Summary
Importance
Advancing paternal age is associated with increased genetic mutations during spermatogenesis, which research suggests may cause psychiatric morbidity in the offspring. The effects of advancing paternal age at childbearing on offspring morbidity remains unclear, however, because of inconsistent epidemiological findings and the inability of previous studies to rigorously rule out confounding factors.
Objective
Examine the associations between advancing paternal age at childbearing and numerous indices of offspring morbidity.
Setting
Population-based cohort study in Sweden.
Participants
All individuals born in Sweden 1973–2001 (N=2,615,081), with subsets of the data used to predict childhood/adolescent morbidity.
Design
We estimated the risk for psychiatric and academic morbidity associated with advancing paternal age using several quasi-experimental designs, including the comparison of differentially exposed siblings, cousins, and first-born cousins.
Exposure
Paternal age at childbearing
Main outcomes
Psychiatric (autism, ADHD, psychosis, bipolar disorder, suicide attempt, and substance use problem) and academic (failing grades and low educational attainment) morbidity.
Results
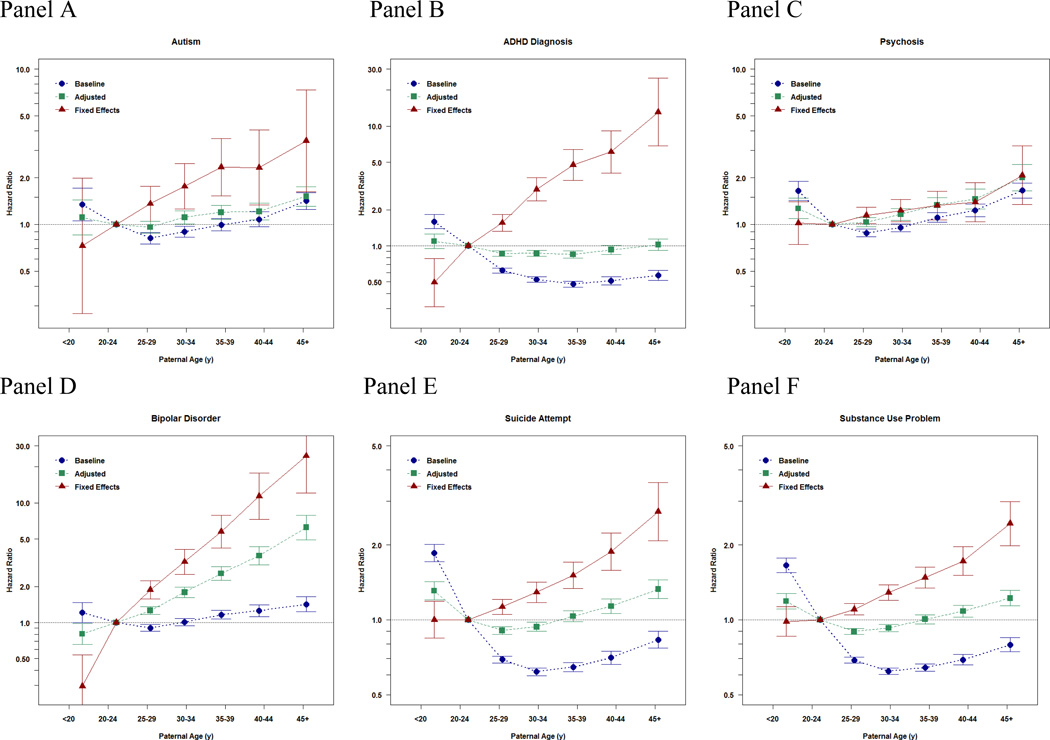
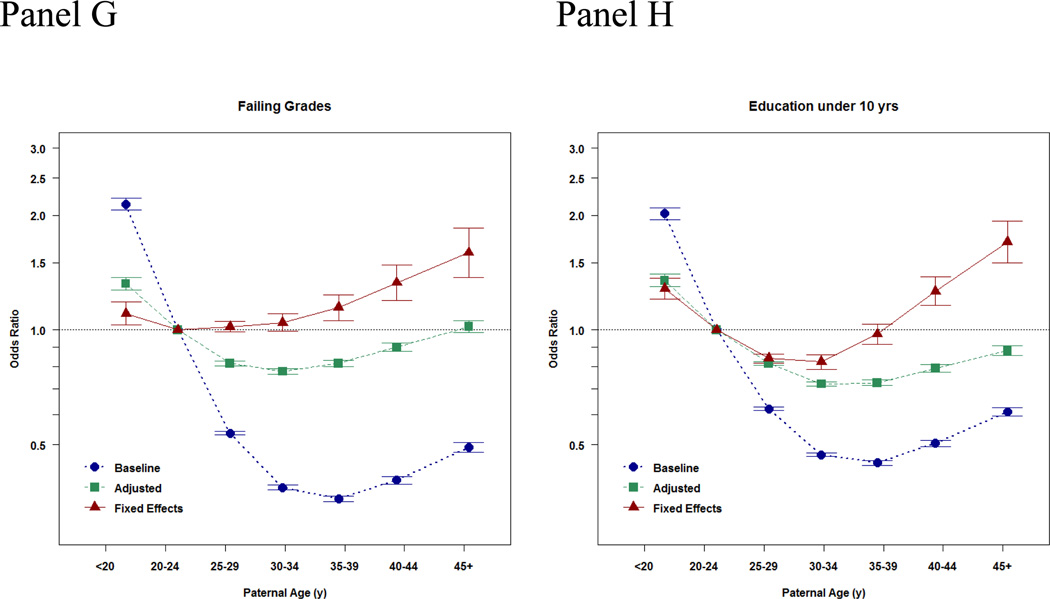
In the population, advancing paternal age was associated with increased risk for some psychiatric disorders (e.g. autism, psychosis, and bipolar disorders) but decreased risk for the other indices of morbidity. In contrast, the sibling-comparison analyses indicated that advancing paternal age had a dose-response relationship with every index of morbidity, with the magnitude of the associations being as large or larger than the estimates in the entire population. Compared to offspring born to fathers 20–25 years old, offspring of fathers 45 years+ were at heightened risk for autism (Hazard Ratio [HR]=3.45, 95% Confidence Intervals [CI]=1.62–7.33), ADHD (HR=13.13, CI=6.85–25.16), psychosis (HR=2.07, CI=1.35–3.20), bipolar disorder (HR=24.70, CI=12.12–50.31), suicide attempts (HR=2.72, CI=2.08–3.56), substance use problems (HR=2.44, CI=1.98–2.99), failing a grade (Odds Ratio [OR]=1.59, CI=1.37–1.85), and low educational attainment (OR=1.70, CI=1.50–1.93) in within-sibling comparisons. Additional analyses using several quasi-experimental designs obtained commensurate results, further strengthening the internal and external validity of the findings.
Conclusions and Relevance
Advancing paternal age is associated with increased risk for psychiatric and academic morbidity, with the magnitude of the risks being as large or larger than previous estimates. These findings are consistent with the hypothesis that new genetic mutations occurring during spermatogenesis are causally related to offspring morbidity.
