Abstract
Objective(s): Transplantation quality improvement and reduction of cellular damage are important goals that are now considered by researchers. Melatonin is secreted from the pineal gland and some organs such as testes. According to beneficial effects of melatonin (such as its antioxidant and antiapoptotic properties), researchers have proposed that the use of melatonin may improve transplantation quality. The aim of this study was to investigate the effects of melatonin on the spermatogonial stem cells transplantation in the azoospermic mice.
Materials and Methods: The testes of the BALB/c mice pups (6-day-old) after vitrified-thawed, were digested with enzymes (collagenase, DNaseΙ, trypsin-EDTA) to disperse the cells. The SSCs, type A, were isolated from the rest of testicular cells by MACS. Spermatogonial stem cells were labeled with PKH26 fluorescent kit. Labeled spermatogonial stem cells were transplanted into the testes of infertile mice (busulfan 40 mg/kg). The mice died two months after transplantation and the efficiency of spermatogenesis was investigated. TNP2 and hematoxyline-eosin staining were used to detect the efficiency of cell transplantation.
Results: TNP2 were detected in the samples that received melatonin and spermatogonial stem cells transplantation, simultaneously. TNP2 was not detectable in the transplant recipient mice that received placebo for 10 weeks (control group). According to hematoxyline-eosin staining, melatonin improved structure of testes.
Conclusion: Administration of melatonin (20 mg/kg) simultaneously with transplantation of spermatogonial stem cells in azoospermia mouse testis increases the efficiency of transplantation and improves structural properties of the testes tissue.
Key Words: Cryopreservation, Melatonin, Spermatogonial stem cells, Transplantation
Introduction
Spermatogenesis is a complex phenomenon in which proliferation and differentiation of spermatog-onial stem cells continuously seek to become mature sperm (1, 2). Spermatogonial stem cell transplant-ation is a technique in which the spermatogonial stem cells, type A, from a donor animal are injected into seminiferous tubules or rete testes of the recipient testis (azoospermic mice) (3, 4). Following the transplantation, spermatogonial stem cells migr-ated to basal compartment of seminiferous tubules via unknown mechanisms and resume spermato-genesis (4, 5). In animal models (rodents, pigs, goats and dogs), spermatogonial stem cell transplantation into the testes of infertile males can lead to the reoccurrence of spermatogenesis (6-14). The current challenge concerning spermatogonial stem cells tran-splantation is increasing the quality and safety of cellular transplantation (1). The application of this technique to children rescued from chemotherapy because of lower success in animal models (e.g. mice, rats, goats, pigs and dogs) don’t extend (1). However, the recipient mice were able to reproduce live offspring but litter sizes were reduced in comparison with control group and the concentration and moti-lity of sperms were decreased (15-17). Improving the quality and reducing the harm to transplanted cells during freezing-thawing and recovery of sperm-atogenesis after transplantation of cells seem to be essential (1). Researchers have suggested that melatonin could be used to improve the quality and performance of organ transplantation (18). Use of melatonin in organ transplantation due to its pro-perties such as antioxidant, antiapoptotic, antibiotic, antiviral and immunosuppressive effects appears to be effective. (19-21). Melatonin is small biological molecule that is secreted from pineal gland and other organs e.g. retina, testis (19, 22, 23). Effects of melat-onin are studied in many regulatory functions of the cells such as immune response, cell signaling, protec-ting fatty acids from oxidation and nuclear DNA from damage, controlling the tumor growth and inhibiting cell proliferation, oncostatic action, antiapoptotic effect on many normal cells, enhancing apoptosis in the tumor cells and significant anti-aging properties (19, 20, 22-32) . Effects of melatonin on cells transp-lantation are not clear. It is not clear whether melato-nin can improve spermatognial stem cell transplant-ation efficiency or not. The aim of this study is assessment of concurrent administration of melato-nin with spermatogonial stem cells transplantation in azoospermic mice.
Materials and Methods
T All experiments were performed in accordance with principles of laboratory animal care. Male 6- day-old- BALB/c mouse pups (N=80) were obtained from physiology research center. Mice were eutha-nized by excessive doses of ketamine HCl (80 mg/kg) and xylazine (10 mg/kg) (Pharmacia and Upiohn, Erlangen, Germany) (21) in accordance with the protocols approved by Ahvaz Jundishapur University Medical Science Animal Care and Use Committee. Testes were vitrified and thawed according to Gholami et al methods (33). Testes were transferred to vitrification solution 1 (V.S 1), 2 (V.S 2) and 3 (V.S 3), respectively (Table 1). Finally, samples were transferred to liquid nitrogen tank.
