Abstract
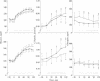
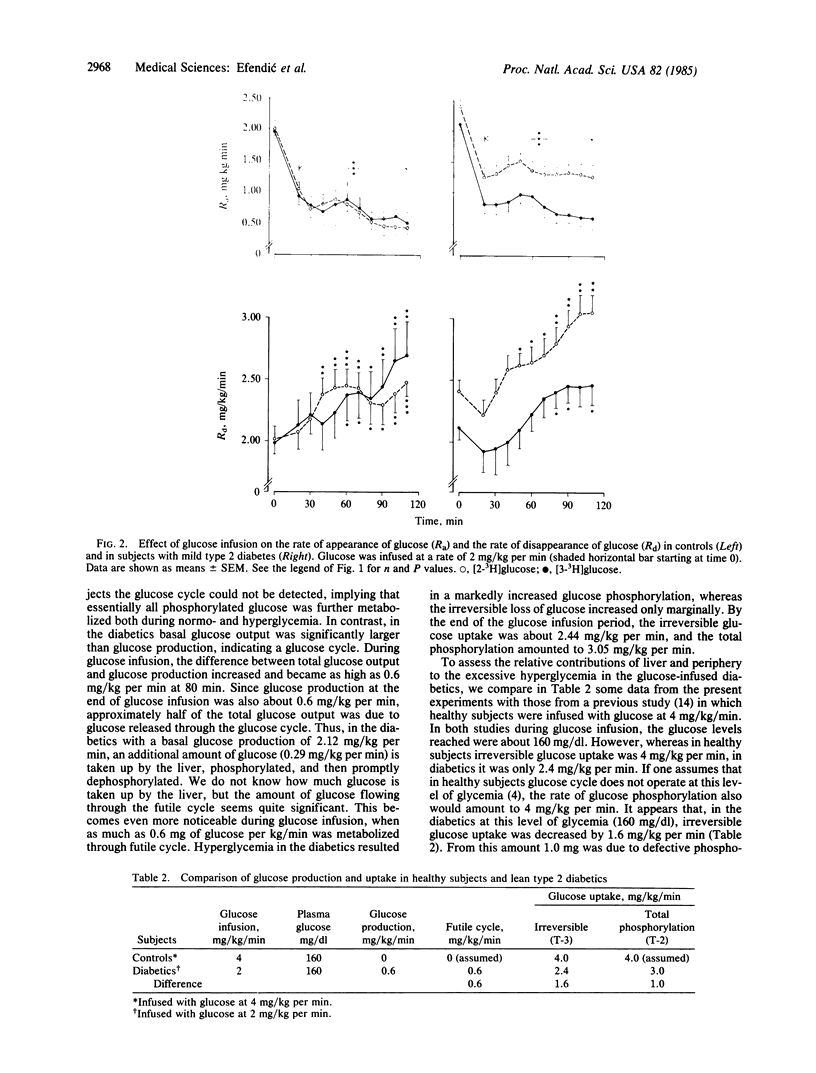
The aims were to assess in the mild, lean, type 2 diabetics the activity of the hepatic futile cycle (glucose cycling) in the basal state and during an infusion of glucose and the overall contribution of futile cycling and the relative contributions of the liver and the periphery to excessive hyperglycemia during a glucose challenge. To determine hepatic futile cycling, we studied seven healthy controls (C) and eight mild, lean, type 2 diabetics with decreased oral glucose tolerance test and blood glucose of 123 +/- 4 mg/dl. Experiments included an equilibration period, followed by a 2-hr infusion of glucose at 2 mg/kg of body weight per min. In each subject, two such experiments were performed randomly with infusions of [2-3H]glucose or [3-3H]glucose to calculate, respectively, total glucose output or total glucose phosphorylation and glucose production or irreversible glucose loss. Futile cycling equals the difference between glucose turnover measured by the two tracers. In controls basal glucose production was 2.0 +/- 0.09 mg/kg per min, and it decreased by 75% during glucose infusion; futile cycling could not be detected. Plasma glucose increased by 30% and plasma C-peptide by 88%. In the diabetics total glucose output (2.41 +/- 0.17 mg/kg per min) was larger than glucose production (2.12 +/- 0.16 mg/kg per min), indicating a glucose cycle. During the glucose infusion, glucose production in the diabetics as well as in the controls decreased by 75% (to 0.6 mg/kg per min) despite higher than normal plasma glucose and C-peptide; futile cycling amounted to 0.6 mg/kg per min, which is half of the total glucose output; increase of glucose uptake was essentially only due to phosphorylation of glucose because irreversible uptake increased only marginally; and most glucose taken up by the liver during the glucose challenge reenters the blood stream without being oxidized or polymerized. These findings, when compared to our previous work in which controls were infused with glucose at 4 mg/kg per min, indicate that excessive hyperglycemia in the diabetics during glucose infusion is due to a decrease in irreversible glucose uptake (impaired phosphorylation and futile cycling) and to a decrease in suppression of glucose production. The relative contributions of the liver and periphery to hyperglycemia seem to be almost equivalent. The mechanism behind the increased glucose cycle activity is not clear; it may be due to a relative decrease of glycogen synthase or increase in glucose-6-phosphatase or both.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altszuler N., Barkai A., Bjerknes C., Gottlieb B., Steele R. Glucose turnover values in the dog obtained with various species of labeled glucose. Am J Physiol. 1975 Dec;229(6):1662–1667. doi: 10.1152/ajplegacy.1975.229.6.1662. [DOI] [PubMed] [Google Scholar]
- Bergman R. N. Integrated control of hepatic glucose metabolism. Fed Proc. 1977 Feb;36(2):265–270. [PubMed] [Google Scholar]
- Cowan J. S., Hetenyi G., Jr Glucoregulatory responses in normal and diabetic dogs recorded by a new tracer method. Metabolism. 1971 Apr;20(4):360–372. doi: 10.1016/0026-0495(71)90098-9. [DOI] [PubMed] [Google Scholar]