Epidemiological studies have indicated that advancing paternal age (APA) at childbearing is associated with increased risk for offspring psychiatric problems, including autism spectrum disorders (ASD),1–3 schizophrenia,4,5 and bipolar disorder,6,7 as well as intellectual8 and academic problems.9 Recent genomic studies have shown that the age of the fathers at conception is an important factor in determining the number of de novo mutations in children10 and also linked de novo mutations to ASD, suggesting a mediating biological pathway leading to ASD.11–13 De novo mutations associated with APA have been linked with numerous offspring outcomes.14
Several major issues remain in the study of APA, however. First, the associations between early risks and offspring psychopathology have been questioned because most studies have not rigorously ruled out plausible confounding factors, which could be competing explanations for the statistical associations.15,16 Epidemiological studies may overestimate the magnitude of the effects because selection factors may account for part of the associations; personality traits associated with postponing parenthood17–19 or birth-order effects2 could better explain the associations. In contrast, increased maturity,20 conscientiousness,21 and social and cultural capital22 associated with delayed childbearing could suppress the statistical estimates of the specific effects of APA found in epidemiological studies. Researchers, therefore, must use quasi-experimental designs, approaches that rely on design features rather than relying solely on statistical controls, to better address confounding factors because of the inability of conduct randomized experiments of APA in humans.15,23,24 Family-based, quasi-experimental designs can help control for unmeasured confounds that family members share.25 For instance, the sibling-comparison design provides an approach that rules out all genetic and environmental factors shared by siblings.24–27 Sibling-comparison studies of APA have been inconsistent, however. Two sibling-comparison studies indicated APA was robustly associated with ASD,2,28 while two sibling-comparison studies found no association between APA and schizophrenia within families.18,19
Second, the existing research has been unable to explore if APA has general effects across numerous indices of morbidity because most studies have explored only one outcome at the time, and several indices of morbidity, such as suicidal behavior, substance use problems, attention deficit-hyperactivity disorder (ADHD), have not yet been extensively studied in relation to APA.
