Abstract
The diabetes pandemic incurs extraordinary public health and financial costs that are projected to expand for the foreseeable future. Consequently, the development of definitive therapies for diabetes is a priority. Currently, a wide spectrum of therapeutic strategies, from implantable insulin-delivery devices to transplantation-based cell replacement therapy, to β-cell regeneration, focus on replacing the lost insulin-production capacity of diabetics. Among these, β-cell regeneration remains promising but heretofore unproven. Indeed, recent experimental work has uncovered surprising biology that underscores the potential therapeutic benefit of β-cell regeneration. These studies have elucidated a variety of sources for the endogenous production of new β-cells from existing cells. First, β-cells, long thought to be post-mitotic, have demonstrate potential for regenerative capacity. Second, the presence of pancreatic facultative endocrine progenitor cells has been established. Third, the malleability of cellular identity has availed the possibility of generating β-cells from other differentiated cell types. Here, we will review the exciting developments surrounding endogenous sources of β-cell production and consider the potential of realizing a regenerative therapy for diabetes from adult tissues.
Introduction
The incidence of diabetes, a disease of disrupted glucose homeostasis, is increasing at an alarming rate. Auto-immune Type 1 diabetes (T1DM) has doubled over the past 20 years and continues to grow annually by 2-4% worldwide.1,2 Simultaneously, the obesity epidemic has led to widespread insulin resistance and Type 2 diabetes (T2DM). Indeed, the health consequences of diabetes cannot be overstated: by the year 2050, an astounding 25% of Americans will be diabetic, diabetes-related costs will exceed $336 billion annually, and for the first time, life expectancy in the United States may shorten because of increased cardiovascular disease complications.3-5 The rapid growth of this life-shortening, intensely disruptive, and potentially curable condition highlights the urgent need to develop definitive treatments.6
Although the pathogenic mechanisms of T1DM and T2DM are distinct, they share the common end-point of decreased β-cell mass, i.e. loss of insulin production capacity. Presently, treatment strategies for diabetes rely upon the chronic administration of exogenous insulin, pharmacologic stimulation of insulin production or insulin sensitivity, and rarely, the transplantation of pancreatic islets or whole pancreas.7,8 Regrettably, these strategies are short-lived and/or fail to sufficiently recapitulate the function of endogenous insulin production. Despite the therapeutic potential of a method to restore adequate insulin production by safely increasing an individual's β-cell mass, no such approach has been established. Consequently, a major goal of current research is to identify methods to either expand the existing β-cell mass or generate new β-cells (Figure 1A). On the one hand, because of the virtually unlimited growth potential of embryonic stem cells and induced-pluripotent stem cells, there has been considerable interest in defining a method for generating new β-cells from stem cells through a sequential process of directed differentiation. This technique relies upon the in vitro recapitulation of the normal developmental process, which has been extensively dissected (Figure 1B). Currently, our ability to produce functional β-cells efficiently and safely remains a challenge.9 On the other hand, strategies for generating new β-cells from adult tissues have received considerably less attention. While these approaches rely upon cells with limited replication capacity, they have the potential to be utilized in situ and, perhaps, carry a reduced risk for introducing neoplastic disease. Here we will consider the multitude of competing regenerative approaches for generating new β-cells from adult tissues.
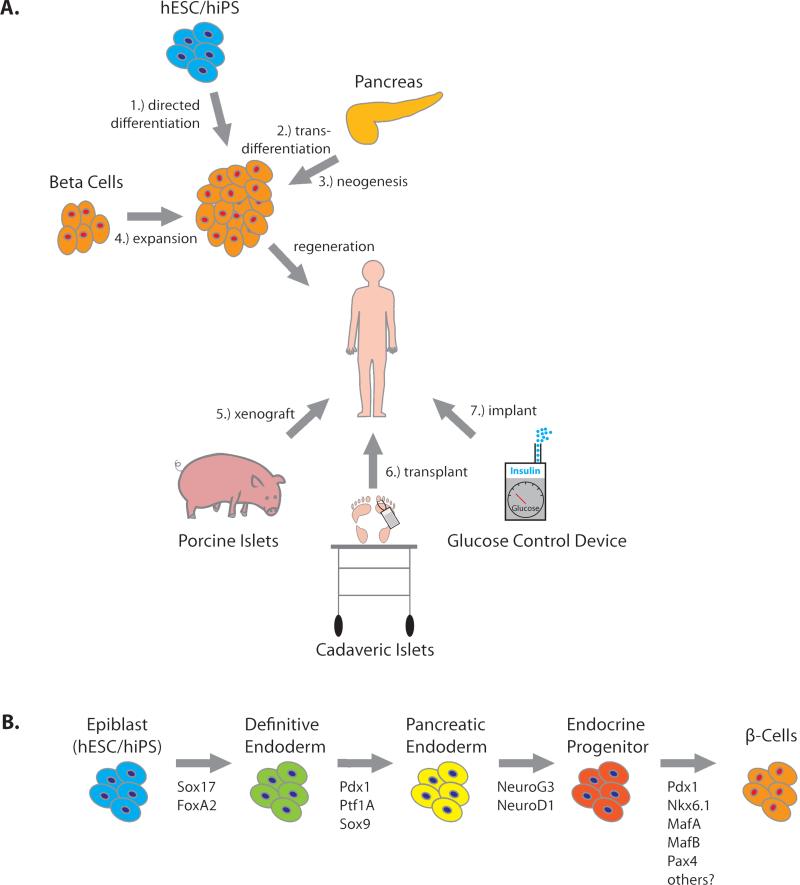
Figure 1. Theoretical Sources of Insulin.
(A) Seven strategies for restoring insulin production. Current research focuses on four methods to restore insulin production through expansion of β-cell mass: 1) directed differentiation of β-cells from human stem cells (hESC or hiPS), 2) transdifferentiation of existing pancreatic cell types to a β-cell fate, 3) generation of new β-cells from existing progenitor cells in the mature pancreas (neogenesis), 4) expansion of β-cell mass from existing β-cell pools (in vitro or in vivo). Three additional strategies may restore insulin production: 5) xenogeneic transplantation, e.g., porcine islets, or 6) allogeneic cadaveric transplantation, and 7) Glucose control devices may be implanted into humans to restore euglycemia. (B) The β-cell differentiation pathway. β-Cell differentiation is directed by the temporally regulated expression of master-regulator transcription factors, shown beneath the arrows. Recapitulation of the normal differentiation pathway is used to direct stem cells toward a β-cell fate. Currently, the full complement of factors required to generate mature, glucose-responsive beta cells from insulin-positive endocrine progenitor cells are unknown.
β-Cell Mass: Fixed or Flexible?
The capacity for humans and rodents to increase their β-cell mass has been recognized for several decades. The earliest observations of β-cell mass expansion were maladaptive in nature. In 1926, Warren observed hyperplastic adenomas of the islands of Langerhans in several post-mortem samples.10 Subsequently, the syndrome of hypoglycemia and hyperinsulinemia was recognized to result from the growth and metastasis of insulin-producing cells.11 More recently, investigators have noted that an adaptive increase in β-cell mass is associated with pregnancy and obesity. An early finding made by Green and Taylor showed that islet size is increased during pregnancy in rats, an observation that was confirmed in humans.12,13 Importantly, the increase in islet mass results from a combination of hypertrophy and hyperplasia.14 Obesity is also associated with an increase in β-cell mass in both rodents and humans.15-17 Studies in humans have documented a 30-60% increase in islet mass in non-diabetic obese individuals that is primarily attributed to hyperplasia rather than hypertrophy of islet cells; however, direct evidence of increased β-cell replication is not consistently observed.18-20 Consequently, the source of new β-cells under conditions of adaptive growth is an essential question in the field of regenerative medicine.
Genetic studies in rodents have highlighted the enormous growth and regenerative capacity of islets. A remarkable observation made by Bruning et al., was that the combined haploinsufficiency for insulin receptor (IR) and Insulin-Receptor Substrate-1 (IRS-1) caused an impressive 10-fold increase in β-cell mass and a 20-fold increase in insulin secretion.21 These mutations cause a modest defect in insulin signaling that mimic the insulin-resistant state of obesity. In a complementary approach, Kulkarni and coworkers generated mice that were insulin resistant as a result of entirely lacking insulin receptor expression in their hepatocytes (LIRKO, Liver Insulin Receptor Knockout).22 The consequences of this mutation on β-cells were equally profound: β-cell replication rates were increased by 8-fold and islet mass by 27-fold. These studies highlight the ability of rodent β-cell mass to adapt to peripheral insulin resistance. Taking another tact, Nir et al. asked whether the β-cells of young mice have the capacity to regenerate after near-total ablation.23 Using the inducible expression of diphtheria toxin specifically within mouse β-cells, these investigators caused an acute injury that caused a 75% loss of β-cells and then observed whether β-cell mass could recover. Indeed, β-cell volume and glucose homeostasis recovered to near-normal levels in a matter of weeks as a result of β-cell regeneration. Furthermore, the regenerative capacity of islets is also maintained by very old mice.24 Importantly, the post-ablation regenerative capacity of rodent islets has been confirmed in a variety of experimental models.25-27 The demonstration that β-cell mass can be regenerated has led to a profound interest in defining pharmacologic approaches to therapeutically control β-cell growth and mass.