Table 1.
Details of the solution used for vitrification
| 0.5 molar sucrose | Ethylene glycol | DMSO | 20% FBS | Time | |
|---|---|---|---|---|---|
| V.S. 1 | + | + (7.5%) | + (7.5%) | _ | 10 min |
| V.S. 2 | + | + (15%) | + (15%) | _ | 10 min |
| V.S 3 | + | + (15%) | + (15%) | + | 10 min |
Thawing procedure
Samples were maintained for 30 seconds at room temperature and were hold in water bath 37°C until defreeze then, samples were transferred to thawing solution1 (T.S.1) containing 0.5 molar sucrose at 4°C. After 5 min, samples were trans-ferred to thawing solution 2 (T.S.2) containing 0.25 molar sucrose at 4°C. After 5 min, samples were transferred to thawing solution 3 (T.S.3) containing 0.125 molar sucrose at 4°C (33).
Digestion of 6-day-old mouse testes
The cells digestion was done according to Milazzo et al, with little modification (34). Briefly, after removal of tunica albogina, 6-day-old mice testes were digested in the two steps. In the first step, 10 testes were incubated in 1 mg/ml collagen-ase type ΙV and 200-700 µg/ml DNaseΙ for 15 min at 37°C with slow pipetting. After centrifuging at 100 g for 5 min, in the second step supernatant was discarded and cells were resusp-ended in 1 ml trypsin-EDTA (sigma) and 200 µg/ml DNaseΙ for 5 min at 37°C. Trypsin was inactivated with adding 10% FBS to cell suspension.
Separation and purification with Laminin and MACS
Petri dishes (60 mm) were incubated with 20 µg/ml laminin, overnight. Supernatants were removed and Petri dishes containing laminin were washed with PBS buffer. Petri dishes were incubated with %0.5 mg/ml BSA, for one hour at 37°C to prevent nonspecific bindings and then they were washed with PBS buffer (35). In next steps, spermatogonial stem cells were purified with CD90.1 antibody. The cells, digested in the previous step, were incubated for 1 hr in the Petri dishes containing laminin at 32°C. Next, Petri dishes were washed with PBS. Cells that were attached to laminin were isolated by using trypsin - EDTA. CD90.1 (Thy1.1+) was used to detect spermatogonial stem cells type A. The procedure was performed according to manufacturer manual (Miltenyi Biotec, order no. 130-094-523). In brief, 107 of total cells were centrifuged at 300 g for 10 min. Cell pellet was resuspended in 90 µl of buffer .Buffer solution contained phosphate-buffered saline (PBS), PH 7.2, 0.5% bovine serum albumin (BSA), and 2 mM EDTA by diluting MACS BSA stock solution (# 130-91-376) 1:20 with autoMACS rinsing solution (# 130-091-222). Also, 10 µl CD90.1 microbeads was added. Then, it was well mixed and incubated for 15 min in the refrigerator (2-8). Cells were washed by adding 1-2 ml of buffer and centrifuged at 300 g for 10 min. Next, up to 108 cells were resuspended in 500 µl of buffer. Then, the cell suspension was loaded onto a MACS column, which is placed in the magnetic field of a MACS separator.
Labeling cells for transplantation
Cells were labeled with real fluorescent cell linker kit according to manufacturer catalogs. In brief, cells were centrifuged at 400 g for 5 min. Dye was prepared at the concentrations of 16 ×10 -6 M and cells were incubated with this dye for 2-7 min at 25C˚. Labeling procedure stops by addition of FBS. Subsequently, cells were washed 3 times in DMEM and kept on ice until transplantation.
Azoospermic mice for cells transplantation
Busulfan, 40 mg/kg, was injected intraper-itonealy to mice for 4-6 weeks. The mice will be ready for transplantation, 2.5 months after injection. The male BALB/c mice in our study, were divided into three groups. The first group (n=5) received transplanted cells and 10 weeks melatonin (20 mg/kg). The second group (n=5) received transplanted cells and 10 weeks placebo. The third group (n=5) of mice that did not receive transplanted cells and were injected with melatonin or placebo.
Spermatogonial stem cell transplantation
Spermatogonial stem cells transplanted to left rete testes by microinjection, using a needle under a stereomicroscope with the appropriate diameter, after anesthetizing the mice (36). Melatonin, 20 mg/kg daily, was injected intraperitoneally to mice for 10 weeks after transplantation.
