH3 mimetic, has in vivo efficacy against aggressive Myc-driven mouse lymphomas without provoking thrombocytopenia. Blood. 2013;121:2285–2288. doi: 10.1182/blood-2013-01-475855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadarajan S, Vogler M, Butterworth M, Dinsdale D, Walensky LD, Cohen GM. Evaluation and critical assessment of putative MCL-1 inhibitors. Cell Death Differ. 2013;20:1475–1484. doi: 10.1038/cdd.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335:440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- Walensky LD, Kung AL, Escher I, Malia TJ, Barbuto S, Wright RD, et al. Activation of apoptosis in vivo by a hydrocarbon-stapled BH3 helix. Science. 2004;305:1466–1470. doi: 10.1126/science.1099191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walensky LD, Pitter K, Morash J, Oh KJ, Barbuto S, Fisher J, et al. A stapled BID BH3 helix directly binds and activates BAX. Mol Cell. 2006;24:199–210. doi: 10.1016/j.molcel.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Wang K, Gross A, Waksman G, Korsmeyer SJ. Mutagenesis of the BH3 domain of BAX identifies residues critical for dimerization and killing. Mol Cell Biol. 1998;18:6083–6089. doi: 10.1128/mcb.18.10.6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Kitada S, Rega M, Stebbins J, Zhai D, Cellitti J, et al. Apogossypol derivatives as pan-active inhibitors of antiapoptotic B-cell lymphoma/leukemia-2 (Bcl-2) family proteins. J Med Chem. 2009a;52:4511–4523. doi: 10.1021/jm900472s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Kitada S, Rega MF, Emdadi A, Yuan HB, Cellitti J, et al. Apogossypol derivatives as antagonists of antiapoptotic Bcl-2 family proteins. Mol Cancer Ther. 2009b;8:904–913. doi: 10.1158/1535-7163.MCT-08-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Stebbins JL, Kitada S, Dash R, Placzek W, Rega MF, et al. BI-97C1, an optically pure apogossypol derivative as pan-active inhibitor of antiapoptotic B-cell lymphoma/leukemia-2 (Bcl-2) family proteins. J Med Chem. 2010;53:8000–8011. doi: 10.1021/jm1001265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells JA, McClendon CL. Reaching for high-hanging fruit in drug discovery at protein-protein interfaces. Nature. 2007;450:1001–1009. doi: 10.1038/nature06526. [DOI] [PubMed] [Google Scholar]
- Westphal D, Dewson G, Czabotar PE, Kluck RM. Molecular biology of Bax and Bak activation and action. Biochim Biophys Acta. 2011;1813:521–531. doi: 10.1016/j.bbamcr.2010.12.019. [DOI] [PubMed] [Google Scholar]
- Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, et al. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19:1294–1305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE, et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315:856–859. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- Wilson WH, O'Connor OA, Czuczman MS, LaCasce AS, Gerecitano JF, Leonard JP, et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol. 2010;11:1149–1159. doi: 10.1016/S1470-2045(10)70261-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Liu D, Huang Z. Synthesis and helical structure of lactam bridged BH3 peptides derived from pro-apoptotic Bcl-2 family proteins. Bioorg Med Chem Lett. 2004;14:1403–1406. doi: 10.1016/j.bmcl.2003.09.101. [DOI] [PubMed] [Google Scholar]
- Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- Zha H, Aime-Sempe C, Sato T, Reed JC. Proapoptotic protein Bax heterodimerizes with Bcl-2 and homodimerizes with Bax via a novel domain (BH3) distinct from BH1 and BH2. J Biol Chem. 1996;271:7440–7444. doi: 10.1074/jbc.271.13.7440. [DOI] [PubMed] [Google Scholar]
- Zhai D, Jin C, Satterthwait AC, Reed JC. Comparison of chemical inhibitors of antiapoptotic Bcl-2-family proteins. Cell Death Differ. 2006;13:1419–1421. doi: 10.1038/sj.cdd.4401937. [DOI] [PubMed] [Google Scholar]
- Zhang H, Nimmer PM, Tahir SK, Chen J, Fryer RM, Hahn KR, et al. Bcl-2 family proteins are essential for platelet survival. Cell Death Differ. 2007;14:943–951. doi: 10.1038/sj.cdd.4402081. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zhu W, Lapolla SM, Miao Y, Shao Y, Falcone M, et al. Bax forms an oligomer via separate, yet interdependent, surfaces. J Biol Chem. 2010;285:17614–17627. doi: 10.1074/jbc.M110.113456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Aguilar A, Chen J, Bai L, Liu L, Meagher JL, et al. Structure-based design of potent Bcl-2/Bcl-xL inhibitors with strong in vivo antitumor activity. J Med Chem. 2012a;55:6149–6161. doi: 10.1021/jm300608w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Chen J, Meagher JL, Yang C-Y, Aguilar A, Liu L, et al. Design of Bcl-2 and Bcl-xL inhibitors with subnanomolar binding affinities based upon a new scaffold. J Med Chem. 2012b;55:4664–4682. doi: 10.1021/jm300178u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Chang DC. Dynamics and structure of the Bax-Bak complex responsible for releasing mitochondrial proteins during apoptosis. J Cell Sci. 2008;121:2186–2196. doi: 10.1242/jcs.024703. [DOI] [PubMed] [Google Scholar]