- Dunn A., Katz J., Golden S., Chenoweth M. Estimation of glucose turnover and recycling in rabbits using various [3H, 14C]glucose labels. Am J Physiol. 1976 Apr;230(4):1159–1162. doi: 10.1152/ajplegacy.1976.230.4.1159. [DOI] [PubMed] [Google Scholar]
- Efendić S., Wajngot A., Cerasi E., Luft R. Insulin release, insulin sensitivity, and glucose intolerance. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7425–7429. doi: 10.1073/pnas.77.12.7425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Refai M., Bergman R. N. Simulation study of control of hepatic glycogen synthesis by glucose and insulin. Am J Physiol. 1976 Nov;231(5 Pt 1):1608–1619. doi: 10.1152/ajplegacy.1976.231.5.1608. [DOI] [PubMed] [Google Scholar]
- Fisher C. J., Stetten M. R. Parallel changes in vivo in microsomal inorganic pyrophosphatase, pyrophosphate-glucose phosphotransferase and glucose 6-phosphatase activities. Biochim Biophys Acta. 1966 May 26;121(1):102–109. doi: 10.1016/0304-4165(66)90352-7. [DOI] [PubMed] [Google Scholar]
- HUGGETT A. S., NIXON D. A. Use of glucose oxidase, peroxidase, and O-dianisidine in determination of blood and urinary glucose. Lancet. 1957 Aug 24;273(6991):368–370. doi: 10.1016/s0140-6736(57)92595-3. [DOI] [PubMed] [Google Scholar]
- Hanson T. L., Nordlie R. C. Liver microsomal inorganic pyrophosphate-glucose phosphotransferase and glucose-6-phosphatase. Effects of diabetes and insulin administration in kinetic parameters. Biochim Biophys Acta. 1970 Jan 14;198(1):66–75. doi: 10.1016/0005-2744(70)90033-1. [DOI] [PubMed] [Google Scholar]
- Hers H. G. The control of glycogen metabolism in the liver. Annu Rev Biochem. 1976;45:167–189. doi: 10.1146/annurev.bi.45.070176.001123. [DOI] [PubMed] [Google Scholar]
- Issekutz B., Jr Studies on hepatic glucose cycles in normal and methylprednisolone-treated dogs. Metabolism. 1977 Feb;26(2):157–170. doi: 10.1016/0026-0495(77)90051-8. [DOI] [PubMed] [Google Scholar]
- Jakobsson S. V., Dallner G. Nature of the increase in liver microsomal glucose-6-phosphatase activity during the early stages of alloxan-induced diabetes. Biochim Biophys Acta. 1968 Oct 15;165(3):380–392. [PubMed] [Google Scholar]
- Katz J., Dunn A. Glucose-2-t as a tracer for glucose metabolism. Biochemistry. 1967 Jan;6(1):1–5. doi: 10.1021/bi00853a001. [DOI] [PubMed] [Google Scholar]
- Katz J., Rognstad R. Futile cycles in the metabolism of glucose. Curr Top Cell Regul. 1976;10:237–289. doi: 10.1016/b978-0-12-152810-2.50013-9. [DOI] [PubMed] [Google Scholar]
- Newgard C. B., Foster D. W., McGarry J. D. Evidence for suppression of hepatic glucose-6-phosphatase with carbohydrate feeding. Diabetes. 1984 Feb;33(2):192–195. doi: 10.2337/diab.33.2.192. [DOI] [PubMed] [Google Scholar]
- Nordlie R. C., Sukalski K. A., Alvares F. L. Responses of glucose 6-phosphate levels to varied glucose loads in the isolated perfused rat liver. J Biol Chem. 1980 Mar 10;255(5):1834–1838. [PubMed] [Google Scholar]
- Wajngot A., Roovete A., Vranić M., Luft R., Efendić S. Insulin resistance and decreased insulin response to glucose in lean type 2 diabetics. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4432–4436. doi: 10.1073/pnas.79.14.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]