Hematoxyline and eosin staining
After 10 weeks of spermatogonial stem cells transplantation, the mice were killed with a high dose of anesthetic and testicular tissue was extracted for analysis. Left testes after fixation, embedded in paraffin, cutting and dehydration stained with hema-toxylin-eosin dye for evaluation of histological changes. The results were analyzed based on a descriptive method and the blind evaluation of tissue sections within similarly staged seminiferous tubules was done by specialists.
Immunohistochemistry
The replacement of histones with protamine is done via transition nuclear protein 2 (TNP2) in the late stages of spermiogenesis. TNP2 appears in the nuclei at step 10 spermatid. Tracing this protein could be a good indicator to show spermatid. Immu-nohistochemical staining was performed according to the method of Zheng et al, with slight modific-ations (37). After fixation, embedded in paraffin and cutting, paraffin was removed from the the specim-ens by Xylene and specimens were dehydrated in ethanol. Antigens retrieval was done in citrate buffer (pH = 6) for 15 min at 98°C. Samples were incubated with PBS-0.3% Triton X-100 with 10% Normal donkey serum for 3 hr. The samples were incubated overnight with primary antibody (Polyclonal IgG Goat). Primary antibodies were diluted in PBS-TS at the ratio of 1:100. At this stage, PBS-TS only was added to the negative control samples. Secondary antibody was diluted at the ratio of 1:50 in PBS-TS. After washing, samples were incubated with DAPI for 12 min. The results were analyzed based on a descriptive method and the blind evaluation of tissue sections within similarly staged seminiferous tubules done by specialists.
Statistical analysis
Results (seminiferous epithelium) of treated group with melatonin and control group (untreated group) were compared with Mean-Whitney U-test and SPSS.16 software. Results are presented as MeanSD and statistical analysis were considered significant at Pv =0.001.
Results
Study of tissue sections after transplantation using hematoxylin – eosin staining
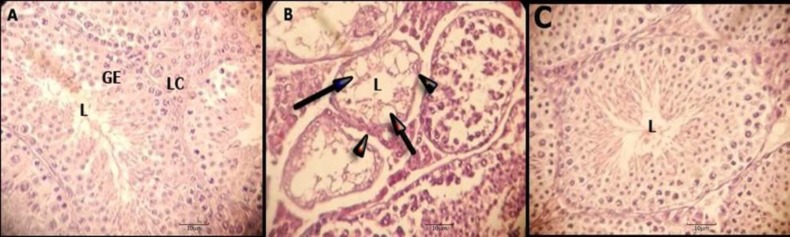
The results were analyzed based on Johnson’s method (38) and the blind evaluation of tissue sections was done by specialists. Statistical semin-iferous epithelium analysis between treated group with melatonin (7.951.85) and control group (1.500.513) according to Johnson's method is significant (Pv=0.001). Tissue sections of the first group that received transplanted cells and melat-onin, showed that large number of sperm was found in the lumen of seminiferous tubes and completes spermatogenesis. Germinal epithelium seminiferous tubes are stratified (Figure 1, section A). Basement membrane is organized and integrated (Figure 1, section A). Leydig cells in the interstitial space are evident (Figure 1, section A). The tubes have a significant thickness of germinal cells and various germ cells were observed including spermatogonia, primary spermatocytes, secondary spermatocytes, round spermatid, spermatozoa and sertoli cells (Figure 1, section A). In the interstitial tissue, visible loose connective tissue with blood vessels, nerves and leydig cells with round nuclei were observed (Figure 1, section A). Tissue sections of the second group that received transplanted cells and placebo, showed that lumen of seminiferous tubes represented a few elongated spermatids (Figure 1, section C). Epithelium of seminiferous is stratified and normal (Figure 1, section C). Basement mem-brane is normal (Figure 1, section C). Leydig cells are showed in the interstitial space (Figure 1, section C). Tissue sections of the third group that did not receive transplanted cells and placebo, showed that lumen of seminiferous tubes are devoid of spermatid and epithelium seminiferous tubes are irregular and destructed (Figure 1, section B). Vacuoles are seen in the epithelium seminiferous tubes and the basement membrane thickening is irregular (Figure 1, section B). Interstitial space is irregular (Figure 1, section B). The destruction of spermatogenesis can be seen clearly (Figure 1, section B).