Although the regenerative capacity of rodent islets is firmly established, the potential of human islets to recover after injury is less clear. Despite evidence of human β-cell mass expansion in the settings of obesity and pregnancy as well as the occurrence of insulinomas, the majority of β-cell generation, as determined by BrdU incorporation, Ki-67 staining and 14C-DNA content of β-cells, occurs within the first years of life and is nearly undetectable beyond the third decade.28-31 At first blush, the low rate of β-cell generation in adults (<0.1% per day) presents a potentially insurmountable barrier to therapeutically expanding β-cell mass. However, there are several reasons to believe that therapeutic β-cell regeneration might be achieved. First, occasional adult individuals demonstrate up to 7% Ki-67-positive β-cell staining, suggesting a wide spectrum of β-cell regenerative capacity. Secondly, the proliferative machinery of mature human β-cells remains intact as indicated by the proliferation observed in islets transplanted into rodents.32-34 Finally, modest genetic manipulation e.g., overexpression of cMyc, ChREBP, NKX6.1, Cdk6 or FoxM1 is sufficient to promote substantial levels of human β-cell replication in vitro.35-39 Notably, one must be cautious to use multiple markers for cellular replication such as Ki-67, BrdU, PCNA and phospho-histone-3 (PH3) to confirm β-cell division in order to counterbalance the limitations of any single replication marker.40 Overall, the therapeutic potential of harnessing the latent growth potential of mature human β-cells for the treatment of diabetes exists; however, it must be emphasized that at present a clinically viable strategy does not exist.
Whereas most researchers recognize a robust β-cell regenerative capacity in rodents and the potential for β-cell regeneration in humans, the source of new β-cells has remained controversial. Presently, potential sources for new β-cells include self-duplication or expansion (β-cells from β-cells, Figure 2A), neogenesis from exocrine progenitor cells (facultative tissue stem-cells located among the ductal, acinar or centroacinar cells, Figure 2B) and transdifferentiation from other mature cell-types (e.g. α-cells, Figure 2C). While only some of these sources may be natural reservoirs for new β-cells, any origin may be made therapeutically useful through targeted manipulation. Here we will consider the evidence that has been acquired to support the various natural and synthetic origins for new β-cells (Table 1).
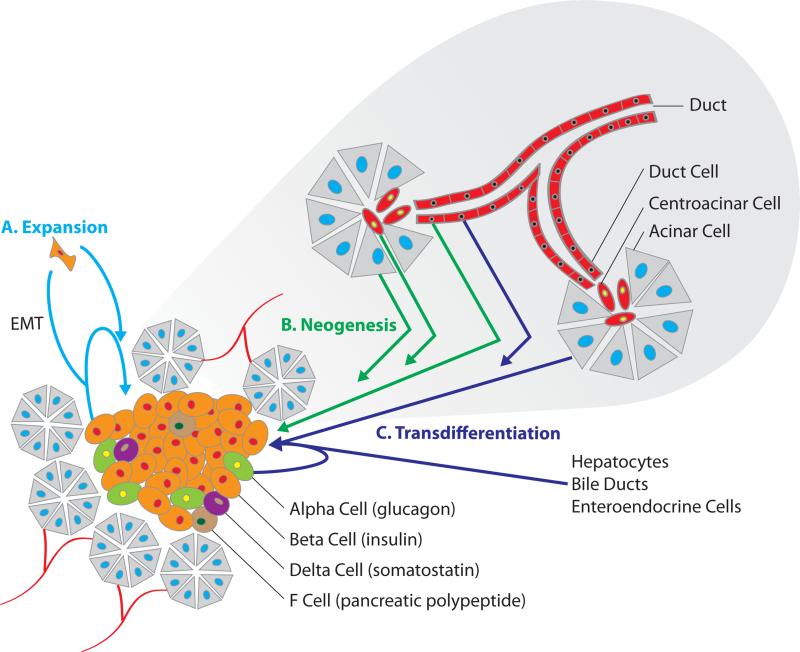
Figure 2. Potential Sources of New Beta Cells.
Three distinct pathways of achieving β-cell mass replenishment have been defined: β-cell expansion, β-cell neogenesis and transdifferentiation of non-β-cells to β-cells. These processes which may occur in-vivo or in-vitro are illustrated within the context of the endocrine and exocrine pancreas. (A) Expansion of pre-existing β-cells may occur directly through beta cell division, or through a mesenchymal cell intermediate produced via an epithelial to mesenchymal transition (EMT). (B) Facultative progenitor cells insulated among non-endocrine epithelial cell populations have the potential to repopulate the endocrine cell population through neogenesis. (C) Pancreatic cells (alpha, duct, and acinar cells), liver cells (hepatocytes and bile ducts), and gut cells (enteroendocrine cells) may be induced to become mature β-cells through a variety of genetic and pharmacologic manipulations.
Table 1.
Pathways Toward β-cell Regeneration
| Pathway | Physiologic Conditions | Environmental Triggers | *Biologic Factors | *Small Molecules/Transcription Factors |
|---|---|---|---|---|
| β-Cell Expansion | Pregnancy12-14 Obesity15-20 Insulin Resistance21-22 Development28-30 |
β-Cell Ablation23-27 Partial Pancreatectomy41 |
Glucose47-48 GLP-1 Betatrophin54 Serotonin62 Lactogens14,58-62 Osteocalcin58-59 |
**cMyc, ChREBP, NKX6.1, Cdk6, FoxM135-39 Glucokinase activators, Sulfonylureas50 L-type calcium channel agonists, phorbol esters, Wnt agonists51 WS664 Adenosine agonists66 Adenosine Kinase Inhibitors (ADKI's)65-66 |
| β-Cell Neogenesis | Development | Pancreatic Duct Ligation/Partial Pancreatectomy27,79-83,88-91 | - | Pax487,91 |
| β-Cell Trans-differentiation | - | β-Cell Ablation23,116 | - | Pdx1101 NeuroD +/− Betacellulin102 Pdx1+NeuroD/Ngn3103 Pdx1+MafA+Ngn3/NeuroD105 **Combinatorial Culture Conditions107 BRD7389110 FoxO1118 |
Biologic factors and small molecules listed are limited to those discussed in the review
In-vitro
β-Cells From β-Cells
In 2004, Dor and Melton published a landmark paper which established pre-existing β-cells rather than specialized progenitor cells as the primary and usual source of new β-cells in adult rodents.41 Using mice engineered to express a tamoxifen-dependent Cre recombinase only within insulin+ mature β-cells, these authors performed an in vivo pulse-chase experiment to follow the fate of existing β-cells and assess the source of new β-cells. Mice treated with tamoxifen indelibly activated the production of alkaline phosphatase within mature β-cells. Subsequently, when animals were allowed to age up to one year, the source of new β-cells was revealed to be previously existing β-cells (alkaline phosphatase+) rather than non-β-cells which would give rise to alkaline phosphatase− β-cells. Similar observations were made when partial pancreatectomy (PPY) was used to stimulate β-cell regeneration. Interestingly, the authors observed no evidence of islet neogenesis, as all insulin+ cell clusters contained alkaline phosphatase+ β-cells and recently generated β-cells (determined by BrdU labeling) were also alkaline phosphatase+ (new β-cells came from dividing old β-cells). Caveats to this work are that (a) tamoxifen-induced recombination was limited to 30% of the β-cell population and (b) tamoxifen-independent recombinase activity is an established problem in the mice used for these experiments.42 Despite these limitations, the primary role of β-cell duplication in β-cell regeneration was established. And perhaps more importantly, β-cells were confirmed not to be post-mitotic and therefore a potential resource for therapeutically expanding an individual's β-cell mass.
The conclusions that (1) new β-cells come from old β-cells and that (2) specialized progenitor cells or a “proliferative compartment” do not substantially contribute to β-cell regeneration in mice have been established by a variety of other rodent studies. Notable are those performed by Teta et al. and Nir et al., which used innovative DNA double-labeling experiments and a genetic β-cell ablation-regeneration systems, respectively, to trace the origins of new β-cells and demonstrate the equally distributed replicative capacity of mature β-cells.43,23 Unlike the case of rodents, the question of whether human β-cells regenerate—and if so, whether they arise from pre-existing β-cells—remains unresolved. However, the cotemporaneous increase in β-cell mass and β-cell replication that occur in early life without islet neogenesis (increasing islet size but stable islet number), indicates that the predominant source of new β-cells in humans are previously existing β-cells, i.e. self-duplication.19
The identification of self-duplication as the primary mechanism for β-cell growth and regeneration has heightened the interest in finding methods to therapeutically stimulate β-cell growth. This interest is furthered by the results of human genome wide association studies (GWAS) that implicate growth-associated loci as contributors to the hereditary risk of T2DM, e.g. CDKN2A/B and CCND2.44 Consequently, a variety of strategies for promoting β-cell duplication are being pursued. These include the identification of (1) growth-factors that are produced in the setting of insulin resistance and/or pregnancy, (2) factors that stimulate neonatal β-cell growth, (3) small molecules that drive mature β-cell replication, and (4) the establishment of a glucose responsive β-cell line for transplantation. Importantly, any therapeutically viable growth-promoting strategy must have a reversible effect on β-cell growth and be highly selective for β-cells to avoid neoplastic transformation.
