Abstract
We have recently demonstrated that the combination of gemcitabine and a superoxide dismutase mimetic protects mice against lung cancer by suppressing the functions of myeloid-derived suppressor cells and by activating memory CD8+ T-cell responses. Persistent memory cells exhibited a glycolytic metabolism, which may have directly enhanced their effector functions. This combinatorial therapeutic regimen may reduce the propensity of some cancer patients to relapse.
Keywords: antitumor immunity, combination therapy, lung cancer, memory response, metabolism, myeloid-derived suppressor cells, oxidative stress
The immunosuppressive activity exerted by myeloid-derived suppressor cells (MDSCs) in both pre-clinical tumor models and cancer patients has been intensively characterized.1 In lung cancer, the levels of circulating MDSCs correlate positively with disease progression and immunosuppression.2,3 Despite consistent progresses in front line therapies, lung neoplasms reign as the leading cause of cancer-related deaths.4,5 In this setting, a major contribution to the growth of primary neoplastic lesions as well as to the relapse of therapy-resistant tumors is provided by the inhibition of host antitumor immune functions, resulting in reduced overall survival. Our goal was to develop a multipronged approach that would reactivate antitumor immune responses to limit tumor growth and extend the survival of lung cancer patients.
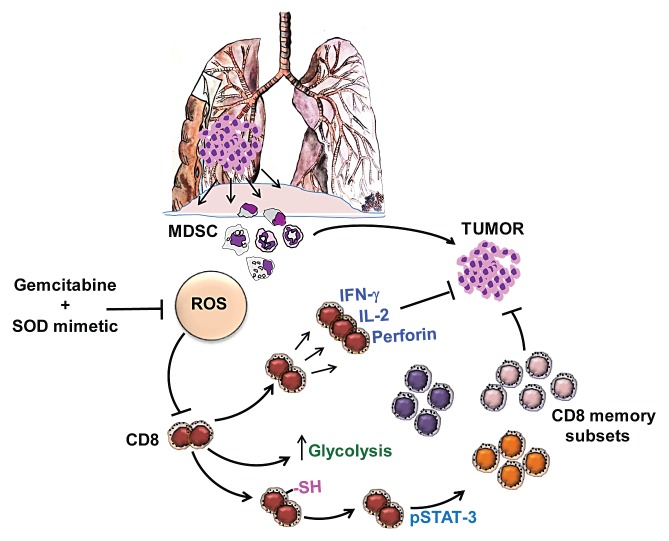
To this aim, we combined gemcitabine, a widely-employed chemotherapeutic agent for lung cancer that depletes MDSCs, with a superoxide dismutase (SOD) mimetic, which inhibits the production of reactive oxygen species (ROS) in the tumor microenvironment (TME). Elevated ROS levels promote indeed a state of oxidative stress that favors tumor progression. Using a syngeneic, immunocompetent murine model, we convincingly demonstrated that this combination therapy effectively reduces the growth of lung cancers by inhibiting the immunosuppressive activity of MDSCs, depleting tumor-infiltrating regulatory T cells (Tregs) and by limiting ROS levels in the TME. Both quantitative and qualitative features of tumor-specific memory CD8+ T-cell responses, including their polyfunctionality and cytotoxic potential, were enhanced by our combinatorial regimen.6 We demonstrated that the activation of signal transducer and activator of transcription 3 (STAT3) and metabolic alterations contributed to such an enhancement. Specifically, we observed increased central memory T (TCM)-, effector memory T (TEM)- and stem cell memory T (TSCM)-cell responses in tumor-bearing mice that received the combination therapy (Fig. 1). In this setting, the adoptive transfer of both TCM and TEM cells increased the survival of tumor-bearing mice by nearly 80%. Most importantly, these cells conferred long-term protection to mice against a re-challenge with living cancer cells of the same type.
Figure 1. Enhancing immune responses against lung cancer by targeting myeloid-derived suppressor cells. The combination of gemcitabine and a superoxide dismutase mimetic inhibits the activity of myeloid-derived suppressor cells (MDSCs), modulates redox signaling and enhances the quantity, quality and persistence of memory CD8+ T cells.
Our combinatorial therapeutic approach has the potential to improve the persistence and function of T cells while inhibiting the immunosuppressive activity of MDSCs, Tregs, and tumor-associated neutrophils (TANs), resulting in robust and protracted antitumor immune responses. This treatment can be extended to malignancies including breast carcinoma, multiple myeloma and ovarian cancer, in all of which the influx of MDSCs is associated with the suppression of antitumor immune responses and disease progression. SOD mimetics are currently being used for the treatment of patients with metastatic renal carcinoma and melanoma. In both these settings, SOD mimetics have been shown to enhance antitumor immunity while reducing the side effects of immunotherapy.7 We strongly believe that combining SOD mimetics with gemcitabine, which is also used to treat lung cancer patients, will significantly reduce tumor burden while protecting these individuals from disease recurrence.