Figure 1.
Testis sections stained with hematoxylin - eosin. Section A, samples that received melatonin and spermatogonial stem cells transplantation. Section B, samples that did not t receive melatonin and spermatogonial stem cells transplantation. Section C, samples that received placebo and spermatogonial stem cells transplantation. In the section B, vacuole is shown with arrows and thickening of the basement membrane is shown with arrow heads. Lumen (L), germinal epithelium (GE), Leydig cells (LC). Hematoxylin -eosin. Magnification X 400
Immunohistochemical results for review TNP2
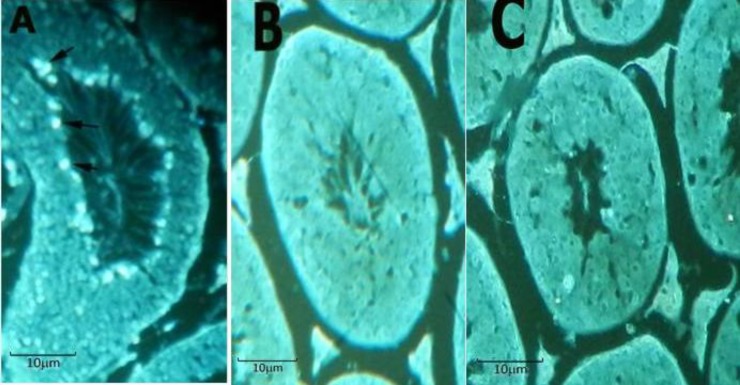
TNP2 is shownin the central part of seminiferous tubules (Figure 2, section A) in the mice that received melatonin and spermatogonial stem cells transplantation (group 1). TNP2 was not detectable in the transplant recipient mice that received a placebo (group 2) for 10 weeks (Figure 2, section C).
Figure 2.
Immunohistological staining for detected TNP2. TNP2, Bright spots in periphery of the central lumen (arrows), detected in section A. Section A, samples that received melatonin and spermatogonial stem cells transplantation, simultaneously. Section B shows the negative control group. Section C, samples that received placebo and spermatogonial stem cells transplantation. TNP2 was not detected in the section C. Magnification X 400
Discussion
Researchers showed that administration busulfan 40 mg/kg to mice lead to complete loss of spermatogenesis (39). Cryopreservation of sperma-togonial stem cells can preserve fertility in children with cancer. Melatonin has antioxidant and anti-apoptotic properties (22). Melatonin seems to be useful in preventing transplant rejection (19). Melatonin can easily cross the cell membrane because of its small size and high lipophilic properties. Very high concentrations of melatonin in the cell nucleus protects DNA against damaging agents (22, 40). Therefore, in this study the effect of melatonin on transplanted spermatogonial stem cells in mice were studied and the results showed that melatonin improves the maturation and survival of spermatogonial stem cells in the recipient testes. Aziz et al, showed that administration of melatonin was more effective than stem cell therapy in busulfan-treated mice (41). Aziz et al, showed that administration of melatonin to azoospermic mice leads to complete regeneration of germ cells with appearance of elongated and round spermatids. In the stem cell-treated mice, germ cells showed partial or incomplete regeneration with invisible round and elongated spermatids (42). Results obtained by Aziz et al, are consistent with the results obtained in this study. Simultaneous injection of melatonin and busulfan reduces the effects of busulfan on spermatogenesis (42). Melatonin administration red-uced testicular damage in the rats that were treated with cisplatin (43) and streptozotocin (44). Researchers showed that melatonin decrease germ cells apoptosis and cadmium-induced testicular stress in testes (45). Administration of melatonin to older men and women may help repairing oxidative damage of gunine DNA (46). Administration of high doses of melatonin (100 mg/kg) protects the testicular against the harmful effects X-rays (47). Melatonin successfully increases the quality of life in patients with tumor (48, 49). This evidence indicates protective effects of melatonin which is consistent with the results of this study. The amount of apoptotic cells in the group receiving melatonin was reduced as compared with the control group. These results confirm finding of other researchers about the anti-apoptotic effects of melatonin. Hemadi et al, showed that daily administration of melatonin to mice that received ectopic testicular transplantation for 2 months at the dose of 20 mg/kg led to the resumption of spermatogenesis, decreased apoptosis and improved transplantation (21). Researchers suggested that antioxidant and anti-apoptotic prope-rties of melatonin can protect sperm, epididymis and seminal vesicle (50). D'Istria et al showed that melatonin has anti-proliferative effect on germ cells (51). Researchers have shown that melatonin has antiproliferative effect on human prostate epithelial cells and neuroblastoma cells (52, 53). In our previous studies, we showed that supplementation of vitrification-thawing media with melatonin does not protect testicular tissue from injury (33) and melatonin may cause apoptosis in cells that were damaged in the process of freezing – thawing (54). Researchers showed that short-term administration of melatonin was beneficial and side effects have not been reported (19). Researchers suggested that melatonin strengthen the immune system (19). Melatonin role in organ transplantation is still not well understood. Jung et al, showed that the administration of melatonin can prevent rejection of transplanted hearts (18). Taken together, it can be said that melatonin may improve the quality of transplantation. In order to examine the effect of melatonin on the process of cells transplantation and to determine the exact mechanism on sperma-togonial stem cells transplantation more closely, we suggest that more studies should be done.