The current study explored the association between APA and numerous indices of offspring psychiatric (ASD, ADHD, psychosis, bipolar disorder, suicide attempt, and substance use problem) and academic morbidity (failing grade and low educational attainment) in a large population-based study of all offspring born in Sweden across 28 years. Rather than focus on a single indicator of functioning, the current study included several indices of psychiatric problems and educational outcomes to explore the scope and specificity of the associations with APA. The main analyses compared differentially exposed siblings (with the exposure being paternal age at childbearing) to more precisely estimate the specific effects of APA. The sibling-comparison design has a number limitations and assumptions, however, which can limit the internal and external validity of the findings.25–27,29,30 We therefore estimated the effects of APA using numerous additional family-based quasi-experimental designs,25 including the comparison of differentially exposed cousins and first-born cousins, in addition to using statistical controls for measured covariates, because the designs have different strengths and limitations. As such, we sought to find converging evidence from multiple designs, each with their own assumptions and limitations, which is critical for making causal inferences.31
Methods
Sample
All data for this population-based study were obtained by linking information available in the following population-based registries: (1) the Medical Birth Register includes data on more than 99% of pregnancies in Sweden since 1973; (2) the Multi-Generation Register contains information about biological relationships for all individuals living in Sweden since 1933; (3) the Migration Register supplies information on dates for migration in or out of Sweden; (4) the Cause of Death Register contains information on dates and causes of all deaths since 1958; (5) the Patient Register provides diagnoses for all inpatient psychiatric hospital admissions in Sweden since 1973 and outpatient care since 2001; (6) the National Crime Register includes detailed information about all criminal convictions since 1973; (7) the National School Register includes grades in all subjects for all students at the end of grade nine since 1983; (8) the Education Register contains information on highest level of completed formal education through 2008; (9) the Military Conscription Register includes cognitive assessments for all 18-year-old men in Sweden between 1970 and 2009; (10) the longitudinal integration database for health insurance and social studies (LISA) contains yearly assessments of income, marital status, employment status, social welfare status, and education for all individuals 15 years or older since 1990. More details about these registers, the variables, and the methods are provided elsewhere.32
All individuals in the analysis cohort were born in Sweden between 1973 and 2001. Birth-related data for 2,917,399 children were obtained from the Swedish Medical Birth Register. We first excluded those from multiple births (66,089), with missing data on gestational age (7,923), with recorded gestational age less than 23 weeks (102), and with recorded gestational age of more than 42 weeks and 6 days (38,588). We then excluded children with at least one recorded emigration from Sweden during this 28-year period (172,535), as well as those with a missing identifier for the biological mother (158), missing or invalid sex (2), or an invalid birth order variable (19). Following linkage with the Multi-Generation Register, we excluded an additional 16,902 individuals with insufficient data to establish a birth date for the biological father. The resulting cohort of 2,615,081 individuals represents 89.6% of the entire population and includes offspring from 1,408,669 distinct fathers and 1,404,489 distinct mothers.
Measures
See Table 1 for descriptive statistics and the birth cohorts used for each outcome. Appendix A in the Supplemental Appendix includes descriptive statistics by subgroups based on APA.
Table 1.
Descriptive Statistics
| Variables | Birth Year | Total N | Cases | ||
|---|---|---|---|---|---|
| Offspring | 1973–2001 | 2,615,081 | na | % | |
| Female | 1,264,248 | 48.34 | |||
| First born | 1,084,993 | 41.49 | |||
| Second born | 963,970 | 36.86 | |||
| Third born | 402,855 | 15.41 | |||
| Fourth born or higher | 163,263 | 6.24 | |||
| Paternal | 1904–1987 | 1,408,669 | |||
| Nationality (Swedish) | 2,291,415 | 87.62 | |||
| Upper Secondary Education (min 3 yrs) | 1,122,661 | 42.93 | |||
| Serious Adult Psychopathology | 46,670 | 1.78 | |||
| Criminality (any) | 1,041,427 | 39.82 | |||
| Father's Age at Birth (yrs)b | 31.27 | 5.96 | |||
| Father's Age at Birth of First Child (yrs)b | 28.92 | 5.54 | |||
| Disposable Income at Birthb | 895,833 | 47.22 | 22.72 | ||
| Maternal | 1924–1987 | 1,404,484 | |||
| Nationality (Swedish) | 2,308,587 | 88.28 | |||
| Upper Secondary Education (min 3 yrs) | 1,336,236 | 51.10 | |||
| Serious Adult Psychopathology | 48,524 | 1.86 | |||
| Criminality (any) | 306,779 | 11.73 | |||
| Mother's Age at Birth (yrs)b | 28.42 | 5.09 | |||
| Psychiatric Morbidity | |||||
| Autismc | 1992–2001 | 900,337 | 2,424 | 0.27 | |
| ADHD Diagnosisc | 1992–2001 | 900,337 | 2,861 | 0.32 | |
| Psychosisd | 1973–1997 | 2,293,032 | 10,739 | 0.89 | |
| Bipolar Disorderd | 1973–1997 | 2,293,032 | 6,819 | 0.61 | |
| Suicide Attemptd | 1973–1997 | 2,293,032 | 29,148 | 2.24 | |
| Substance Use Problemd | 1973–1997 | 2,293,032 | 43,377 | 3.04 | |
| Academic Achievement | |||||
| Failing Grades | 1973–1992 | 1,767,091 | 264132 | 14.95 | |
| Education under 10 yrs | 1973–1991 | 1,679,921 | 484799 | 28.86 | |
| Higher Education | 1973–1983 | 906,458 | 288374 | 31.81 | |
| Low IQ (males) | 1973–1992 | 641,020 | 62646 | 9.77 | |
Note.
n = number of identified cases; % = percentage of cases,
M = mean; SD = standard deviation,
Kaplan-Meier estimate by the age of 15 years,
Kaplan-Meier estimate by 30 years of age.