Circulating factors that stimulate β-cell regeneration
A strong stimulant for β-cell replication is the insulin resistant state. Insulin resistance is defined by a loss of insulin potency that results in an increased requirement for insulin to achieve euglycemia.45 The connection between insulin resistance and β-cell replication is well illustrated by the LIRKO mouse model which fails to suppress hepatic gluconeogenesis in response to insulin and thereby models the insulin resistant state. The inability to suppress hepatic glucose production causes a massive hyperinsulinemia (>20-fold at 2 months) and a robust compensatory β-cell expansion (>6-fold at 2 months).22,46 Presently, the underlying mechanism of insulin-resistance-dependent β-cell replication remains only partially elucidated.
From a teleological perspective, glucose is a logical controller of β-cell mass: insulin production capacity should match insulin needs. Indeed, glucose has been recognized as a stimulant for β-cell replication in vitro and in vivo for many decades.47,48 However, the observation that β-cell replication is increased in islets transplanted into euglycemic insulin resistant animals has led some to suggest that a circulating factor other than glucose is responsible for driving β-cell replication.49 This notion was challenged by Porat et al., who demonstrated that β-cell replication is triggered by “β-cell work,” i.e. the metabolic activity of the β-cell and not by hyperglycemia per se.50 Interestingly, the β-cell's ability to monitor its workload is connected to its ability to generate the calcium channel-dependent action potentials that trigger insulin secretion: calcium channel antagonists such as nifedipine inhibit β-cell replication and insulin release. Indeed, compounds which increase β-cell work by (a) promoting glucose metabolism (glucokinase activators) or (b) triggering β-cell depolarization (sulfonylureas and calcium channel activators) are sufficient to increase β-cell replication.50,51 The integral connection between β-cell work (metabolic flux), insulin secretion and β-cell replication may explain why molecules that amplify insulin secretion, e.g. glucagon like-1 peptide (GLP-1), do not prevent β-cell failure: medications that augment insulin secretion also increase the β-cell workload which, already hyper-active in the setting of hyperglycemia, might accelerate β-cell failure rather than prevent it. Consequently, we propose that therapeutically viable stimulants for β-cell growth must function without increasing the β-cell workload. Such a stimulant might work by controlling the β-cells’ perception of its workload without actually increasing the metabolic demands placed upon the cell or via workload independent pathways. Consideration of the first strategy leads to an important and unresolved question: how does the β-cell measure its workload over time? In other words, how does a β-cell know how hard it has worked and when it should divide? Although the answer to this question is not known, intracellular calcium-dependent signaling and autocrine insulin signaling are two candidates for further investigation.52,22
Despite mounting evidence that β-cell work controls β-cell replication in the insulin resistant state, investigators have continued to look for additional circulating factors that promote adaptive β-cell replication. Recently, a β-cell growth factor was found in the serum of LIRKO mice which can promote rodent and human β-cell replication independent of glucose.53 Subsequently, the Melton laboratory identified a β-cell selective secreted growth factor, ANGPTL8 / Betatrophin, that is up-regulated in mouse models of insulin resistance (infusion of an insulin receptor antagonist) and during pregnancy in mice.54 This work raises the extraordinary possibility of a β-cell selective growth factor of terrific therapeutic potential. Whether Betatrophin increases β-cell work, has off-target effects, and/or promotes human β-cell replication, are questions that remain to be answered. Additionally, mouse studies have shown that β-cell mass may be regulated by osteocalcin (OCN), a bone-derived hormone that stimulates β-cell replication.55,56 OCN is stored in an inactive form in the bone matrix until it is released and activated by bone resorption.57 Interestingly, hyperinsulinemia enhances bone resorption and, consequently, β-cell replication via bone-derived OCN. Therefore, a feed-forward bone to β-cell loop provides a potential mechanism for insulin demand and insulin production capacity (β-cell mass) to be aligned.
In addition to insulin resistance, pregnancy has been identified as a metabolic state that triggers β-cell mass expansion. Work by the Sorenson group identified lactogens (prolactin and placental lactogen) as key promoters of adaptive β-cell growth during pregnancy in rodents.14 Indeed, transgenic mice that express placental lactogen in their islet β-cells (Rip-mPL1) demonstrate an increased islet area and an augmented β-cell replication rate despite being hypoglycemic.58 Conversely, prolactin receptor deficiency leads to a non-progressive defect in β-cell mass in male and female mice, indicating an in utero role for lactogens in β-cell mass establishment in addition to its effect on maternal β-cell mass expansion.59 Recent rodent studies have suggested that lactogens promote β-cell replication via the action of FOXM1 by down-regulating Menin and, consequently, the cell-cycle inhibitors p18 and p27.60,61 Surprisingly, the enhanced β-cell replication that is observed during pregnancy peaks around gestational day 14 and then returns toward baseline despite persistently elevated lactogen levels. Recent work from the German laboratory may connect the transient proliferative effect of lactogens during pregnancy with serotonin signaling- a driver for β-cell replication.62 These investigators, through global islet gene expression analysis during pregnancy, noted a dramatic lactogen-dependent increase in the islet's ability to synthesize (Tph1, Tph2) and respond (Htr2b) to serotonin during the gestational period that is associated with enhanced β-cell replication. However, the expression of Htr1d, a negative regulator of serotonin signaling, is induced in late gestation. These results raise the possibility of preventing gestational diabetes by therapeutically manipulating β-cell mass during pregnancy with medications that enhance serotonin signaling; such medications are available and have been safely used by pregnant women.
Small Molecule Stimulation of β-cell Replication
An alternative to finding endogenous factors that promote β-cell replication is to identify small molecules that stimulate β-cell growth. This approach was first published using a reversibly transformed rodent β-cell line that retains some of the functional properties of β-cells.63,51 Initially, the molecules found to stimulate β-cell replication were nonselective growth promoters (wnt agonists and phorbol esters) and β-cell depolarizing agents (L-type calcium channel activators), which have limited therapeutic potential. However, follow-up work using the same screening platform identified a small molecule (WS6) that binds to Erb3 binding protein-1 (also called PA2G4, EBP1) and promotes rodent and human β-cell proliferation.64 Whether this molecule is suitable for in vivo use is unknown. To overcome the limitations of using an immortalized cell line, which exhibit unlimited growth potential and altered metabolic behavior, we performed small molecule screening with primary rodent islet cultures.65 These studies led to the surprising identification of adenosine kinase inhibitors (ADKis) as novel β-cell growth promoting factors. Importantly, ADKis stimulate the growth of β-cells, in vivo and in vitro, but not a spectrum of other cell types (α-cells, hepatocytes or myocytes). Using a zebrafish model of diabetes, Andersson et al. confirmed the ability of ADKis to promote β-cell regeneration.66 These findings suggest a conserved role for ADK in controlling β-cell growth and regeneration. Future studies will need to address whether ADKis promote human β-cell replication and have therapeutic potential. To date, primary human islet-cell-based screens have been hampered by the limited availability, variability, and high cost of human islets as well as their very low basal replication rate.67
A β-cell Line for Transplantation
Given the limitations of mature human β-cell replication, alternative strategies for generating large quantities of glucose-responsive insulin-producing cells are being pursued. Recently, Ravassard and co-workers established a functional human β-cell line from human fetal pancreatic cultures.68 Although these cells are transformed, expressing SV40LT and TERT, they secrete insulin in response to elevated glucose levels and can rescue diabetes in mice. These cells set the stage for the development of a renewable source of insulin-producing cells that might be used for transplantation. In the future, human β-cell lines might be reversibly transformed and encapsulated for transplantation to remove oncologic risks. Alternatively, fetal β-cells retain substantial growth potential and therefore may be expanded ex vivo before being used for transplantation.69 A major limitation to the use of an allogeneic β-cell transplantation strategy is the need for immunosuppression. Consequently, an effective strategy of expanding the endogenous β-cell mass may require a pharmacologic mechanism for alleviating β-cell selective growth-suppressive signals, e.g. the inhibition of Menin.70 Despite the absence of a method to treat diabetes by expanding an individual's β-cell mass, many promising strategies are advancing through pre-clinical stages. The potential to stimulate clinically relevant levels of adult human β-cell replication is bolstered by reports of nesidioblastosis developing after gastric-bypass surgery in some patients.71 However, a major challenge to the strategy of stimulating β-cell growth is whether an acceptable safety profile can be achieved- optimism may be rooted in the hope that a transient therapeutic period may be all that is required.
