Abstract
Subacute sclerosing panencephalitis (SSPE) is a slow infection caused by measles virus in which several years separate recovery from typical acute measles and the development of a slowly progressive neurological disease. We have investigated replication of measles virus in brain tissue obtained after the onset of neurological disease and in the terminal phase. With a hybridization tomographic technique that combines in situ hybridization with macroradioautographic screening of large areas of tissue, we analyzed the spatial and temporal distribution of virus genes in vivo, using region- and strand-specific probes for the nucleocapsid and matrix genes. We show that early in the course of SSPE there is a global repression in the synthesis and expression of the genome. In the final stage of SSPE most infected cells still have depressed levels of plus- and minus-strand viral RNA and contain nucleocapsid protein but lack matrix protein. These findings provide further evidence for a unified view of slow infections of the nervous system, where the general constraints on virus gene expression provide an explanation for persistence of virus in the face of the host's immune response, and the slow evolution of pathological change. In the final phases of SSPE the more specific block in virus replication accounts for the cell-associated state of the virus and the difficulty in virus isolation.
Full text
PDF

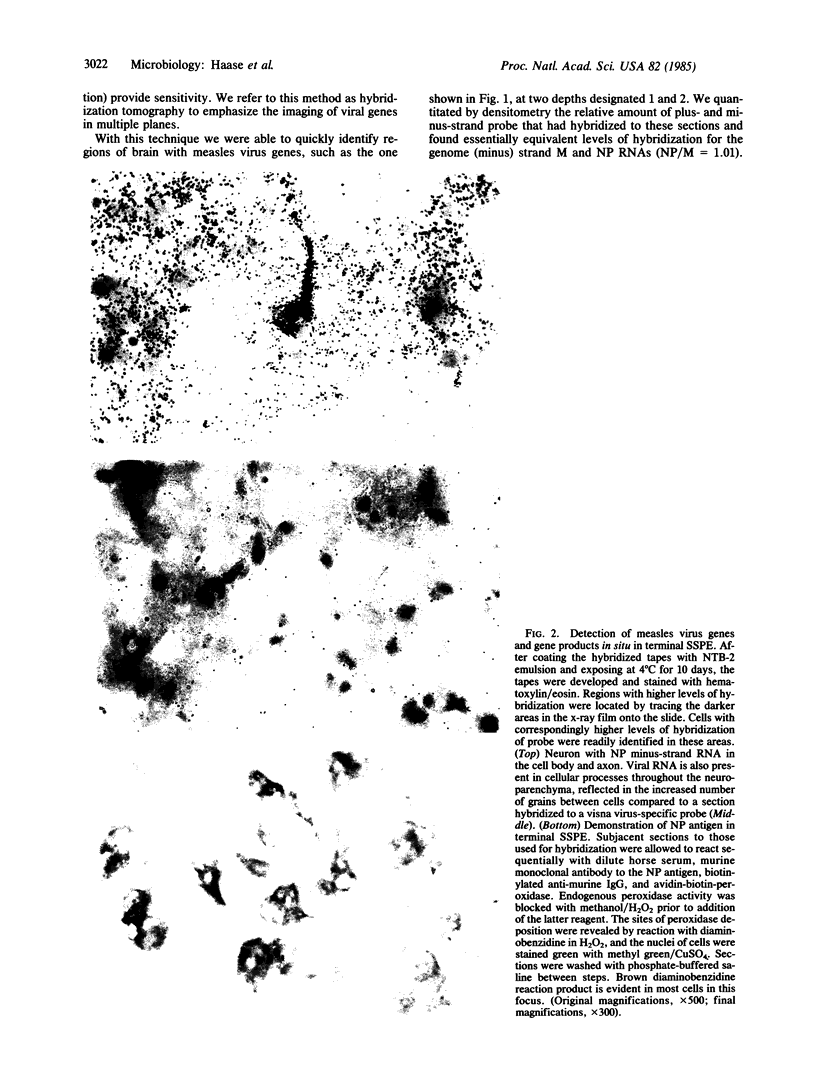
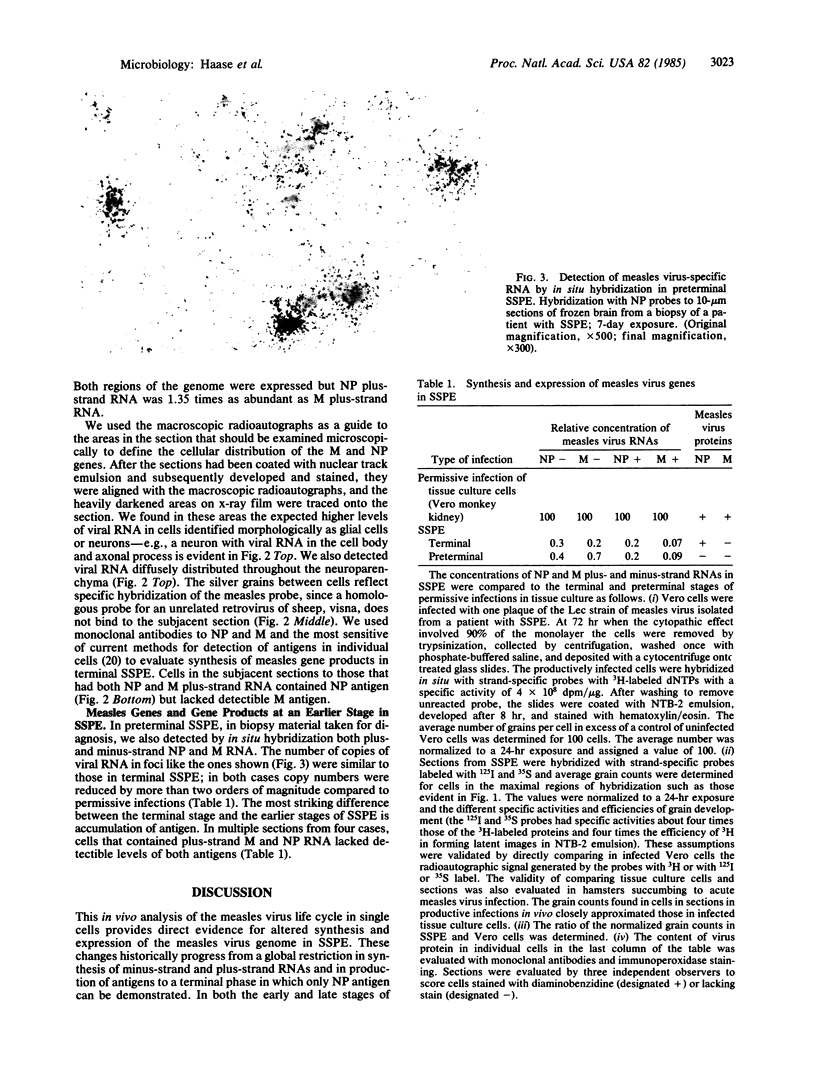

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brahic M., Stowring L., Ventura P., Haase A. T. Gene expression in visna virus infection in sheep. Nature. 1981 Jul 16;292(5820):240–242. doi: 10.1038/292240a0. [DOI] [PubMed] [Google Scholar]
- Brahic M., Stroop W. G., Baringer J. R. Theiler's virus persists in glial cells during demyelinating disease. Cell. 1981 Oct;26(1 Pt 1):123–128. doi: 10.1016/0092-8674(81)90040-4. [DOI] [PubMed] [Google Scholar]
- Brown D. M., Frampton J., Goelet P., Karn J. Sensitive detection of RNA using strand-specific M13 probes. Gene. 1982 Dec;20(2):139–144. doi: 10.1016/0378-1119(82)90032-4. [DOI] [PubMed] [Google Scholar]
- Burk K. H., Oefinger P. E., Dreesman G. R. Detection of non-A, non-B hepatitis antigen by immunocytochemical staining. Proc Natl Acad Sci U S A. 1984 May;81(10):3195–3199. doi: 10.1073/pnas.81.10.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter M. J., Willcocks M. M., ter Meulen V. Defective translation of measles virus matrix protein in a subacute sclerosing panencephalitis cell line. Nature. 1983 Sep 8;305(5930):153–155. doi: 10.1038/305153a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser N. W., Lawrence W. C., Wroblewska Z., Gilden D. H., Koprowski H. Herpes simplex type 1 DNA in human brain tissue. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6461–6465. doi: 10.1073/pnas.78.10.6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner R. C., Howarth A. J., Hahn P., Brown-Luedi M., Shepherd R. J., Messing J. The complete nucleotide sequence of an infectious clone of cauliflower mosaic virus by M13mp7 shotgun sequencing. Nucleic Acids Res. 1981 Jun 25;9(12):2871–2888. doi: 10.1093/nar/9.12.2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorecki M., Rozenblatt S. Cloning of DNA complementary to the measles virus mRNA encoding nucleocapsid protein. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3686–3690. doi: 10.1073/pnas.77.6.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase A. T., Gantz D., Blum H., Stowring L., Ventura P., Geballe A., Moyer B., Brahic M. Combined macroscopic and microscopic detection of viral genes in tissues. Virology. 1985 Jan 15;140(1):201–206. doi: 10.1016/0042-6822(85)90462-3. [DOI] [PubMed] [Google Scholar]
- Haase A. T., Stowring L., Ventura P., Burks J., Ebers G., Tourtellotte W., Warren K. Detection by hybridization of viral infection of the human central nervous system. Ann N Y Acad Sci. 1984;436:103–108. doi: 10.1111/j.1749-6632.1984.tb14780.x. [DOI] [PubMed] [Google Scholar]
- Haase A. T., Swoveland P., Stowring L., Ventura P., Johnson K. P., Norrby E., Gibbs C. J., Jr Measles virus genome in infections of the central nervous system. J Infect Dis. 1981 Aug;144(2):154–160. doi: 10.1093/infdis/144.2.154. [DOI] [PubMed] [Google Scholar]
- Hall W. W., Choppin P. W. Evidence for lack of synthesis of the M polypeptide of measles virus in brain cells in subacute sclerosing panencephalitis. Virology. 1979 Dec;99(2):443–447. doi: 10.1016/0042-6822(79)90026-6. [DOI] [PubMed] [Google Scholar]
- Hall W. W., Choppin P. W. Measles-virus proteins in the brain tissue of patients with subacute sclerosing panencephalitis: absence of the M protein. N Engl J Med. 1981 May 7;304(19):1152–1155. doi: 10.1056/NEJM198105073041906. [DOI] [PubMed] [Google Scholar]
- Hall W. W., Lamb R. A., Choppin P. W. Measles and subacute sclerosing panencephalitis virus proteins: lack of antibodies to the M protein in patients with subacute sclerosing panencephalitis. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2047–2051. doi: 10.1073/pnas.76.4.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall W. W., Sahgal V., Harter D. H., Choppin P. W. Abnormal levels of antibodies to measles-virus proteins in patient with mental retardation and seizures 24 years after measles encephalitis. Lancet. 1979 Nov 3;2(8149):967–968. doi: 10.1016/s0140-6736(79)92669-2. [DOI] [PubMed] [Google Scholar]