Ordinal paternal age
An ordered categorical representation of APA at childbearing was used as the main explanatory variable in our statistical models. Consistent with previous research,18 we grouped APA for each offspring into seven categories ranging from 20 years or younger to 45 years or older in five-year intervals with 20–24 years as the reference group.
Offspring Outcomes
A total of ten offspring outcomes from two different outcome domains were analyzed in this study. The six indices of psychiatric morbidity, which have been shown to be valid in previous studies, were: (1) Autism spectrum disorder33 and (2) Attention-deficit/hyperactivity disorder (ADHD)34 were identified using inpatient and outpatient diagnoses according to ICD -9 and ICD-10; (3) Psychosis was measured as the age of first inpatient hospitalization for schizophrenia or other non-organic (i.e., affective) psychotic disorders according to ICD-8, -9, and -10 criteria35, (4) Bipolar Disorder was measured as first hospitalization using ICD criteria35; (5) Suicide Attempt was identified as age of first attempt using the ICD codes for any primary or secondary diagnosis for individuals 12 years of age or older in the Patient Register36; and (6) Substance use problem was defined as first inpatient hospitalization involving a primary or secondary diagnosis of alcohol- or any other, non-nicotine, substance use disorder for individuals 12 years of age or older.37,38
The four dichotomous outcomes indexing academic achievement (i.e., cognitive ability) were: (1) Failing grades was measured as poor school performance as assessed in Grade 9 using standardized numerical scales, which is commensurate with receiving an overall failing grade across 16 academic subjects39,40; (2) Education under 10 years compared offspring with a low level of academic achievement (i.e., no more than 9 years of primary and secondary education) to those with higher levels of achievement (i.e., completed 10 years or more of education); (3) Higher education indexed individuals who completed at least 3 years or more of postsecondary education; and (4) Low IQ (male conscripts only) indexed general intellectual performance including logical-inductive reasoning, verbal ability, visual-spatial perception, as well as theoretical-technical skills. Offspring in the low IQ group were assessed with stanine scores within the lowest 10th percentile.
Covariates
Measured offspring covariates were sex, birth parity (categorized into second, third, or fourth born or higher with first born as the reference group), and year of birth. Measured maternal and paternal covariates included indices of Swedish nationality, highest level of completed education (categorized into five levels), lifetime history of psychiatric hospitalization, and lifetime history of any criminal conviction. Additional covariates included maternal age at childbearing (ordinal and grouped into the same seven bins as APA) and paternal disposable household-level income in the proband birth year (categorized into quintiles to control for inflation and other time-varying socio-economic events with the lowest, i.e., 0–20th percentile serving as the reference class). Correlations between APA and the covariates can be found in Supplemental Appendix B.
Statistical Analyses
We used Cox proportional-hazards regressions to estimate hazard ratios associated with APA for the right-censored psychiatric outcomes; we used information on the date of death or the end of the assessment period (2009) to calculate age at censoring. We used logistic regression to estimate odds ratios for the dichotomous academic achievement outcomes. All of the analyses controlled for offspring gender and birth order while using robust (“sandwich”) standard errors to account for the clustered nature of the data. We fitted three main models. First, we used a baseline model to estimate the crude associations in the population. The logistic models additionally controlled for proband birth year. Second, the adjusted model included all of the covariates. Note that only ASD and ADHD were adjusted by paternal income because valid information regarding disposable income was not available for birth years earlier than 1992. Third, the sibling fixed-effects model (a stratified Cox or conditional logistic regression model)41 accounted for all genetic and environmental factors shared among paternal siblings by controlling for unmeasured cluster-level covariates. The model also included offspring-specific and maternal measured covariates. The fixed effects model extends the Cox and logistic models by stratifying (conditioning) on the set of siblings, adjusting for all of factors that are shared within each sibling set.
We also ran several sensitivity analyses using various designs, including cousin- and first-born cousin comparisons, to test the robustness of the results.
Results
Main Analyses
The panels in Figure 1 present the results by outcome. In line with previous studies, APA was associated with greater risk for ASD (baseline model), with the association being relatively unaffected by adjustment for measured covariates (adjusted model). For example, after adjustment for the covariates, offspring born to fathers older than 45 years had an increased risk of having a diagnosis of ASD of about 75% (Hazard Ratio [HR]=1.76, 95% Confidence Interval [CI]=1.36–2.28) compared to fathers 20–25 years old (Panel A). In contrast, the fixed effects sibling model suggests that APA was even more strongly associated with autism in the offspring; when accounting for all factors shared by siblings and several measured covariates, parental age 45 years+ was associated with a 3.5 time increased risk of offspring ASD (HR=3.45, CI=1.62–7.33).