Conclusion
Simultaneous administration of melatonin and spermatogonial stem cells transplantation can improve spermatogenesis quality.
Acknowledgment
This study was approved with grant number PRC-85 in the physiology research center. Authors thank Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran for the financial support.
References
- 1.Geens M, Goossens E, De Block G, Ning L, Van Saen D, Tournaye H. Autologus spermatogonial stem cell transplantation in man: current obstacles for a future clinical application. Hum Reprod Update. 2008;14:121–129. doi: 10.1093/humupd/dmm047. [DOI] [PubMed] [Google Scholar]
- 2.Russell LD, Ettlin RA, Sinha Hikim AP, Clegg ED. Histological and Histopathological Evaluation of the Testis. 1st ed. Clearwater, FL: Cache River Press; 1990. [Google Scholar]
- 3.cLean DJ, Johnston D, Russell L, Griswold M. Germ cell transplantation and the study of testicular function. Trends Endocrinol Metab. 2001;12:16–21. doi: 10.1016/s1043-2760(00)00330-1. [DOI] [PubMed] [Google Scholar]
- 4.Nagano M, Avarbock MR, Brinster RL. Pattern and kinetics of mouse donor spermatogonial stem cell colonization in recipient testes. Biol Reprod. 1999;60:1429–1436. doi: 10.1095/biolreprod60.6.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hermann B, Sukhwani M, Lin C, Sheng Y, Tomko J, Rodriguez M, Shuttleworth J, McFarland D, Hobbs R, Pandolfi P, Schatten G, Orwig K. Characterization, cryopreservation, and ablation of spermatogonial stem cells in adult rhesus macaques. Stem Cells . 2007;25:2330–2338. doi: 10.1634/stemcells.2007-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brinster CJ, Ryu BY, Avarbock MR, Karagenc L, Brinster RL, Orwig KE. Restoration of fertility by germ cell transplantation requires effective recipient preparation. Biol Reprod. 2003;69:412–420. doi: 10.1095/biolreprod.103.016519. [DOI] [PubMed] [Google Scholar]
- 7.Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci . 1994;91:11298–11302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Honaramooz A, Behboodi E, Blash S, Megee SO, Dobrinski I. Germ cell transplantation in goats. Mol Reprod Dev . 2003;64:422–428. doi: 10.1002/mrd.10205. [DOI] [PubMed] [Google Scholar]
- 9.Kim Y, Turner D, Nelson J, Dobrinski I, McEntee M, Travis AJ. Production of donor-derived sperm after spermatogonial stem cell transplantation in the dog. Reproduction. 2008;136:823–831. doi: 10.1530/REP-08-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mikkola M, Sironen A, Kopp C, Taponen J, Sukura A, Vilkki J, et al. Transplantation of normal boar testicular cells resulted in complete focal spermatogenesis in a boar affected by the immotile short-tail sperm defect. Reprod Domest Anim. 2006;41:124–128. doi: 10.1111/j.1439-0531.2006.00651.x. [DOI] [PubMed] [Google Scholar]
- 11.Nagano M, Mccarrey JR, Brinster RL. Primate spermatogonial stem cells colonize mouse testes. Biol Reprod. 2001;64:1409–1416. doi: 10.1095/biolreprod64.5.1409. [DOI] [PubMed] [Google Scholar]
- 12.Ogawa T, Dobrinski I, Avarbock MR, Brinster RL. Transplantation of male germ line stem cells restores fertility in infertile mice. Nat Med. 2000;6:29–34. doi: 10.1038/71496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orwig KE, Schlatt S. Cryopreservation and transplantation of spermatogonia and testicular tissue for preservation of male fertility. J Natl Cancer Inst Monogr. 2005:51–56. doi: 10.1093/jncimonographs/lgi029. [DOI] [PubMed] [Google Scholar]