- Heidecker G., Messing J., Gronenborn B. A versatile primer for DNA sequencing in the M13mp2 cloning system. Gene. 1980 Jun;10(1):69–73. doi: 10.1016/0378-1119(80)90145-6. [DOI] [PubMed] [Google Scholar]
- Koch E. M., Neubert W. J., Hofschneider P. H. Lifelong persistence of paramyxovirus Sendai-6/94 in C129 mice: detection of a latent viral RNA by hybridization with a cloned genomic cDNA probe. Virology. 1984 Jul 15;136(1):78–88. doi: 10.1016/0042-6822(84)90249-6. [DOI] [PubMed] [Google Scholar]
- Koch E. M., Neubert W. J., Hofschneider P. H. Lifelong persistence of paramyxovirus Sendai-6/94 in C129 mice: detection of a latent viral RNA by hybridization with a cloned genomic cDNA probe. Virology. 1984 Jul 15;136(1):78–88. doi: 10.1016/0042-6822(84)90249-6. [DOI] [PubMed] [Google Scholar]
- Lavi E., Gilden D. H., Highkin M. K., Weiss S. R. Persistence of mouse hepatitis virus A59 RNA in a slow virus demyelinating infection in mice as detected by in situ hybridization. J Virol. 1984 Aug;51(2):563–566. doi: 10.1128/jvi.51.2.563-566.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrby E., Chen S. N., Togashi T., Shesberadaran H., Johnson K. P. Five measles virus antigens demonstrated by use of mouse hybridoma antibodies in productively infected tissue culture cells. Arch Virol. 1982;71(1):1–11. doi: 10.1007/BF01315171. [DOI] [PubMed] [Google Scholar]
- Ricca G. A., Taylor J. M., Kalinyak J. E. Simple rapid method for the synthesis of radioactively labeled cDNA hybridization probes utilizing bacteriophage M13mp7. Proc Natl Acad Sci U S A. 1982 Feb;79(3):724–728. doi: 10.1073/pnas.79.3.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux L., Waldvogel F. A. Instability of the viral M protein in BHK-21 cells persistently infected with Sendai virus. Cell. 1982 Feb;28(2):293–302. doi: 10.1016/0092-8674(82)90347-6. [DOI] [PubMed] [Google Scholar]
- Rozenblatt S., Gesang C., Lavie V., Neumann F. S. Cloning and characterization of DNA complementary to the measles virus mRNA encoding hemagglutinin and matrix protein. J Virol. 1982 Jun;42(3):790–797. doi: 10.1128/jvi.42.3.790-797.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togashi T., Orvell C., Vartdal F., Norrby E. Production of antibodies against measles virions by use of the mouse hybridoma technique. Arch Virol. 1981;67(2):149–157. doi: 10.1007/BF01318598. [DOI] [PubMed] [Google Scholar]
- ter Meulen V., Hall W. W. Slow virus infections of the nervous system: virological, immunological and pathogenetic considerations. J Gen Virol. 1978 Oct;41(1):1–25. doi: 10.1099/0022-1317-41-1-1. [DOI] [PubMed] [Google Scholar]
- ter Meulen V., Katz M., Müller D. Subacute sclerosing panencephalitis: a review. Curr Top Microbiol Immunol. 1972;57:1–38. doi: 10.1007/978-3-642-65297-4_1. [DOI] [PubMed] [Google Scholar]