Figure 1. Baseline, Adjusted, and Fixed Effects Associations between Paternal Age at Childbearing and Offspring Psychiatric and Academic Morbidity.
Panels A–G present the point estimates (with 95% Confidence Intervals presented with the error bars) of the association between the paternal age at childbearing and each index of offspring morbidity using paternal age 20–24 years old as the reference category. The x-axis presents the ordinal bins of paternal age at childbearing. The y-axis presents effect sizes, either Hazard Ratios or Odds Ratios, which quantify the magnitude of the associations. The effect size estimates are depicted at the median age within each bin of APA. The blue estimates provide the baseline relations, the associations in the population. The green estimates provide the adjusted relations, the associations when statistically controlling for statistical covariates, which is what is standard in ordinary epidemiological studies of APA. The red estimates present the fixed effects relations, the association when accounting for all factors shared by siblings and measured covariates. The final estimates, therefore, provide the estimates when comparing differentially exposed siblings and statistically controlling for measured covariates. Because these estimates account for both measured and unmeasured covariates, these results are thought to be closer to the potential true causal effect.
Similar patterns of associations were observed for the other indices of psychiatric and academic morbidity (Figure 1, panel B–G) with stronger effects of APA when accounting for all factors shared by siblings. For example, in the fixed-effects sibling models APA (45 years+) was associated with increased risk of ADHD (HR=13.13, CI=6.85–25.16), psychosis (HR=2.07, CI=1.35–3.20), bipolar disorder (HR=24.70, CI=12.12–50.31), suicide attempts (HR=2.72, CI=2.08–3.56), substance use problems (HR=2.44, CI=1.98–2.99), failing a grade (OR=1.59, CI=1.37–1.85), and low educational attainment (OR=1.70, CI=1.50–1.93) compared to offspring born to fathers 20–25 years old.
Sensitivity Analyses
Because of the need to examine the robustness of the findings and test the limitations and assumptions inherent in sibling-comparisons25 we ran a series of sensitivity analyses. First, we fitted models predicting completion of higher education (in the full sample) and IQ (in males) to examine whether the results would be commensurate with the main analyses predicting academic morbidity in the main text (Supplemental Appendix C). Second, we fitted fixed effect cousin models (i.e., comparing differentially exposed cousins) to examine the generalizability of the sibling-comparison results (Supplemental Appendix D, panels D). Third, we compared differentially exposed first-born cousins to account for birth order effects (in addition to statistically controlling for birth-order in all of the main analyses) and the possibility that ‘stoppage’2 would explain the sibling-comparison results (Supplemental Appendix D, panels E). Fourth, we fitted models predicting ASD and ADHD by the age of 8 years old to assure all siblings in a family lived through the same risk period (Supplemental Appendix E). The models also controlled for year of birth to account for changing diagnostic practices and prevalence, which could confound the results.42 Fifth, we fitted models that controlled for paternal age at first childbearing while estimating the association between focal age of childbearing and the outcomes for all second- and later-born offspring (Supplemental Appendix F). This explored the possibility that carry-over effects could confound the sibling-comparison estimates and provided the opportunity to directly compare the results with previous studies of APA that used this analytical approach.18 Sixth, we fitted models predicting continuous indices of grades and IQ (in males) to examine the results were comparable to the dichotomous indices used in the main analyses (Supplemental Appendix G). Seventh, we examined whether the results from the baseline models were comparable in offspring with and without siblings to further test the generalizability of the sibling-comparison results (Supplemental Appendix H).25,32 The sensitivity analyses as a whole provide commensurate results to the main analyses and provide support for stronger causal inferences. Finally, the parameter estimates associated with maternal age at childbearing in the adjust models are presented in Supplemental Appendix I.
Discussion
The current study showed clear associations between APA and indices of psychiatric morbidity (e.g., ASD and psychosis), in line with most previous studies that have used ordinary comparisons of unrelated individuals.1–7 Yet, we have extended the previous literature in several critical ways.
First, we analyzed the largest study of APA to date, with coverage across an entire country and valid indices of offspring morbidity. This enabled us to more precisely estimate the risks for rare indices of psychiatric and academic morbidity.
Second, the results suggest that unmeasured genetic and environmental selection factors shared by siblings, as well as the influence of several measured covariates, do not account for the associations between APA and offspring morbidity, which is consistent with a causal hypothesis. Researchers have suggested the association between APA and offspring morbidity is not causal and that the associations could be better explained by selection factors;2,17–19 the extant sibling-comparison studies were, in fact, inconsistent.2,18,19,28 The current study, however, found robust within-sibling associations. The inconsistent findings from sibling-comparison studies of schizophrenia may be due to the differences in ages of the samples (e.g., a previous study was based on offspring born 1955–1992)18 and different diagnostic criteria (i.e. we used a broad definition of psychosis).