- 14.Shinohara T, Orwig KE, Avarbock MR, Brinster RL. Remodeling of the postnatal mouse testis is accompanied by dramatic changes in stem cell number and niche accessibility. Proc Natl Acad Sci . 2001;98:6186–6191. doi: 10.1073/pnas.111158198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goossens E, Frederickx V, De Block G, Van Steirteghem AC, Tournaye H. Reproductive capacity of sperm obtained after germ cell transplantation in a mouse model. Hum Reprod. 2003;18:1874–1880. doi: 10.1093/humrep/deg360. [DOI] [PubMed] [Google Scholar]
- 16.Goossens E, Frederickx V, De Block G, Van Steirteghem A, Tournaye H. Evaluation of in vivo conception after testicular stem cell transplantation in a mouse model shows altered post-implantation development. Hum Reprod. 2006;21:2057–2060. doi: 10.1093/humrep/del105. [DOI] [PubMed] [Google Scholar]
- 17.Goossens E, De Block G, Tournaye H. Computer-assisted motility analysis of spermatozoa obtained after spermatogonial stem cell transplantation in the mouse. Fertil Steril . 2008a;90:1411–1416. doi: 10.1016/j.fertnstert.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 18.Jung FJ, Yang L, Harter L, Inci I, Schneiter D, Lardinois D, et al. Melatonin in vivo prolongs cardiac allograft survival in rats. J Pineal Res. 2004;37:36–41. doi: 10.1111/j.1600-079X.2004.00133.x. [DOI] [PubMed] [Google Scholar]
- 19.Fildes JE, Yonan N, Keevil BG. Melatonin--a pleiotropic molecule involved in pathophysiological processes following organ transplantation. Immunology. 2009;127:443–449. doi: 10.1111/j.1365-2567.2009.03113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hemadi M, Abolhassani F, Akbari M, Sobhani A, Pasbakhsh P, Ahrlund-Richter L, et al. Melatonin promotes the cumulus-oocyte complexes quality of vitrified-thawed murine ovaries; with increased mean number of follicles survival and ovary size following heterotopic transplantation. Eur J Pharmacol. 2009;618:84–90. doi: 10.1016/j.ejphar.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 21.Hemadi M, Zargar M, Sobhani A. Assessment of morphological and functional changes in neonate vitrified testis grafts after host treatment with melatonin. Folia Morphol (Warsz) 2011;70:95–102. [PubMed] [Google Scholar]
- 22.Hardeland R, Cardinali DP, Srinivasan V, Spence DW, Brown GM, Pandi-Perumal SR. Melatonin--a pleiotropic, orchestrating regulator molecule. Prog Neurobiol. 2011;93:350–384. doi: 10.1016/j.pneurobio.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Zawilska JB, Skene DJ, Arendt J. Physiology and pharmacology of melatonin in relation to biological rhythms. Pharmacol Rep. 2009;61:383–410. doi: 10.1016/s1734-1140(09)70081-7. [DOI] [PubMed] [Google Scholar]
- 24.Ahmad R, Haldar C. Melatonin and androgen receptor expression interplay modulates cell-mediated immunity in tropical rodent Funambulus pennanti: an in vivo and in vitro study. Scand J Immunol. 2010;71:420–430. doi: 10.1111/j.1365-3083.2010.02396.x. [DOI] [PubMed] [Google Scholar]
- 25.Dasilva CM, Macias-garcia B, Miro-moran A, González-Fernández L, Morillo-Rodriguez A, Ortega- Ferrusola C, et al. Melatonin reduces lipid peroxidation and apoptotic-like changes in stallion spermatozoa. J Pineal Res. 2011;51:172–179. doi: 10.1111/j.1600-079X.2011.00873.x. [DOI] [PubMed] [Google Scholar]
- 26.Martin-renedo J, Mauriz JL, Jorquera F, Ruiz-Andrés O, González P, González-Gallego J. Melatonin induces cell cycle arrest and apoptosis in hepatocarcinoma HepG2 cell line. J Pineal Res. 2008;45:532–540. doi: 10.1111/j.1600-079X.2008.00641.x. [DOI] [PubMed] [Google Scholar]