Islet Neogenesis
Potential endogenous sources of new β-cells are previously existing β-cells, a facultative progenitor cell population that can give rise to new islets (neogenesis), and other mature cells that can be coaxed into becoming β-cells (trans-differentiation). As the evidence for cell-type plasticity increases, the distinction between neogenesis and transdifferentiation is increasingly blurred. For the purposes of this discussion, β-cells generated through a process that recapitulates development is considered neogenesis (Figure 2B) and all other non-β-cell-derived pathways are considered to be transdifferentiation (Figure 2C). As discussed above, while the primary postnatal source of new β-cells are previously existing β-cells, there is mounting evidence that supports the existence of facultative progenitor cell populations within the pancreas. Given that adult β-cells may have a limited replicative capacity, identifying a method for expanding a β-cell progenitor population may be important. Currently, it is probable but not definitively shown that endocrine progenitor cells exist within the adult pancreas. Although there are several studies supporting the existence of such cells, there is also an opposing body of literature. How these apparently diametrically opposed experimental observations will be cohesively integrated is an exciting and ongoing mystery.72
An extensive body of literature has defined the developmental origins of β-cells and established the identity of multi-potent progenitors present during development.9 This work has revealed the importance of several master transcription factors that define pancreatic progenitor cells. The pancreas duodenal homeobox gene, PDX1, is the earliest such marker and is required to maintain all of the pancreatic cell populations (endocrine, exocrine and duct cells) in mice and humans.73,74 Additionally, the transcription factor neurogenin 3 (Neurog3) has been established as the master regulator of endocrine cell formation in mice.75,76 During development, Neurog3+ cells are found in duct-like structures that give rise to all endocrine cell lineages. However, Neurog3+ cells are transient progenitors as they beget a very restricted population of daughter endocrine cells (possibly at a 1:1 ratio), are nearly gone by E16.5, and are rarely found in the islets and exocrine/duct cells of adult animals.77 These results suggest that NEUROG3 is a marker of islet progenitor cells which, if retained in the adult animal, have only a limited capacity to support islet neogenesis. Consequently, a therapeutically useful adult endocrine progenitor cell must be capable of continuously producing new NEUROG3+ cells. Interestingly, Zhou et al. have identified a population of cells (tip cells, PDX1+PTF1a+Cpa1+) in mice that give rise to all pancreatic epithelial lineages including NEUROG3+-ductal cells during development.78 These cells are believed to terminally differentiate into exocrine cells by E14 but could represent the origin of a facultative progenitor cell population if they persist in the adult pancreas.
Pancreatic Facultative Endocrine Progenitor Cells: In Vivo
Following pancreatic injury, PPY and pancreatic duct ligation (PDL), numerous investigators have found evidence for islet neogenesis from precursor cells located within pancreatic ducts of rodents.79 Based upon the proximity of insulin+-cells to pancreatic ductules within regions of high proliferation (termed foci of regeneration), the source of the new islets has generally been identified as precursor cells located within the ductal epithelium. In the rat PPY model, foci of regeneration appear to give rise to new islets and new pancreatic lobes within one week.80 Importantly, studies of the human pancreas have yielded similar observations: increased ductal cell proliferation and duct-associated insulin+-cells are observed in association with obesity, type 1 diabetes, and type 2 diabetes in humans.81,82 These data, while inconclusive, raise the possibility of identifying a pancreatic progenitor cell that may be leveraged for therapeutic purposes.
Recently, increasingly rigorous experiments in rodents have yielded conflicting evidence for the presence of endocrine progenitor cells located within the pancreatic ducts. Using PDL to stimulate pancreatic regeneration and transgenic Neurog3 reporter mice to identify endocrine progenitor cells, Xu et al. found facultative progenitor cells located within the pancreatic ducts of mice.27 As seen previously, PDL caused acinar cell apoptosis, pancreatic duct proliferation, and a two-fold β-cell mass expansion that plateaued within 7 days. Interestingly, these authors also observed a 50-fold induction of the Neurog3 transcript and identified insulin− Neurog3+-cells located within the pancreatic ducts of injured pancreata that expressed a variety of endocrine progenitor cell markers (PDX1, Ptf1a, Sox9, HNF6).27,83 Furthermore, based upon the perdurance of the Neurog3-LacZ reporter, NeurogG3+ cells were found to become hormone+-cells after migrating away from the duct and into islet structures.27 This observation was confirmed in vitro where NEUROG3+ insulin−-cells formed functional islets when injected into an embryonic Neurog3−/− pancreatic bud which cannot autonomously generate mature endocrine cells. Given the transient nature of Neurog3+-cells and the continued expression of Neurog3 for >1 month, one might expect persistent endocrine cell generation and islet expansion; however, islet mass does not expand beyond 7 days. Experimental data addressing this apparent conundrum has not been obtained.
In contrast to Xu et al., several lineage tracing experiments performed post-pancreatic injury in mice failed to identify a source of new β-cells other than previously existing β-cells.41, 43 Using an identical PDL protocol as Xu et al., Rankin and coworkers confirmed a 70% loss of distal pancreatic mass, increased ductal proliferation and the induction of Neurog3 expression but failed to identify an increase in β-cell mass or replication.84 Similarly, Xiao et al. failed to identify new β-cells that arise from non-β-cells after PPY or PDL.85 These scientists used an inventive reporter system that relies upon insulin promoter-dependent Cre expression to switch cells from expressing membrane-targeted Tomato (red) to membrane-targeted EGFP (Green). Consequently, new β-cells (yellow) can be identified by the overlapping presence of red and green fluorescent proteins. Indeed, yellow cells were found during development but not beyond P5. To determine whether acinar cells might harbor facultative endocrine progenitor cells, Desai et al. subjected mice that harbor elastase-CreERT2 and Rosa26-lox-stop-lox-LacZ cassettes to a variety of pancreatic injury models (cerulien-induced pancreatic injury, PPY, and PDL).86 Indeed, these mice provided no evidence that new β-cells arise from acinar cells within the examined time frame of 1 week. Similar results were obtained when mice harboring a pancreatic duct-specific lineage marker (HNF1β dependent Cre-ER expression) were challenged with PDL or alloxan-dependent β-cell ablation.87 These lineage tracing experiments compellingly support the conclusions that pancreatic injury models, e.g. PDL, cause increased ductal cell proliferation and induction of Neurog3 within ductal cells but did not support the differentiation of Neurog3+ cells into β-cells.
However, the findings of Xu et al. are bolstered by several lineage tracing experiments in rodents that support the existence of facultative stem cells within the pancreatic ducts or acinar tissues. First, Inada et al. used human carbonic anhydrase II (CAII)-driven CRE-ER expression to show that ductal cells can give rise to mature endocrine cells in the PDL mouse model.88 Although the faithfulness of the CAII reporter system is imperfect, the conclusions are not easily dismissed. Second, Van de Casteele et al. have used a Neurog3-Cre-ER reporter system to demonstrate that some of the new β-cells that arise after PDL descend from non-β-cells that activate Neurog3 expression.89 It is noted by the authors that similarly to the CAII reporter system, the induction of Neurog3 promoter activity within mature β-cells that are stressed by the inflammation associated with PDL could not be excluded. Third, Pan et al. have used indelibly marked exocrine cells (PTF1a-Cre-ER) and the PDL model to demonstrate that insulin+-cells can descend from exocrine cells which dedifferentiate and transiently acquire a duct cell-like progenitor phenotype that is reminiscent of the developmental tip cells described by Zhou et al.78,90 Notably, these insulin+-cells are primarily located within ducts and arose >30d post-injury. While these results are very different from the dramatic post-PDL islet neogenesis that occurs within one week, it is reminiscent of the staining occasionally found on human pancreatic sections.82 Fourth, and perhaps most compelling, is the observation that the misexpression of PAX4 within adult α-cells leads to continuous transdifferentiation of α-cells to β-cells (discussed below) and the mobilization of a NEUROG3+ ductal cell population that attempts to replenish the unstable α-cell population.91 Amazingly, and contrary to prior published work with the same reporter mice, these authors utilize HNF1a-Cre-ER to indelibly label duct cells and demonstrate their ability to express NEUROG3, undergo EMT, and become endocrine cells (β-cells).87,91 In aggregate, these experiments strongly suggest that under some experimental circumstances, facultative progenitor cells capable of giving rise to insulin+-cells do exist in rodents.
Pancreatic Facultative Endocrine Progenitor Cells: In Vitro
Whether pancreatic facultative progenitor cells have therapeutic potential remains uncertain. However, a first step toward realizing their utility might be the establishment of an in vitro method for β-cell generation from a facultative progenitor cell population. Prior efforts have indicated that purified mouse ductal cells contain rare (≈1/8,500) pancreas-derived multipotent precursors (PMP) that express neural and pancreatic markers and generate cell types of both lineages including β-cells.92 Interestingly, these authors subsequently determined that PMP cells express low levels of insulin.93 While this finding might explain why Dor et al. found new β-cells (insulin+-cells) to arise exclusively from pre-existing β-cells (insulin+-cells), it contradicts other studies showing that all β-cells (insulin+-cells) are equally likely to undergo cellular replication and that no highly replicative insulin+ progenitor is present.43,94 Recently, Rovira et al. demonstrated that murine centroacinar/terminal duct cells, located at the junction between ducts and acinar cell clusters, represent a distinct endocrine progenitor population capable of in vitro clonal expansion, self-renewal, and mature endocrine cell generation.95 These cells express Aldh1, a progenitor cell marker, as well as additional progenitor cell markers not characteristic of PMPs (Sca-1, SDF1, c-Met, Nestin and sox9). Interestingly, adult human pancreatic epithelial cells (ductal and exocrine cells), depleted of mature endocrine cells, and placed into tissue culture, can subsequently give rise to mature endocrine cells when transplanted into mice in conjunction with human fetal endocrine progenitor cells or pancreatic stromal cells, or are maintained under three-dimensional culture conditions.96-98 These results indicate that mammalian facultative endocrine progenitor cells may exist within the pancreas but require specific environmental conditions to realize their potential of endocrine cell differentiation. Perhaps it is the stringency of the required conditions that has, to date, prevented the reproducible isolation and identification of pancreatic endocrine progenitor cells.
β-Cells From Mature Non-β-Cells
Cellular identity, once thought to be highly stable, is now viewed as highly mutable. This paradigm shift was partially driven by the work of Yamanaka and colleagues who demonstrated that terminally differentiated cells could be reprogrammed to pluripotent stem cells by the forced expression of transcription factors.99 Indeed, the related phenomenon of transdifferentiation (lineage conversion between mature cell types) has also received increasing attention as a method for generating mature cell types that are of short supply, e.g. β-cells.100 The principle behind directed transdifferentiation is that certain transcription factors act as “master regulators” and have a dominant effect on cellular identity.