Third, although APA has been associated with severe psychiatric morbidity, relatively few studies have explored other indices of morbidity. The current study also explored offspring ADHD, bipolar disorder, suicidal behavior, substance use problems, and academic problems.8,9 As such, the findings suggest APA is also associated with morbidity across several psychiatric disorders and developmental domains.
Fourth, and perhaps most importantly, the current study suggests that the specific risks associated with APA follow a dose-response relationships (i.e., the increased risk was not solely present in extremely advanced paternal age) and that the magnitude of most of the associations were stronger than previous estimates. In fact, the magnitudes of the associations with ADHD and bipolar disorder, in particular, were quite large in the sibling-comparisons. Furthermore, the direction of the associations with many outcomes (e.g., suicide, substance, and academic problems) went in the opposite direction after controlling for familial confounding. These finding indicates that the factors shared by siblings that are correlated with APA captures “beneficial” factors (e.g., increased maturity,20 conscientiousness,21 and social and cultural capital22) that suppress the specific negative effects of APA on psychiatric and academic morbidity. Future research will need to specify these familial factors. The finding, however, suggest that APA is a greater risk factor for psychiatric morbidity than has been previously reported. It is important to note that we replicated the findings using several advanced family-based quasi-experimental designs, each with their own strengths, limitations, and assumptions,25 which further strengthens both the internal and external validity of the findings.23,31
The sibling-comparison results are consistent with the hypothesis that genetic mutations during spermatogenesis associated with APA influence offspring morbidity across numerous indices of morbidity.14 If de novo point mutations are important genetic risk factors then molecular genetic studies should involve exome sequencing from both affected individuals and parents; relying on genome-wide association studies would not identify such genetic factors. It is important to note that environmental factors specifically associated with APA also could account for the findings. Early paternal age also was associated with increased risk for some offspring problems, notably the educational outcomes, but not others.
The findings must be considered in light of several limitations and assumptions. Sibling-comparison studies include several assumptions. Sibling-comparisons are sensitive to (a) unreliable measurement of the risk factor, (b) carry-over effects from one sibling to another, (c) sibling-contagion effects, (d) concerns about the non-generalizability of differentially exposed siblings, and (e) birth-order effects.25–27,29,30 We believe that unreliability is a minor issue, given APA is based on official records, and we addressed many of the other assumptions in the extensive sensitivity analyses (e.g., comparison of first-born cousins). Nevertheless, observational studies can never prove causality; the sibling-comparisons could not rule out within-family confounds associated with APA and the outcomes. Randomized control trials are necessary for causal conclusions. Because of the inability to conduct such studies of APA, causal inferences must be made from commensurate results using different designs and samples.31 Together with studies exploring grandparental age43 and the association between APA and criminality,44 the current results, which are based on several family-based quasi-experimental designs, provide strong evidence that APA has a specific influence on offspring morbidity.
In sum, the findings suggest APA represents a risk for numerous public health and societal problems. Regardless of whether these results should lead to policy changes, clarification of the associations with APA would inform future basic neuroscience research, medical practice, and personal decision-making about childbearing.45,46
Supplementary Material
Acknowledgements
All authors contributed extensively to the work presented in this paper. B.M.D., M.E.R., R.K-H., E.F., and P.L. helped design the methods and analytical strategy. M.E.R. completed all of the analyses. B.M.D. and P.L. secured funding for the merging of the registries and database management of the project. C.A., H.L., and P.L. provided oversight for the database management of the project. B.M.D. prepared the initial draft of the manuscripts, and all authors commented on the manuscript at all stages, including the interpretation and implications of the findings.
The manuscript was supported by grants from the National Institute of Child Health and Human Development (HD061817), the Swedish Research Council (Medicine), and the Swedish Council for Working Life and Social Research. The funding agencies provided support for merging the registries, database management, analyses, and manuscript preparation. The funding agencies played no role in the design and conduct of the analyses or the interpretation of the data.
Footnotes
Dr. Martin Rickert had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors declare no competing financial interests.
Neither this manuscript nor one with substantially similar content under their authorship has been published or is being considered for publication elsewhere.
Preliminary results for autism were presented at the 2013 Society for Research in Child Development Conference in Seattle, WA (April 2013) by B.M.D.
Supplementary information can be found in the Supplemental Appendices.