- 27.Millan-plano S, Piedrafita E, Miana-mena FJ, Fuentes-Broto L, Martínez-Ballarín E, López-Pingarrón L, et al. Melatonin and structurally-related compounds protect synaptosomal membranes from free radical damage. Int J Mol Sci. 2010;11:312–328. doi: 10.3390/ijms11010312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radogna F, Cristofanon S, Paternoster L, D'Alessio M, De Nicola M, Cerella C, et al. Melatonin antagonizes the intrinsic pathway of apoptosis via mitochondrial targeting of Bcl-2. J Pineal Res. 2008;44:316–325. doi: 10.1111/j.1600-079X.2007.00532.x. [DOI] [PubMed] [Google Scholar]
- 29.Radogna F, Paternoster L, Albertini MC, Accorsi A, Cerella C, D'Alessio M, et al. Melatonin as an apoptosis antagonist. Ann N Y Acad Sci . 2006;1090:226–233. doi: 10.1196/annals.1378.025. [DOI] [PubMed] [Google Scholar]
- 30.Radogna F, Paternoster L, Albertini MC, Cerella C, Accorsi A, Bucchini A, et al. Melatonin antagonizes apoptosis via receptor interaction in U937 monocytic cells. J Pineal Res. 2007;43:154–162. doi: 10.1111/j.1600-079X.2007.00455.x. [DOI] [PubMed] [Google Scholar]
- 31.Reiter RJ, Tan DX, Manchester LC, Paredes SD, Mayo JC, Sainz RM. Melatonin and reproduction revisited. Biol Reprod. 2009;81:445–456. doi: 10.1095/biolreprod.108.075655. [DOI] [PubMed] [Google Scholar]
- 32.Zhao Y, Qi LW, Wang WM, Saxena PK, Liu CZ. Melatonin improves the survival of cryopreserved callus of Rhodiola crenulata. J Pineal Res. 2010;50:83–88. doi: 10.1111/j.1600-079X.2010.00817.x. [DOI] [PubMed] [Google Scholar]
- 33.Gholami M, Saki G, Hemadi M, Khodadadi A, Mohammadi-asl J. Supplementation vitrified-thawed media with melatonin do not protecting immature mouse testicular tissue from vitrified-thawed induced injury. Asian J Anim Veterinary Advances . 2012;7:940–949. [Google Scholar]
- 34.Milazzo JP, Vaudreuil L, Cauliez B, Gruel E, Massé L, Mousset-Siméon N, et al. Comparison of conditions for cryopreservation of testicular tissue from immature mice. Hum Reprod. 2008;23:17–28. doi: 10.1093/humrep/dem355. [DOI] [PubMed] [Google Scholar]
- 35.Shinohara K, Avarbock MR, Brinster RL. beta1- and alpha6-integrin are surface markers on mouse spermatogonial stem cells. Proc Natl Acad Sci USA . 1999;96:5504–5509. doi: 10.1073/pnas.96.10.5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ogawa T, Arechaga JM, Avarbock MR, Brinster RL. Transplantation of testis germinal cells into mouse seminiferous tubules. Int J Dev Biol. 1997;41:111–122. [PubMed] [Google Scholar]
- 37.Zheng J, Xia X, Ding H, Yan A, Hu S, Gong X, Zong S, Zhang Y, Sheng HZ. Erasure of the paternal transcription program during spermiogenesis: the first step in the reprogramming of sperm chromatin for zygotic development. Dev Dyn. 2008;237:1463–1476. doi: 10.1002/dvdy.21499. [DOI] [PubMed] [Google Scholar]
- 38.Lewis-Johnes DI, Kerrigan DD. A modified johnsen's count for evaluation of Spermatogenesis in the rat. IRCS Med Sci. 1985;13:510–511. [Google Scholar]
- 39.Anjamrooz SH, Movahedin M, Mowla SJ, anvand SP. Assessment of morphological and functional changes in the mouse testis and epididymal sperms following busulfan treatment. Iran Biomed J. 2007;11:15–22. [PubMed] [Google Scholar]
- 40.Sanchez-hidalho M, De La Lastra CA, Carrascosa-salmoral M, P et al. Age-related changes in melatonin synthesis in rat extrapineal tissues. Exp Gerontol . 2009;44:328–334. doi: 10.1016/j.exger.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 41.Aziz A, Metwally DH, Halla GB. The effect of stem cell therapy versus melatonin on the changes induced by busulfan in the testes of adult rat: histological and immunohistochemical studies. Egypt J Histol. 2013;36:175–184. [Google Scholar]