This principle was first applied to β-cell generation by Ferber et al., who demonstrated that mouse liver cells could be converted into insulin-producing cells via the forced expression of PDX-1.101 Similarly, insulin-producing liver cells have been generated by adenoviral delivery of NeuroD +/− Betacellulin or a combination of PDX-1 (modified with VP16 to enhance transcriptional transactivation) and NeuroD or NEUROG3 into mice.102,103 Although these in vivo studies presumed insulin+-cells arose from hepatocytes, Sox9+ bile duct cells might have been the origin.104 More recently, Zhou et al. demonstrated that mouse pancreatic exocrine cells forced to express the combination of PDX1, MafA, and Neurog3 or NeuroD, can be converted into insulin-producing cells with an efficiency approaching 25% of infected cells.105
While a variety of in vivo strategies for generating β-cells by transdifferentiation in mice have been successful, ex vivo transdifferentiation has been more challenging, as demonstrated with human liver cells.106 However, recent progress has been made by Lima et al. through the combined use of transcription factors (Pdx1, Neurog3, MafA, Pax4), culture medium supplements (betacellulin, exendin-4 and nictoninamide), and compounds aimed at preventing human exocrine cells from undergoing epithelial to mesenchymal transdifferentiation (EMT) (serum-free media, SB43152, Y27632, 5-AZA-2'deoxycytidine).107 To realize the therapeutic potential of manipulating cellular identity, the protocols for transdifferentiation will need to be made scalable, reproducible, and safe.
β-Cells From α-Cells
The close lineage relationship among the pancreatic endocrine cell types (α-,β-,δ- and PP-cells) may provide an opportunity for efficient transdifferentiation among these cell types. Indeed, it has been observed that α-cell-specific deletion of the tumor suppressor gene Menin, the molecular basis for the multiple endocrine neoplasia syndrome 1 (MEN1), in adult mice gives rise to both glucagonomas and α-cell-derived insulinomas.108 Recent studies have suggested a similar capacity for transdifferentiation between pancreatic endocrine cells in humans. Genome wide analysis of the epigenetic landscape (activating H3K4me3 and repressive H3K27me3 modifications) of human α- and β-cells identified a preponderance of bivalent loci in α-cells.109 The presence of bivalence is characteristic of progenitor cells and might indicate that α-cells carry a more plastic epigenetic state that is susceptible to lineage conversion. Given the pathogenic role of glucagon in the progression of diabetes, a method for simultaneously decreasing α-cells and increasing β-cells by directed lineage conversion is a therapeutically attractive strategy. Indeed, Formina-Yadlin and co-workers have identified a small molecule with a limited ability to induce insulin expression by human α-like cells.110
The observation that constitutive removal of Pax4 in mice prevents the development of β- and δ-cells while causing a corresponding increase in the α-cell population raised the possibility that forced expression of Pax4 in α-cells might have the reciprocal effect.111 Amazingly, Pax4 expression in adult murine α-cells causes the predicted conversion of α-cells to β-cells, and as discussed above, the futile de novo generation of α-cells from facultative progenitor cells located within the pancreatic ducts.112 Furthermore, forced α-cell-specific expression of Pax4 in mice leads to a large increase in islet size (10x after 20 months) and supports multiple rounds of β-cell regeneration following β-cell ablation.91 The ability of Pax4 to trans-differentiate murine α-cells to β-cells may relate to the antagonistic functions of Pax4 and Arx in maintaining β-cell and α-cell identity, respectively.113 The repression of Arx by Dnmt1-dependent methylation is required to maintain β-cell identity in mice, and forced expression of Arx is sufficient to transdifferentiate β-cells to α-cells and δ-cells.114,115 These studies suggest that cell-type specific transcription factors act as master regulators to maintain cellular identity and that perturbation of these transcriptional programs is sufficient to destabilize differentiated cellular phenotypes.
The potential utility of α-cell to β-cell conversion has been boosted by the observation that environmental cues (e.g. extreme β-cell destruction, as opposed to genetic manipulation) are sufficient to direct a change in cellular identity.26 Using a murine genetic model to ablate >99% of the β-cell mass in mature animals, the authors were able to follow the recovery of insulin producing cells and determine the origin of the new β-cells. Surprisingly, the β-cell population was replenished by α-cell transdifferentiation rather than by β-cell proliferation, which had been observed previously in a similar β-cell ablation study in mice.23 However, a third mouse study which used a combination of PDL and Alloxan treatment to ablate β-cells noted both α-cell to β-cell conversion (based upon the presence of insulin and glucagon double positive cells) and increased β-cell proliferation.116 Presently, the bases for these discrepancies are unknown.
β-Cells From De-differentiated β-Cells
The difficulty of expanding mature β-cells has led to an interest in an alternative strategy that circumvents the growth-inhibited state of differentiated mature β-cells. In 2004 Gershengorn and colleagues suggested that human insulin-expressing cells that are maintained in adherent culture conditions in the presence of serum undergo de-differentiation, initiate the expression of mesenchymal cell markers such as vimentin (EMT), undergo exponential expansion, and retain the ability to return to a hormone+ state when grown as clusters in serum-free media.117 Subsequently, similar experiments performed with lineage-marked murine β-cells replicated the de-differentiation of β-cells but failed to demonstrate that these cells have a substantial proliferative capacity.118 In contrast, lentiviral-based lineage tracing with human β-cells confirmed the proliferative potential of de-differentiated human β-cells and emphasized the possibility of species-specific cellular behavior.119
Recently, pancreatic β-cell de-differentiation to a progenitor-like state has been highlighted as an event that may contribute to the pathogenesis of type 2 diabetes (e.g. β-cell failure) in murine disease models.118 Whereas loss of the transcription factor FoxO1 in Neurog3-expressing gut progenitor cells leads to the potentially beneficial ectopic generation of insulin+-cells, loss of FoxO1 expression in mature β-cells causes a predisposition to metabolic stress and de-differentiation.121 Interestingly, de-differentiated β-cells might be used to reconstitute a functional β-cell mass by expanding these “progenitor-like” cells and then re-differentiating them. While this strategy is intriguing, many hurdles remain. First, the contribution of β-cell de-differentiation to human disease remains to be established. Second, the therapeutic benefit of β-cell rest has been explored for several decades with mixed success.122 The ability of β-cell rest to remedy de-differentiation remains unsubstantiated. Third, a method for in vivo re-differentiation must be established. Recent work based on cultured human islet cells identified the role of Notch pathway inhibition in facilitating the reestablishment of β-cell identity of de-differentiated β-cells. Perhaps this provides a starting point for realizing the goal of maintaining β-cell identity and preventing β-cell failure.123
Conclusion
The exploding incidence of diabetes in the U.S. and abroad has made the disease one of the world's top public health burdens. While the projected economic and human costs are increasing, the concurrent boom in regenerative medicine offers hope for a sustainable, scalable treatment. Diabetes is a disease of β-cell degeneration, and is ideally suited for treatments aimed at regeneration. Recently, there has been a seismic shift in our understanding of cellular identity: under appropriate conditions one cell type may become any other cell type. Consequently, β-cells, which usually have limited growth potential, may be cajoled toward an identity of exponential growth capacity or reverted to a progenitor-like state for expansion. Alternatively, non-β-cells, whether they are considered to be facultative stem cells or terminally differentiated, may be tapped as a vast reservoir for new β-cells. The extraordinary potential of manipulating cellular identity for therapeutic purposes has just begun to be explored. Presently, the future is undifferentiated.