References
- 1.Reichenberg A, et al. Advancing paternal age and autism. Arch. Gen. Psychiatry. 2006;63:1026–1032. doi: 10.1001/archpsyc.63.9.1026. [DOI] [PubMed] [Google Scholar]
- 2.Hultman CM, Sandin S, Levine SZ, Lichtenstein P, Reichenberg A. Advancing paternal age and risk of autism: new evidence from a population-based study and a meta-analysis of epidemiological studies. Mol. Psychiatry. 2011;16:1203–1212. doi: 10.1038/mp.2010.121. [DOI] [PubMed] [Google Scholar]
- 3.Lundstrom S, et al. Trajectories leading to autism spectrum disorders are affected by paternal age: findings from two nationally representative twin studies. J Child Psychol Psychiatry. 2010;51:850–856. doi: 10.1111/j.1469-7610.2010.02223.x. [DOI] [PubMed] [Google Scholar]
- 4.Miller B, et al. Meta-analysis of Paternal Age and Schizophrenia Risk in Male Versus Female Offspring. Schizophr. Bull. 2011;37:1039–1047. doi: 10.1093/schbul/sbq011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malaspina D, et al. Advancing paternal age and the risk of schizophrenia. Arch. Gen. Psychiatry. 2001;58:361–367. doi: 10.1001/archpsyc.58.4.361. [DOI] [PubMed] [Google Scholar]
- 6.Frans EM, et al. Advancing Paternal Age and Bipolar Disorder. Archives of General Psychiatry. 2008;65:1034–1040. doi: 10.1001/archpsyc.65.9.1034. [DOI] [PubMed] [Google Scholar]
- 7.Menezes PR, et al. Paternal and maternal ages at conception and risk of bipolar affective disorder in their offspring. Psychological Medicine. 2010;40:477–485. doi: 10.1017/S003329170999064X. [DOI] [PubMed] [Google Scholar]
- 8.Saha S, et al. Advanced Paternal Age Is Associated with Impaired Neurocognitive Outcomes during Infancy and Childhood. PLoS Med. 2009;6:e1000040. doi: 10.1371/journal.pmed.1000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Svensson AC, Abel K, Dalman C, Magnusson C. Implications of Advancing Paternal Age: Does It Affect Offspring School Performance? PLOS One. 2011;6:e24771. doi: 10.1371/journal.pone.0024771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kong A, et al. Rate of de novo mutations and the importance of father's age to disease risk. Nature. 2012;488:471–475. doi: 10.1038/nature11396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neale BM, et al. Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature. 2012;485:242–245. doi: 10.1038/nature11011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Roak BJ, et al. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature. 2012;485:246–250. doi: 10.1038/nature10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanders SJ, et al. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature. 2012;485:237–241. doi: 10.1038/nature10945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veltman JA, Brunner HG. De novo mutations in human genetic disease. Nature Reviews Genetics. 2012;13:565–575. doi: 10.1038/nrg3241. [DOI] [PubMed] [Google Scholar]
- 15.Academy of Medical Sciences Working Group. Identifying the environmental causes of disease: How should we decide what to believe and when to take action? Academy of Medical Sciences; 2007. [Google Scholar]
- 16.Thapar A, Rutter M. Do prenatal risk factors cause psychiatric disorder? Be wary of causal claims. British Journal of Psychiatry. 2009;195:100–101. doi: 10.1192/bjp.bp.109.062828. [DOI] [PubMed] [Google Scholar]
- 17.Puleo CM, Reichenberg A, Smith CJ, Kryzak LA, Silverman JM. Do autism-related personality traits explain higher paternal age in autism? Mol. Psychiatry. 2008;13:243–244. doi: 10.1038/sj.mp.4002102. [DOI] [PubMed] [Google Scholar]
- 18.Petersen L, Mortensen PB, Pedersen CB. Paternal age at birth of first child and risk of schizophrenia. American Journal of Psychiatry. 2011;168:82–88. doi: 10.1176/appi.ajp.2010.10020252. [DOI] [PubMed] [Google Scholar]
- 19.Granville-Grossman KL. Paternal age and schizophrenia. British Journal of Psychiatry. 1966;112:899–905. doi: 10.1192/bjp.112.490.899. [DOI] [PubMed] [Google Scholar]
- 20.Roberts BW, Caspi A, Moffitt TE. The kids are alright: Growth and stability in personality development from adolescence to adulthood. Journal of Personality and Social Psychology. 2001;81:670–683. [PubMed] [Google Scholar]
- 21.Roberts BW, Walton KE, Viechtbauer W. Patterns of mean-level change in personality traits across the life course: A meta-analysis of longitudinal studies. Psychological Bulletin. 2006;132:1–25. doi: 10.1037/0033-2909.132.1.1. [DOI] [PubMed] [Google Scholar]
- 22.Powell B, Steelman LC, Carini RM. Advancing Age, Advantaged Youth: Parental Age and the Transmission of Resources to Children. Soc. Forces. 2006;84:1359–1390. [Google Scholar]
- 23.Shadish WR, Cook TD, Campbell DT. Experimental and quasi-experimental designs for generalized causal inference. Houghton Mifflin: 2002. [Google Scholar]
- 24.Rutter M. Proceeding from observed correlation to causal inference: The use of natural experiments. Perspectives on Psychological Science. 2007;2:377–395. doi: 10.1111/j.1745-6916.2007.00050.x. [DOI] [PubMed] [Google Scholar]