- 42.Mohammadghasemi F, Faghani M, Khajeh S. The protective effects of melatonin on the histological changes of testis in busulfan-treated adult mice. J Reprod Infertil. 2010;11:67–76. [Google Scholar]
- 43.Atessahin A, Sahna E, Turk G, Ceribaşi AO, Yilmaz S, Yüce A, et al. Chemoprotective effect of melatonin against cisplatin-induced testicular toxicity in rats. J Pineal Res. 2006;41:21–27. doi: 10.1111/j.1600-079X.2006.00327.x. [DOI] [PubMed] [Google Scholar]
- 44.Gunelie E, Tugyan K, Ozturk H, Gumustekin M, Cilaker S, Uysal N. Effect of melatonin on testicular damage in streptozotocin-induced diabetes rats. Eur Surg Res. 2008;40:354–360. doi: 10.1159/000118032. [DOI] [PubMed] [Google Scholar]
- 45.Ji YL, Wang H, Meng C, Zhao XF, Zhang C, Zhang Y, et al. Melatonin alleviates cadmium-induced cellular stress and germ cell apoptosis in testes. J Pineal Res. 2012;52:71–79. doi: 10.1111/j.1600-079X.2011.00921.x. [DOI] [PubMed] [Google Scholar]
- 46.Davanipour Z, Poulsen HE, Weimann A, Sobel E. Endogenous melatonin and oxidatively damaged guanine in DNA. BMC Endocr Disord. 2009;9:22. doi: 10.1186/1472-6823-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Take G, Erdogan D, Helvacioglu F, Göktas G, Ozbey G, Uluoglu C, et al. Effect of melatonin and time of administration on irradiation-induced damage to rat testes. Braz J Med Biol Res. 2009;42:621–628. doi: 10.1590/s0100-879x2009000700006. [DOI] [PubMed] [Google Scholar]
- 48.Lissoni P, Chilelli M, Villa S, Cerizza L, Tancini G. Five years survival in metastatic non-small cell lung cancer patients treated with chemotherapy alone or chemotherapy and melatonin: a randomized trial. J Pineal Res. 2003;35:12–15. doi: 10.1034/j.1600-079x.2003.00032.x. [DOI] [PubMed] [Google Scholar]
- 49.Vijayalaxmi , Thpmas CR, Reiter RJ, Herman TS. Melatonin: from basic research to cancer treatment clinics. J Clin Oncol. 2002;20:2575–2601. doi: 10.1200/JCO.2002.11.004. [DOI] [PubMed] [Google Scholar]
- 50.Mohammadghasemi F, Faghani M, Falahkarkan M. The protective effect of melatonin on sperm parameters, epididymis and seminal vesicle morphology in adult mouse treated with busulfan. J Iran Anatomi Sci. 2010;8:25–36. [Google Scholar]
- 51.D'istria M, Serino I, Izzo G, Ferrara D, De Rienzo G, Minucci S. Effects of melatonin treatment on Leydig cell activity in the testis of the frog Rana esculenta. Zygote. 2004;12:293–299. doi: 10.1017/s0967199404002898. [DOI] [PubMed] [Google Scholar]
- 52.Pizarro JG, Yeste-velasco M, Esparza JL, Verdaguer E, Pallàs M, Camins A, et al. The antiproliferative activity of melatonin in B65 rat dopaminergic neuroblastoma cells is related to the downregulation of cell cycle-related genes. J Pineal Res. 2008;45:8–16. doi: 10.1111/j.1600-079X.2007.00548.x. [DOI] [PubMed] [Google Scholar]
- 53.Tam CW, Chan KW, Liu VW, Pang B, Yao KM, Shiu SY. Melatonin as a negative mitogenic hormonal regulator of human prostate epithelial cell growth: potential mechanisms and clinical significance. J Pineal Res. 2008;45:403–412. doi: 10.1111/j.1600-079X.2008.00608.x. [DOI] [PubMed] [Google Scholar]
- 54.Gholami M, Saki G, Hemadi M, Khodadadi A, Mohamma-di-Asl J. Melatonin effect on expression apoptotic genes in vitrified-thawed 6-old-days mouse spermatogonial stem cells type A. Iran J Basic Med Sci. 2013;16:906–909. [PMC free article] [PubMed] [Google Scholar]