Acknowledgements
RJN was supported by a training grant from the National Human Genome Research Institute (T32 HG000044) and is now supported by a postdoctoral fellowship from the A.P. Giannini Foundation. JA is supported by grants from The National Institute of Diabetes and Digestive and Kidney Diseases (DK0884206 and DK098143). All authors have read the journal's policy on disclosure of potential conflicts of interest, and none have a conflict of interest to declare.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Vehik K, Hamman RF, Lezotte D, et al. Increasing incidence of type 1 diabetes in 0- to 17-year-old Colorado youth. Diabetes care. 2007;30(3):503–9. doi: 10.2337/dc06-1837. [DOI] [PubMed] [Google Scholar]
- 2.Onkamo P, Vaananen S, Karvonen M, Tuomilehto J. Worldwide increase in incidence of Type I diabetes--the analysis of the data on published incidence trends. Diabetologia. 1999;42(12):1395–403. doi: 10.1007/s001250051309. [DOI] [PubMed] [Google Scholar]
- 3.Huang ES, Basu A, O'Grady M, Capretta JC. Projecting the future diabetes population size and related costs for the U.S. Diabetes care. 2009;32(12):2225–9. doi: 10.2337/dc09-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Population health metrics. 2011;8(1):29. doi: 10.1186/1478-7954-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olshansky SJ, Passaro DJ, Hershow RC, et al. A potential decline in life expectancy in the United States in the 21st century. The New England journal of medicine. 2005;352(11):1138–45. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- 6.Miller RG, Secrest AM, Sharma RK, Songer TJ, Orchard TJ. Improvements in the life expectancy of type 1 diabetes: the pittsburgh epidemiology of diabetes complications study cohort. Diabetes. 2012;61(11):2987–92. doi: 10.2337/db11-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harlan DM, Kenyon NS, Korsgren O, Roep BO. Current advances and travails in islet transplantation. Diabetes. 2009;58(10):2175–84. doi: 10.2337/db09-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nath DS, Gruessner AC, Kandaswamy R, Gruessner RW, Sutherland DE, Humar A. Outcomes of pancreas transplants for patients with type 2 diabetes mellitus. Clinical transplantation. 2005;19(6):792–7. doi: 10.1111/j.1399-0012.2005.00423.x. [DOI] [PubMed] [Google Scholar]
- 9.Pagliuca FW, Melton DA. How to make a functional beta-cell. Development (Cambridge, England) 2013;140(12):2472–83. doi: 10.1242/dev.093187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warren S. Adenomas of the Islands of Langerhans. The American journal of pathology. 1926;2(4):335–403.. [PMC free article] [PubMed] [Google Scholar]
- 11.Wilder RM, Allan FN, Power MH, Robertson HE. Carcinoma of the islands of the pancreas - Hyperinsulinism and hypoglycemia. J Amer Med Assoc. 1927;89:348–55. [Google Scholar]
- 12.Green IC, Taylor KW. Effects of pregnancy in the rat on the size and insulin secretory response of the islets of Langerhans. The Journal of endocrinology. 1972;54(2):317–25. doi: 10.1677/joe.0.0540317. [DOI] [PubMed] [Google Scholar]
- 13.Van Assche FA, Aerts L, De Prins F. A morphological study of the endocrine pancreas in human pregnancy. British journal of obstetrics and gynaecology. 1978;85(11):818–20. doi: 10.1111/j.1471-0528.1978.tb15835.x. [DOI] [PubMed] [Google Scholar]
- 14.Sorenson RL, Brelje TC. Adaptation of islets of Langerhans to pregnancy: beta-cell growth, enhanced insulin secretion and the role of lactogenic hormones. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 1997;29(6):301–7. doi: 10.1055/s-2007-979040. [DOI] [PubMed] [Google Scholar]
- 15.Bonner-Weir S. Islet growth and development in the adult. Journal of molecular endocrinology. 2000;24(3):297–302. doi: 10.1677/jme.0.0240297. [DOI] [PubMed] [Google Scholar]
- 16.Kloppel G, Lohr M, Habich K, Oberholzer M, Heitz PU. Islet pathology and the pathogenesis of type 1 and type 2 diabetes mellitus revisited. Survey and synthesis of pathology research. 1985;4(2):110–25. doi: 10.1159/000156969. [DOI] [PubMed] [Google Scholar]
- 17.Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes, obesity & metabolism. 2008;10(Suppl 4):32–42. doi: 10.1111/j.1463-1326.2008.00969.x. [DOI] [PubMed] [Google Scholar]
- 18.Ritzel RA, Butler AE, Rizza RA, Veldhuis JD, Butler PC. Relationship between beta-cell mass and fasting blood glucose concentration in humans. Diabetes care. 2006;29(3):717–8. doi: 10.2337/diacare.29.03.06.dc05-1538. [DOI] [PubMed] [Google Scholar]
- 19.Meier JJ, Butler AE, Saisho Y, et al. Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes. 2008;57(6):1584–94. doi: 10.2337/db07-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saisho Y, Butler AE, Manesso E, Elashoff D, Rizza RA, Butler PC. beta-cell mass and turnover in humans: effects of obesity and aging. Diabetes care. 2013;36(1):111–7. doi: 10.2337/dc12-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruning JC, Winnay J, Bonner-Weir S, Taylor SI, Accili D, Kahn CR. Development of a novel polygenic model of NIDDM in mice heterozygous for IR and IRS-1 null alleles. Cell. 1997;88(4):561–72. doi: 10.1016/s0092-8674(00)81896-6. [DOI] [PubMed] [Google Scholar]
- 22.Okada T, Liew CW, Hu J, et al. Insulin receptors in beta-cells are critical for islet compensatory growth response to insulin resistance. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(21):8977–82. doi: 10.1073/pnas.0608703104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nir T, Melton DA, Dor Y. Recovery from diabetes in mice by beta cell regeneration. The Journal of clinical investigation. 2007;117(9):2553–61. doi: 10.1172/JCI32959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stolovich-Rain M, Hija A, Grimsby J, Glaser B, Dor Y. Pancreatic beta cells in very old mice retain capacity for compensatory proliferation. The Journal of biological chemistry. 2012;287(33):27407–14. doi: 10.1074/jbc.M112.350736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cano DA, Rulifson IC, Heiser PW, et al. Regulated -cell regeneration in the adult mouse pancreas. Diabetes. 2007 doi: 10.2337/db07-0913. [DOI] [PubMed] [Google Scholar]
- 26.Thorel F, Nepote V, Avril I, et al. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature. 2010;464(7292):1149–54. doi: 10.1038/nature08894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu X, D'Hoker J, Stange G, et al. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132(2):197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 28.Perl S, Kushner JA, Buchholz BA, et al. Significant human beta-cell turnover is limited to the first three decades of life as determined by in vivo thymidine analog incorporation and radiocarbon dating. The Journal of clinical endocrinology and metabolism. 2010;95(10):E234–9. doi: 10.1210/jc.2010-0932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.In't Veld P, De Munck N, Van Belle K, et al. Beta-cell replication is increased in donor organs from young patients after prolonged life support. Diabetes. 2010;59(7):1702–8. doi: 10.2337/db09-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reers C, Erbel S, Esposito I, et al. Impaired islet turnover in human donor pancreata with aging. European journal of endocrinology / European Federation of Endocrine Societies. 2009;160(2):185–91. doi: 10.1530/EJE-08-0596. [DOI] [PubMed] [Google Scholar]
- 31.Gregg BE, Moore PC, Demozay D, et al. Formation of a human beta-cell population within pancreatic islets is set early in life. The Journal of clinical endocrinology and metabolism. 2012;97(9):3197–206. doi: 10.1210/jc.2012-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tyrberg B, Eizirik DL, Hellerstrom C, Pipeleers DG, Andersson A. Human pancreatic beta-cell deoxyribonucleic acid-synthesis in islet grafts decreases with increasing organ donor age but increases in response to glucose stimulation in vitro. Endocrinology. 1996;137(12):5694–9. doi: 10.1210/endo.137.12.8940401. [DOI] [PubMed] [Google Scholar]
- 33.Tyrberg B, Ustinov J, Otonkoski T, Andersson A. Stimulated endocrine cell proliferation and differentiation in transplanted human pancreatic islets: effects of the ob gene and compensatory growth of the implantation organ. Diabetes. 2001;50(2):301–7. doi: 10.2337/diabetes.50.2.301. [DOI] [PubMed] [Google Scholar]
- 34.Levitt HE, Cyphert TJ, Pascoe JL, et al. Glucose stimulates human beta cell replication in vivo in islets transplanted into NOD-severe combined immunodeficiency (SCID) mice. Diabetologia. 2011;54(3):572–82. doi: 10.1007/s00125-010-1919-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takane KK, Kleinberger JW, Salim FG, Fiaschi-Taesch NM, Stewart AF. Regulated and reversible induction of adult human beta-cell replication. Diabetes. 2012;61(2):418–24. doi: 10.2337/db11-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karslioglu E, Kleinberger JW, Salim FG, et al. cMyc is a principal upstream driver of beta-cell proliferation in rat insulinoma cell lines and is an effective mediator of human beta-cell replication. Molecular endocrinology (Baltimore, Md. 2011;25(10):1760–72. doi: 10.1210/me.2011-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Metukuri MR, Zhang P, Basantani MK, et al. ChREBP mediates glucose-stimulated pancreatic beta-cell proliferation. Diabetes. 2012;61(8):2004–15. doi: 10.2337/db11-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schisler JC, Fueger PT, Babu DA, et al. Stimulation of human and rat islet beta-cell proliferation with retention of function by the homeodomain transcription factor Nkx6.1. Mol Cell Biol. 2008;28(10):3465–76. doi: 10.1128/MCB.01791-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davis DB, Lavine JA, Suhonen JI, et al. FoxM1 is up-regulated by obesity and stimulates beta-cell proliferation. Molecular endocrinology (Baltimore, Md. 2010;24(9):1822–34. doi: 10.1210/me.2010-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rieck S, Zhang J, Li Z, et al. Overexpression of hepatocyte nuclear factor-4alpha initiates cell cycle entry, but is not sufficient to promote beta-cell expansion in human islets. Molecular endocrinology (Baltimore, Md. 2012;26(9):1590–602. doi: 10.1210/me.2012-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429(6987):41–6. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 42.Liu Y, Suckale J, Masjkur J, et al. Tamoxifen-independent recombination in the RIP CreER mouse. PLoS ONE. 2010;5(10):e13533. doi: 10.1371/journal.pone.0013533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Teta M, Rankin MM, Long SY, Stein GM, Kushner JA. Growth and regeneration of adult beta cells does not involve specialized progenitors. Developmental cell. 2007;12(5):817–26. doi: 10.1016/j.devcel.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 44.Morris AP, Voight BF, Teslovich TM, et al. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nature genetics. 2012;44(9):981–90. doi: 10.1038/ng.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muoio DM, Newgard CB. Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nature reviews Molecular cell biology. 2008;9(3):193–205. doi: 10.1038/nrm2327. [DOI] [PubMed] [Google Scholar]