- 25.D’Onofrio BM, Lahey BB, Turkheimer E, Lichtenstein P. The critical need for family-based, quasi-experimental research in integrating genetic and social science research. American Journal of Public Health. 2013;103:S46–S55. doi: 10.2105/AJPH.2013.301252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lahey BB, D’Onofrio BM. All in the family: Comparing siblings to test causal hypotheses regarding environmental influences on behavior. Current Directions in Psychological Science. 2010;19:319–323. doi: 10.1177/0963721410383977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donovan SJ, Susser E. Commentary: Advent of sibling designs. International Journal of Epidemiology. 2011;40:345–349. doi: 10.1093/ije/dyr057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parner ET, et al. Parental Age and Autism Spectrum Disorders. Ann. Epidemiol. 2012;22:143–150. doi: 10.1016/j.annepidem.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Susser E, Eide MG, Begg M. Invited Commentary: The Use of Sibship Studies to Detect Familial Confounding. Am. J. Epidemiol. 2010;172:537–539. doi: 10.1093/aje/kwq196. [DOI] [PubMed] [Google Scholar]
- 30.Frisell T, Oberg S, Kuja-Halkola R, Sjolander A. Sibling comparison designs: Bias from non-shared confounders and measurement error. Epidemiology. 2012;23:713–720. doi: 10.1097/EDE.0b013e31825fa230. [DOI] [PubMed] [Google Scholar]
- 31.Rutter M, Pickles A, Murray R, Eaves LJ. Testing hypotheses on specific environmental causal effects on behavior. Psychological Bulletin. 2001;127:291–324. doi: 10.1037/0033-2909.127.3.291. [DOI] [PubMed] [Google Scholar]
- 32.D'Onofrio BM, et al. Preterm birth and mortality and morbidity: A population-based quasi-experimental study. JAMA Psychiatry. 2013 doi: 10.1001/jamapsychiatry.2013.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Idring S, et al. Autism Spectrum Disorders in the Stockholm Youth Cohort: Design, Prevalence and Validity. PLOS One. 2012;7:e41280. doi: 10.1371/journal.pone.0041280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larsson H, et al. Does attention deficit hyperactivity disorder share etiologic factors with bipolar disorder and schizophrenia? British Journal of Psychiatry. (in press). [Google Scholar]
- 35.Lichtenstein P, et al. Common genetic influences for schizophrenia and bipolar disorder: A population-based study of 2 million nuclear families. Lancet. 2009;373:234–239. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tidemalm D, Långström N, Lichtenstein P, Runeson B. Risk of suicide after suicide attempt according to coexisting psychiatric disorder: Swedish cohort study with long term follow-up. BMJ. 2008;337 doi: 10.1136/bmj.a2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.D’Onofrio BM, et al. Familial confounding of the associations between maternal smoking during pregnancy and offspring substance use problems: Converging evidence across samples and measures. Archives of General Psychiatry. 2012;69:1140–1150. doi: 10.1001/archgenpsychiatry.2011.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kendler KS, Sundquist K, Ohlsson H, et al. Genetic and familial environmental influences on the risk for drug abuse: A national swedish adoption study. Archives of General Psychiatry. 2012;69:690–697. doi: 10.1001/archgenpsychiatry.2011.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.D'Onofrio BM, et al. A quasi-experimental study of maternal smoking during pregnancy and offspring academic achievement. Child Development. 2010;81:80–100. doi: 10.1111/j.1467-8624.2009.01382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lambe M, Hultman C, Torrang A, MacCabe J, Cnattingius S. Maternal smoking during pregnancy and school performance at age 15. Epidemiology. 2006;17:524–530. doi: 10.1097/01.ede.0000231561.49208.be. [DOI] [PubMed] [Google Scholar]
- 41.Allison PD. Fixed effects regression models. Sage; 2009. [Google Scholar]
- 42.King MD, Fountain C, Dakhlallah D, Bearman PS. Estimated Autism risk and older reproductive age. American Journal of Public Health. 2009;99:1673–1679. doi: 10.2105/AJPH.2008.149021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frans Em SSRA, et al. Autism risk across generations: A population-based study of advancing grandpaternal and paternal age. JAMA Psychiatry. 2013;70:516–521. doi: 10.1001/jamapsychiatry.2013.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuja-Halkola R, Pawitan Y, D'Onofrio BM, Langstrom N, Lichtenstein P. Advancing paternal age and offspring violent offending: A sibling-comparison study. Development and Psychopathology. 2012;24:739–753. doi: 10.1017/S095457941200034X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toriello HV, Meck JM. Statement on guidance for genetic counseling in advanced paternal age. Genet Med. 2008;10:457–460. doi: 10.1097/GIM.0b013e318176fabb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lewis BH, Legato M, Fisch H. Medical implications of the male biological clock. JAMA: The Journal of the American Medical Association. 2006;296:2369–2371. doi: 10.1001/jama.296.19.2369. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.