One of the highlights of our study is the ability of gemcitabine combined with a SOD mimetic to improve the quantity and quality of tumor-targeting memory CD8+ T-cell responses. Preclinical studies based on the adoptive transfer of purified CD8+ T-cell populations, including ours, have revealed that less-differentiated cells including TSCM and TCM lymphocytes can mediate superior antitumor responses as compared with more-differentiated memory cells such as TE lymphocytes. Presumably, this is due to the increased ability of the former 2 T-cell populations to persist and proliferate in vivo. Hence, there has been great interest in understanding the molecular mechanisms that govern the generation of long-lived memory T-cell subsets for developing potent immunotherapies against cancer and infectious diseases. We noted that the TCM and TEM cells isolated from mice treated with our combinatorial regimen are metabolically distinct (notably, more dependent on glycolysis) than those obtained from mice treated with gemcitabine or a SOD mimic alone. Gubser et al. have recently reported that an early glycolytic switch in memory CD8+ T cells enables the rapid acquisition of effector functions during recall responses.8 Such a glycolytic metabolism may also increase the lifespan of memory T cells by limiting oxidative stress. Since memory T-cell subsets migrate and occupy various microenvironmental niches, a glycolytic metabolism may provide them with an improved ability to adapt to changes in nutrient availability and local conditions like oxidative stress. Memory CD8+ T cells have a bioenergetic advantage that endows them with a rapid recall potential.9 Sukumar et al. have recently demonstrated that an increased glycolytic flux enhances the cytolytic functions of TCM and TEM cells, including their ability to secrete perforin and granzyme B.10 In our model, we obtained similar effects by treating tumor-bearing mice with gemcitabine and a SOD mimetic. Alterations in the redox potential of the TME might contribute to the preservation of an optimal glycolytic metabolism in memory T cells. ROS are known to play a role in activation-induced cell death. As SOD mimetics dramatically reduce ROS levels, they may allow for the improved proliferation of activated T cells. Gemcitabine, while depleting MDSCs, also limits the levels of ROS in the TME. Hence, it is possible that memory cells from mice treated with our combinatorial therapy exhibit enhanced antitumor properties, a possibility that will be investigated in the near future.
Memory T-cell populations obtained from tumor-bearing mice subjected to our combinatorial therapy also conferred protection against a re-challenge with cancer cells of the same type. Adoptively transferred memory T cells efficiently proliferated upon re-encountering cancer cells, and in this setting immunosuppression was reduced in the absence of gemcitabine and SOD mimetic. As mentioned earlier, lung cancer has a high relapse rate, mostly due to chemoresistance. The use of novel combinatorial therapies such as the one we have commented herein might result in optimal antineoplastic effects by directly inhibiting tumor growth while exerting an immunostimulatory activity. Tumor-targeting memory T-cell responses developing in cancer patients may keep neoplasms under remission and hence limit the rate of relapse. In summary, the treatment regimen developed by our research group might perhaps be employed as an adjuvant to current treatment options for the treatment of lung cancer patients.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Glossary
Abbreviations:
- MDSC
myeloid-derived suppressor cell
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- STAT3
signal transducer and activator of transcription 3
- TAN
tumor-associated neutrophils
- TCM
central memory T cell
- TEM
effector memory T cell
- TME
tumor microenvironment
- Treg
regulatory T cell
- TSCM
stem cell memory T cell
Citation: Sawant A, Schafer CC, Ponnazhagan S, Deshane JS. The dual targeting of immunosuppressive cells and oxidants promotes effector and memory T-cell functions against lung cancer. OncoImmunology 2013; 2:e27401; 10.4161/onci.27401
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/27401
References
- 1.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–68. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu T, Ramakrishnan R, Altiok S, Youn JI, Cheng P, Celis E, Pisarev V, Sherman S, Sporn MB, Gabrilovich D. Tumor-infiltrating myeloid cells induce tumor cell resistance to cytotoxic T cells in mice. J Clin Invest. 2011;121:4015–29. doi: 10.1172/JCI45862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagaraj S, Youn JI, Weber H, Iclozan C, Lu L, Cotter MJ, Meyer C, Becerra CR, Fishman M, Antonia S, et al. Anti-inflammatory triterpenoid blocks immune suppressive function of MDSCs and improves immune response in cancer. Clin Cancer Res. 2010;16:1812–23. doi: 10.1158/1078-0432.CCR-09-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, Cho H, et al., eds. SEER Cancer Statistics Review.(2975-2010) National Cancer Institute; http://seer.cancer.gov/csr/1975_2010/
- 5.American Cancer Society. Cancer Facts & Figures 2012. American Cancer Society 2012
- 6.Sawant A, Schafer CC, Jin TH, Zmijewski J, Tse HM, Roth J, Sun Z, Siegal GP, Thannickal VJ, Grant SC, et al. Enhancement of antitumor immunity in lung cancer by targeting myeloid-derived suppressor cell pathways. Cancer Res. 2013;73:6609–20. doi: 10.1158/0008-5472.CAN-13-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samlowski WE, Petersen R, Cuzzocrea S, Macarthur H, Burton D, McGregor JR, Salvemini D. A nonpeptidyl mimic of superoxide dismutase, M40403, inhibits dose-limiting hypotension associated with interleukin-2 and increases its antitumor effects. Nat Med. 2003;9:750–5. doi: 10.1038/nm874. [DOI] [PubMed] [Google Scholar]
- 8.Gubser PM, Bantug GR, Razik L, Fischer M, Dimeloe S, Hoenger G, Durovic B, Jauch A, Hess C. Rapid effector function of memory CD8+ T cells requires an immediate-early glycolytic switch. Nat Immunol. 2013;14:1064–72. doi: 10.1038/ni.2687. [DOI] [PubMed] [Google Scholar]
- 9.van der Windt GJ, O’Sullivan D, Everts B, Huang SC, Buck MD, Curtis JD, Chang CH, Smith AM, Ai T, Faubert B, et al. CD8 memory T cells have a bioenergetic advantage that underlies their rapid recall ability. Proc Natl Acad Sci U S A. 2013;110:14336–41. doi: 10.1073/pnas.1221740110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sukumar M, Liu J, Ji Y, Subramanian M, Crompton JG, Yu Z, Roychoudhuri R, Palmer DC, Muranski P, Karoly ED, et al. Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. J Clin Invest. 2013;123:4479–88. doi: 10.1172/JCI69589. [DOI] [PMC free article] [PubMed] [Google Scholar]