- 46.Michael MD, Kulkarni RN, Postic C, et al. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Molecular cell. 2000;6(1):87–97. [PubMed] [Google Scholar]
- 47.Chick WL, Lauris V, Flewelling JH, Andrews KA, Woodruff JM. Effects of glucose on beta cells in pancreatic monolayer cultures. Endocrinology. 1973;92(1):212–8. doi: 10.1210/endo-92-1-212. [DOI] [PubMed] [Google Scholar]
- 48.Bonner-Weir S, Deery D, Leahy JL, Weir GC. Compensatory growth of pancreatic beta-cells in adult rats after short-term glucose infusion. Diabetes. 1989;38(1):49–53. doi: 10.2337/diab.38.1.49. [DOI] [PubMed] [Google Scholar]
- 49.Flier SN, Kulkarni RN, Kahn CR. Evidence for a circulating islet cell growth factor in insulin-resistant states. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(13):7475–80. doi: 10.1073/pnas.131192998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Porat S, Weinberg-Corem N, Tornovsky-Babaey S, et al. Control of pancreatic beta cell regeneration by glucose metabolism. Cell metabolism. 2011;13(4):440–9. doi: 10.1016/j.cmet.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang W, Walker JR, Wang X, et al. Identification of small-molecule inducers of pancreatic beta-cell expansion. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(5):1427–32. doi: 10.1073/pnas.0811848106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heit JJ, Apelqvist AA, Gu X, et al. Calcineurin/NFAT signalling regulates pancreatic beta-cell growth and function. Nature. 2006;443(7109):345–9. doi: 10.1038/nature05097. [DOI] [PubMed] [Google Scholar]
- 53.El Ouaamari A, Kawamori D, Dirice E, et al. Liver-Derived Systemic Factors Drive beta Cell Hyperplasia in Insulin-Resistant States. Cell reports. 2013 doi: 10.1016/j.celrep.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yi P, Park JS, Melton DA. Betatrophin: A Hormone that Controls Pancreatic beta Cell Proliferation. Cell. 2013;153(4):747–58. doi: 10.1016/j.cell.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 55.Lee NK, Sowa H, Hinoi E, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130(3):456–69. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wei J, Hanna T, Suda N, Karsenty G, Ducy P. Osteocalcin promotes beta-cell proliferation during development and adulthood through Gprc6a. Diabetes. 2013 doi: 10.2337/db13-0887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferron M, Wei J, Yoshizawa T, et al. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010;142(2):296–308. doi: 10.1016/j.cell.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vasavada RC, Garcia-Ocana A, Zawalich WS, et al. Targeted expression of placental lactogen in the beta cells of transgenic mice results in beta cell proliferation, islet mass augmentation, and hypoglycemia. The Journal of biological chemistry. 2000;275(20):15399–406. doi: 10.1074/jbc.275.20.15399. [DOI] [PubMed] [Google Scholar]
- 59.Freemark M, Avril I, Fleenor D, et al. Targeted deletion of the PRL receptor: effects on islet development, insulin production, and glucose tolerance. Endocrinology. 2002;143(4):1378–85. doi: 10.1210/endo.143.4.8722. [DOI] [PubMed] [Google Scholar]
- 60.Karnik SK, Chen H, McLean GW, et al. Menin controls growth of pancreatic beta-cells in pregnant mice and promotes gestational diabetes mellitus. Science (New York, NY. 2007;318(5851):806–9. doi: 10.1126/science.1146812. [DOI] [PubMed] [Google Scholar]
- 61.Zhang H, Zhang J, Pope CF, et al. Gestational diabetes mellitus resulting from impaired beta-cell compensation in the absence of FoxM1, a novel downstream effector of placental lactogen. Diabetes. 2010;59(1):143–52. doi: 10.2337/db09-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim H, Toyofuku Y, Lynn FC, et al. Serotonin regulates pancreatic beta cell mass during pregnancy. Nature medicine. 2010;16(7):804–8. doi: 10.1038/nm.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fleischer N, Chen C, Surana M, et al. Functional analysis of a conditionally transformed pancreatic beta-cell line. Diabetes. 1998;47(9):1419–25. doi: 10.2337/diabetes.47.9.1419. [DOI] [PubMed] [Google Scholar]
- 64.Shen W, Tremblay MS, Deshmukh VA, et al. Small-molecule inducer of beta cell proliferation identified by high-throughput screening. Journal of the American Chemical Society. 2013;135(5):1669–72. doi: 10.1021/ja309304m. [DOI] [PubMed] [Google Scholar]
- 65.Annes JP, Ryu JH, Lam K, et al. Adenosine kinase inhibition selectively promotes rodent and porcine islet beta-cell replication. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(10):3915–20. doi: 10.1073/pnas.1201149109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Andersson O, Adams BA, Yoo D, et al. Adenosine signaling promotes regeneration of pancreatic beta cells in vivo. Cell metabolism. 2012;15(6):885–94. doi: 10.1016/j.cmet.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Walpita D, Hasaka T, Spoonamore J, et al. A human islet cell culture system for high-throughput screening. Journal of biomolecular screening. 2012;17(4):509–18. doi: 10.1177/1087057111430253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ravassard P, Hazhouz Y, Pechberty S, et al. A genetically engineered human pancreatic beta cell line exhibiting glucose-inducible insulin secretion. The Journal of clinical investigation. 2011;121(9):3589–97. doi: 10.1172/JCI58447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen H, Gu X, Liu Y, et al. PDGF signalling controls age-dependent proliferation in pancreatic beta-cells. Nature. 2011;478(7369):349–55. doi: 10.1038/nature10502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang Y, Gurung B, Wu T, Wang H, Stoffers DA, Hua X. Reversal of preexisting hyperglycemia in diabetic mice by acute deletion of the Men1 gene. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(47):20358–63. doi: 10.1073/pnas.1012257107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Service GJ, Thompson GB, Service FJ, Andrews JC, Collazo-Clavell ML, Lloyd RV. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. The New England journal of medicine. 2005;353(3):249–54. doi: 10.1056/NEJMoa043690. [DOI] [PubMed] [Google Scholar]
- 72.Ziv O, Glaser B, Dor Y. The plastic pancreas. Developmental cell. 2013;26(1):3–7. doi: 10.1016/j.devcel.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 73.Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature. 1994;371(6498):606–9. doi: 10.1038/371606a0. [DOI] [PubMed] [Google Scholar]
- 74.Stoffers DA, Zinkin NT, Stanojevic V, Clarke WL, Habener JF. Pancreatic agenesis attributable to a single nucleotide deletion in the human IPF1 gene coding sequence. Nature genetics. 1997;15(1):106–10. doi: 10.1038/ng0197-106. [DOI] [PubMed] [Google Scholar]
- 75.Gradwohl G, Dierich A, LeMeur M, Guillemot F. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(4):1607–11. doi: 10.1073/pnas.97.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schwitzgebel VM, Scheel DW, Conners JR, et al. Expression of neurogenin3 reveals an islet cell precursor population in the pancreas. Development (Cambridge, England) 2000;127(16):3533–42. doi: 10.1242/dev.127.16.3533. [DOI] [PubMed] [Google Scholar]
- 77.Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development (Cambridge, England) 2002;129(10):2447–57. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- 78.Zhou Q, Law AC, Rajagopal J, Anderson WJ, Gray PA, Melton DA. A multipotent progenitor domain guides pancreatic organogenesis. Developmental cell. 2007;13(1):103–14. doi: 10.1016/j.devcel.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 79.Bonner-Weir S, Li WC, Ouziel-Yahalom L, Guo L, Weir GC, Sharma A. Beta-cell growth and regeneration: replication is only part of the story. Diabetes. 2010;59(10):2340–8. doi: 10.2337/db10-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bonner-Weir S, Baxter LA, Schuppin GT, Smith FE. A second pathway for regeneration of adult exocrine and endocrine pancreas. A possible recapitulation of embryonic development. Diabetes. 1993;42(12):1715–20. doi: 10.2337/diab.42.12.1715. [DOI] [PubMed] [Google Scholar]
- 81.Butler AE, Galasso R, Matveyenko A, Rizza RA, Dry S, Butler PC. Pancreatic duct replication is increased with obesity and type 2 diabetes in humans. Diabetologia. 2010;53(1):21–6. doi: 10.1007/s00125-009-1556-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Martin-Pagola A, Sisino G, Allende G, et al. Insulin protein and proliferation in ductal cells in the transplanted pancreas of patients with type 1 diabetes and recurrence of autoimmunity. Diabetologia. 2008;51(10):1803–13. doi: 10.1007/s00125-008-1105-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li WC, Rukstalis JM, Nishimura W, et al. Activation of pancreatic-duct-derived progenitor cells during pancreas regeneration in adult rats. Journal of cell science. 2010;123(Pt 16):2792–802. doi: 10.1242/jcs.065268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rankin MM, Wilbur CJ, Rak K, Shields EJ, Granger A, Kushner JA. beta-Cells are not generated in pancreatic duct ligation-induced injury in adult mice. Diabetes. 2013;62(5):1634–45. doi: 10.2337/db12-0848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xiao X, Chen Z, Shiota C, et al. No evidence for beta cell neogenesis in murine adult pancreas. The Journal of clinical investigation. 2013;123(5):2207–17. doi: 10.1172/JCI66323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Desai BM, Oliver-Krasinski J, De Leon DD, et al. Preexisting pancreatic acinar cells contribute to acinar cell, but not islet beta cell, regeneration. The Journal of clinical investigation. 2007;117(4):971–7. doi: 10.1172/JCI29988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Solar M, Cardalda C, Houbracken I, et al. Pancreatic exocrine duct cells give rise to insulin-producing beta cells during embryogenesis but not after birth. Developmental cell. 2009;17(6):849–60. doi: 10.1016/j.devcel.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 88.Inada A, Nienaber C, Katsuta H, et al. Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(50):19915–9. doi: 10.1073/pnas.0805803105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Van de Casteele M, Leuckx G, Baeyens L, et al. Neurogenin 3+ cells contribute to beta-cell neogenesis and proliferation in injured adult mouse pancreas. Cell death & disease. 2013;4:e523. doi: 10.1038/cddis.2013.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pan FC, Bankaitis ED, Boyer D, et al. Spatiotemporal patterns of multipotentiality in Ptf1a-expressing cells during pancreas organogenesis and injury-induced facultative restoration. Development (Cambridge, England) 2013;140(4):751–64. doi: 10.1242/dev.090159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Al-Hasani K, Pfeifer A, Courtney M, et al. Adult Duct-Lining Cells Can Reprogram into beta-like Cells Able to Counter Repeated Cycles of Toxin-Induced Diabetes. Developmental cell. 2013;26(1):86–100. doi: 10.1016/j.devcel.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 92.Seaberg RM, Smukler SR, Kieffer TJ, et al. Clonal identification of multipotent precursors from adult mouse pancreas that generate neural and pancreatic lineages. Nature biotechnology. 2004;22(9):1115–24. doi: 10.1038/nbt1004. [DOI] [PubMed] [Google Scholar]
- 93.Smukler SR, Arntfield ME, Razavi R, et al. The adult mouse and human pancreas contain rare multipotent stem cells that express insulin. Cell stem cell. 2011;8(3):281–93. doi: 10.1016/j.stem.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 94.Brennand K, Huangfu D, Melton D. All beta Cells Contribute Equally to Islet Growth and Maintenance. PLoS biology. 2007;5(7):e163. doi: 10.1371/journal.pbio.0050163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rovira M, Scott SG, Liss AS, Jensen J, Thayer SP, Leach SD. Isolation and characterization of centroacinar/terminal ductal progenitor cells in adult mouse pancreas. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(1):75–80. doi: 10.1073/pnas.0912589107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hao E, Tyrberg B, Itkin-Ansari P, et al. Beta-cell differentiation from nonendocrine epithelial cells of the adult human pancreas. Nature medicine. 2006;12(3):310–6. doi: 10.1038/nm1367. [DOI] [PubMed] [Google Scholar]
- 97.Yatoh S, Dodge R, Akashi T, et al. Differentiation of affinity-purified human pancreatic duct cells to beta-cells. Diabetes. 2007;56(7):1802–9. doi: 10.2337/db06-1670. [DOI] [PubMed] [Google Scholar]
- 98.Bonner-Weir S, Taneja M, Weir GC, et al. In vitro cultivation of human islets from expanded ductal tissue. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(14):7999–8004. doi: 10.1073/pnas.97.14.7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 100.Vierbuchen T, Wernig M. Direct lineage conversions: unnatural but useful? Nature biotechnology. 2011;29(10):892–907. doi: 10.1038/nbt.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ferber S, Halkin A, Cohen H, et al. Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nature medicine. 2000;6(5):568–72. doi: 10.1038/75050. [DOI] [PubMed] [Google Scholar]
- 102.Kojima H, Fujimiya M, Matsumura K, et al. NeuroD-betacellulin gene therapy induces islet neogenesis in the liver and reverses diabetes in mice. Nature medicine. 2003;9(5):596–603. doi: 10.1038/nm867. [DOI] [PubMed] [Google Scholar]
- 103.Kaneto H, Nakatani Y, Miyatsuka T, et al. PDX-1/VP16 fusion protein, together with NeuroD or NGN3, markedly induces insulin gene transcription and ameliorates glucose tolerance. Diabetes. 2005;54(4):1009–22. doi: 10.2337/diabetes.54.4.1009. [DOI] [PubMed] [Google Scholar]
- 104.Banga A, Akinci E, Greder LV, Dutton JR, Slack JM. In vivo reprogramming of Sox9+ cells in the liver to insulin-secreting ducts. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(38):15336–41. doi: 10.1073/pnas.1201701109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455(7213):627–32. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sapir T, Shternhall K, Meivar-Levy I, et al. Cell-replacement therapy for diabetes: Generating functional insulin-producing tissue from adult human liver cells. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(22):7964–9. doi: 10.1073/pnas.0405277102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lima MJ, Muir KR, Docherty HM, et al. Suppression of Epithelial-to-Mesenchymal Transitioning Enhances Ex Vivo Reprogramming of Human Exocrine Pancreatic Tissue Toward Functional Insulin-Producing beta-Like Cells. Diabetes. 2013;62(8):2821–33. doi: 10.2337/db12-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lu J, Herrera PL, Carreira C, et al. Alpha cell-specific Men1 ablation triggers the transdifferentiation of glucagon-expressing cells and insulinoma development. Gastroenterology. 2010;138(5):1954–65. doi: 10.1053/j.gastro.2010.01.046. [DOI] [PubMed] [Google Scholar]
- 109.Bramswig NC, Everett LJ, Schug J, et al. Epigenomic plasticity enables human pancreatic alpha to beta cell reprogramming. The Journal of clinical investigation. 2013;123(3):1275–84. doi: 10.1172/JCI66514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fomina-Yadlin D, Kubicek S, Walpita D, et al. Small-molecule inducers of insulin expression in pancreatic alpha-cells. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(34):15099–104. doi: 10.1073/pnas.1010018107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sosa-Pineda B, Chowdhury K, Torres M, Oliver G, Gruss P. The Pax4 gene is essential for differentiation of insulin-producing beta cells in the mammalian pancreas. Nature. 1997;386(6623):399–402. doi: 10.1038/386399a0. [DOI] [PubMed] [Google Scholar]
- 112.Collombat P, Xu X, Ravassard P, et al. The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into alpha and subsequently beta cells. Cell. 2009;138(3):449–62. doi: 10.1016/j.cell.2009.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Collombat P, Mansouri A, Hecksher-Sorensen J, et al. Opposing actions of Arx and Pax4 in endocrine pancreas development. Genes & development. 2003;17(20):2591–603. doi: 10.1101/gad.269003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dhawan S, Georgia S, Tschen SI, Fan G, Bhushan A. Pancreatic beta cell identity is maintained by DNA methylation-mediated repression of Arx. Developmental cell. 2011;20(4):419–29. doi: 10.1016/j.devcel.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Collombat P, Hecksher-Sorensen J, Krull J, et al. Embryonic endocrine pancreas and mature beta cells acquire alpha and PP cell phenotypes upon Arx misexpression. The Journal of clinical investigation. 2007;117(4):961–70. doi: 10.1172/JCI29115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chung CH, Hao E, Piran R, Keinan E, Levine F. Pancreatic beta-cell neogenesis by direct conversion from mature alpha-cells. Stem cells (Dayton, Ohio) 2010;28(9):1630–8. doi: 10.1002/stem.482. [DOI] [PubMed] [Google Scholar]
- 117.Gershengorn MC, Hardikar AA, Wei C, Geras-Raaka E, Marcus-Samuels B, Raaka BM. Epithelial-to-mesenchymal transition generates proliferative human islet precursor cells. Science (New York, NY. 2004;306(5705):2261–4. doi: 10.1126/science.1101968. [DOI] [PubMed] [Google Scholar]
- 118.Weinberg N, Ouziel-Yahalom L, Knoller S, Efrat S, Dor Y. Lineage tracing evidence for in vitro dedifferentiation but rare proliferation of mouse pancreatic beta-cells. Diabetes. 2007;56(5):1299–304. doi: 10.2337/db06-1654. [DOI] [PubMed] [Google Scholar]
- 119.Russ HA, Bar Y, Ravassard P, Efrat S. In vitro proliferation of cells derived from adult human beta-cells revealed by cell-lineage tracing. Diabetes. 2008;57(6):1575–83. doi: 10.2337/db07-1283. [DOI] [PubMed] [Google Scholar]
- 120.Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic beta cell dedifferentiation as a mechanism of diabetic beta cell failure. Cell. 2012;150(6):1223–34. doi: 10.1016/j.cell.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Talchai C, Xuan S, Kitamura T, DePinho RA, Accili D. Generation of functional insulin-producing cells in the gut by Foxo1 ablation. Nature genetics. 2012;44(4):406–12. S1. doi: 10.1038/ng.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Weng J, Li Y, Xu W, et al. Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet. 2008;371(9626):1753–60. doi: 10.1016/S0140-6736(08)60762-X. [DOI] [PubMed] [Google Scholar]
- 123.Bar Y, Russ HA, Sintov E, Anker-Kitai L, Knoller S, Efrat S. Redifferentiation of expanded human pancreatic beta-cell-derived cells by inhibition of the NOTCH pathway. The Journal of biological chemistry. 2012;287(21):17269–80. doi: 10.1074/jbc.M111.319152. [DOI] [PMC free article] [PubMed] [Google Scholar]